Citation:
Yan SHI, Zhi Feng FU, Yu Dong ZHANG, Shu Ke JIAO. Synthesis of Comblike Poly(methyl methacrylate) by Atom Transfer Radical Polymerization with Poly(ethyl 2-bromoacrylate) as Macroinitiator[J]. Chinese Chemical Letters,
;2003, 14(12): 1289-1292.

-
Comblike poly(methyl methacrylate) was synthesized by atom transfer radical polymerization of methyl methacrylate with poly(ethyl 2-bromoacrylate) as a macroinitiator, which was prepared by conventional free radical polymerization of ethyl 2-bromoacrylate. The obtained comblike polymers were characterized by GPC and 1H NMR.
-
INTRODUCTION
The improvement of human living standard is inseparable from the development of modern industry followed by a large number of organic pollutants.[1, 2] The pollutants are difficult to degrade and easy to diffuse, which has become a common challenge faced by all mankind.[3, 4] The traditional methods for removing organic pollutants, including physical adsorption, chemical treatment and biological decomposition, have many disadvantages, such as high cost, harmful by-products and complex process.[5-10] Therefore, the semiconductor photocatalytic technology has attracted increasing attention for its potential in wastewater treatment due to its green and energy saving characteristics.[11-17] So far, many photocatalysts have been developed, such as TiO2 and its nanocomposites.[18, 19] TiO2 is a kind of widely used commercial photocatalyst, but it can only absorb ultraviolet light and has no visible light response.[20-24] Therefore, photocatalysts with visible light response have become an important research topic in recent years, including ZnO, [25] SnO2, [26] CdS, [27] BiVO4, [28] Ta3N5, [29, 30] g-C3N4[31-33] and so on. Among them, g-C3N4 as a non-metallic organic semiconductor material has become an extremely attractive visible light photocatalyst due to its suitable band structure (2.7 eV), high chemical stability, and low production cost.[34-36] However, the original g-C3N4 has some inherent disadvantages, such as small specific surface area, poor visible light utilization and high carrier recombination rate.[37-39]
In order to overcome the above drawbacks, researchers have developed many strategies to improve the photocatalytic perfor-mance of g-C3N4, such as metal and non-metal doping, [40-43] in troduction of vacancy defects, [44] morphology modulation, [45, 46] construction of heterojunctions, [47-49] etc. Among these methods, non-metallic doping is an attractive strategy to adjust the composition and morphology of g-C3N4-based photocatalysts, which can maintain the non-metallic properties of g-C3N4 with low cost, earth-abundant as well as stable and adjustable properties. By doping non-metallic elements, the band structure of g-C3N4 can be adjusted to tune its light-absorption ability and redox capacity, and the photocatalytic activity can be improved.[50] Lv et al.[51] prepared S-doped g-C3N4 nanosheets using thiourea as precursor system, and the obtained S-doped g-C3N4 nanosheets have a high specific surface area with a reduced bandgap and increased surface N defects. Kumar et al.[52] synthesized a novel in-situ Se-doped g-C3N4 nanosheet by a simple one-pot two-step strategy. The Se doping generated an intermediate state in g-C3N4, which could significantly extend the light absorption to 570 nm. Thus, the Se-doped g-C3N4 nanosheet showed an enhanced efficiency for solar fuel production from CO2. Although most previous reports have focused on single-element doping, some studies have shown that co-doping with more than one nonmetallic atom is more efficient than single-element doping. For example, (P, S) co-doping, [53] (S, O) co-doping[54] and (C, I) co-doping[55] all show a higher photocatalytic activity and unusual physicochemical characteristic. Therefore, it is necessary to study the multi-element doping in g-C3N4 framework and explore the mechanism of its enhanced photocatalytic activity.[56]
In this work, sulfur and selenium co-doped graphitic carbon nitride (SSCN) with efficient photocatalytic activity was synthesized by synchronously introducing sulfur and selenium atoms into the melon structure of g-C3N4 (GCN) via a facile solid-phase thermal reaction of GCN and SeS2. The as-prepared SSCN possesses a larger specific surface area with a richer pore structure that provides more active centers for catalytic reaction. More importantly, the asymmetric structure of SSCN due to introducing sulfur and selenium not only maintains the easier activation of π-π* electron transition but also awakens the n-π* electron transition in g-C3N4. Moreover, the n-π* electron transition of SSCN can be controlled through changing the amount of SeS2, which can greatly extend the photo-response range to 600 nm. As a result, the SSCN samples show an excellent photo-degradation performance for typical antibiotic of tetracycline hydrochloride (TC).
RESULTS AND DISCUSSION
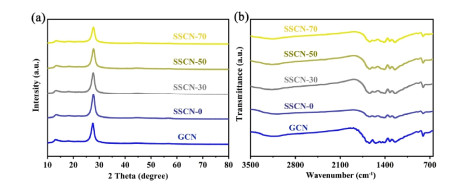
Structure and Property Analysis. Figure 1a displays the XRD patterns of the synthesized samples. Two distinct diffraction peaks of all the samples locate at ~13.2° and 27.5°, corresponding to the (1 0 0) and (0 0 2) planes of g-C3N4, respectively. Compared with GCN, SSCN samples display a lower intensity and broader half peak of the (0 0 2) crystal plane, which is ascribed to the fact that the S and Se doping affects the periodic structure of g-C3N4. This decreased periodic structure not only maintains the easier activation of π-π* electron transition, but also awakens the n-π* electron transition in g-C3N4. The chemical structures of the GCN and SSCN samples were further investigated by the FT-IR spectrum (Figure 1b). The characteristic vibration peaks of as-prepared photocatalysts at 810, 1200~1600 and 3000~3500 cm-1 are attributed to the characteristics from tri-s-triazine ring units, stretching modes of aromatic C-N heterocycle, and stretching vibration of amine groups (NHx), respectively. No obvious C-S and C-Se peaks could be observed, mainly due to the low content of SeS2 and another factor which may be that the characteristic peaks of C-S and C-Se are overlapped with the stretching vibration mode of g-C3N4.
Figure 1
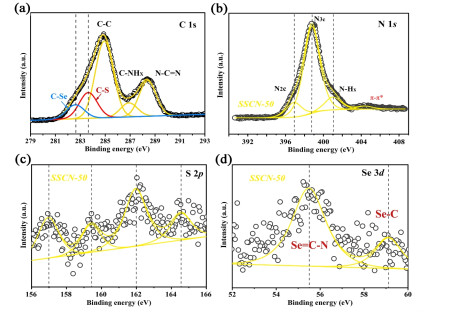
The surface compositions and chemical states of SSCN-50 sample are examined by XPS characterization. Figure 2a shows the C 1s spectrum with three characteristic peaks at about 284.8, 286.9 and 288.4 eV corresponding to C-C, C-NHX and N-C=N, respectively. It is worth noting that two new peaks appear at 282.8 and 283.6 eV, attributed to C-Se and C-S, respectively. As shown in Figure 2b, the N 1s spectrum of SSCN-50 displays three peaks at 396.9, 398.7 and 401.1 eV, which can be respectively associated with N2C, N3C and N-Hx. The peak of S element in Figure 2c can be divided into 157.0, 159.4, 161.9 and 164.5 eV assigned to S 2p in SSCN-50. The Se 3d spectrum is deconvoluted into two main peaks with binding energies of 55.5 and 59.1 eV, corresponding to Se=C-N and C-Se (Figure 2d), respectively.[57, 58] The above results indicate that the sulfur and selenium co-doped g-C3N4 has been synthesized.
Figure 2
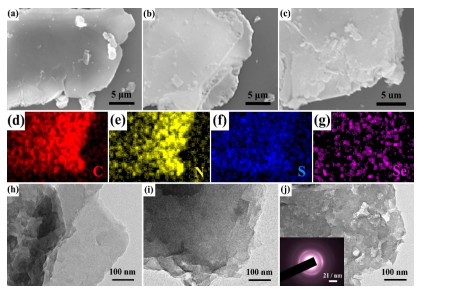
The morphologies and microstructures of GCN, SSCN-0 and SSCN-50 photocatalysts are characterized by SEM and TEM. As shown in Figure 3a-c, the SEM images of GCN, SSCN-0 and SSCN-50 photocatalysts show the 2D structure characteristic. However, compared with GCN and SSCN-0, the surface of SSCN-50 becomes slightly rough due to the doping of S and Se in g-C3N4. As shown in Figure 3d-g, the EDX element mapping images display that the C, N, S and Se are homogeneously distributed in the SSCN-50 sample. The TEM images of GCN, SSCN-0 and SSCN-50 samples are shown in Figure 3h-j, and some appeared pores increase the specific surface area of SSCN-50, which is consistent with the N2 adsorption-desorption result. In addition, the two diffraction rings in SAED pattern (inset of Figure 3j) correspond to the (1 0 0) and (0 0 2) planes of SSCN-50.
Figure 3
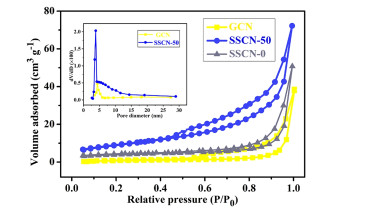
The N2 adsorption-desorption isotherms and pore size distribution curves of GCN and SSCN-50 are shown in Figure 4. It is noted that the samples show a similar type-IV adsorption-desorption isotherms and H3 hysteresis loop, implying the presence of mesopore in photocatalysts. Notably, the specific surface area of SSCN-50 is about 32.43 m2 g-1, which is almost 4 times higher than that of GCN (8.06 m2 g-1). Moreover, the specific surface area of SSCN-0 has been also tested to be 8.67 m2 g-1. The increased surface area is attributed to the rich pore structure (inset of Figure 4) resulting from S and Se co-doping. It is well known that the large specific surface area with more active sites is beneficial for improving the photocatalytic performance.
Figure 4
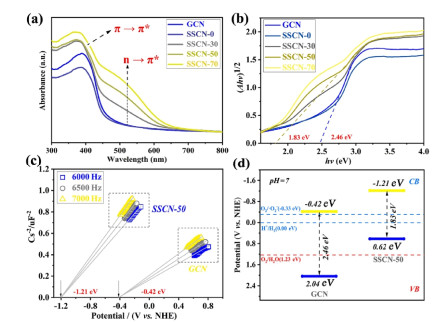
The light absorption and utilization of the prepared samples are characterized by UV-vis DRS. As shown in Figure 5a, the SSCN samples show an enhanced light absorption in both UV and visible light regions. A typical absorption edge around 390 nm is identified as the intrinsic electronic transition (π-π*) of g-C3N4. Notably, a new absorption peak at about 490 nm appears in the UV-vis DRS of SSCN samples, which is ascribed to the (n-π*) electronic transition.[59] The generation of (n-π*) electronic transition can be attributed to the asymmetrical structure of SSCN samples resulting from S and Se co-doping. According to the Kubelka-Munk function (F(R)hv)n versus the energy of exciting light (hv), the bandgap of GCN and SSCN-50 is calculated to be 2.46 and 1.83 eV, respectively (Figure 5b). The Mott-Schottky curves are applied to determine the conduction band (CB) potential of GCN and SSCN-50 samples. As shown in Figure 5c, the GCN and SSCN-50 samples exhibit typical n-type semiconductor characteristics. The CB potentials of GCN and SSCN-50 are determined to be -0.42 and -1.21 V (vs. NHE), respectively. Through combining the value of bandgap and CB potential, the valence band (VB) potentials of GCN and SSCN-50 are determined to be 2.04 and 0.62 V, respectively. The band structure of as-prepared samples is shown in Figure 5d.
Figure 5
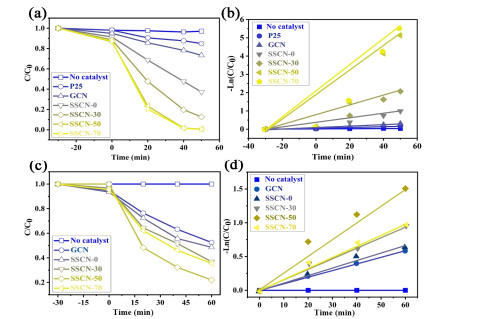
Photocatalytic Activity. The photocatalytic activity of prepared samples was analyzed by degrading antibiotic of TC and typical dye of RhB under visible light irradiation. As depicted in Figure 6a, no significant degradation of RhB is observed without the addition of any photocatalyst. For P25 and GCN samples, only 15.1% and 26.7% of RhB can be respectively removed after 45 minutes of illumination. The photocatalytic activities of all SSCN samples are greatly improved and the highest efficiency of RhB degradation of SSCN-50 can attain 99.4%. The kinetic profile of RhB can be approximated by linearly transforming ln(C0/Ct) = kt to a quasi-level process shown in Figure 6b. The result shows that the degradation rate constant for SSCN-50 (0.067 min-1) is about 16.8 times that of GCN (0.004 min-1). In addition, the antibiotic of TC is also used to evaluate the photocatalytic performance in this work. The SSCN-50 sample also exhibits the highest photocatalytic activity (Figure 6c). Especially, 78% of TC could be degraded after 60 min light irradiation. As shown in Figure 6d, the kinetic curve of TC can be approximated as a pseudo first-order process by linear transformation ln (C0/Ct) = kt, indicating the degradation rate of SSCN-50 (0.025 min-1) to be about 2.5 times that of GCN (0.010 min-1). The above results show that the photocatalytic activity of g-C3N4 could be significantly improved by S and Se co-doping.
Figure 6
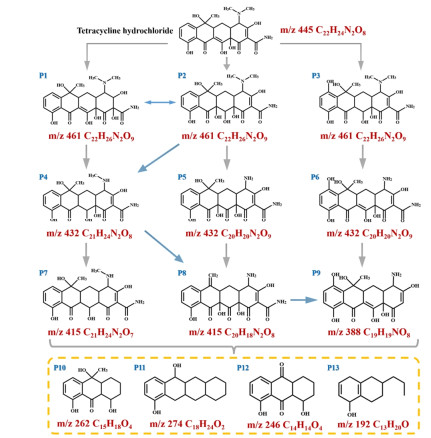
The specific degradation route for TC based on major intermediates is also proposed by LC-MS characterization. As shown in Figure 7, the intermediates P1 (m/z 461), P2 (m/z 461) and P3 (m/z 461) originate from TC cleavage by double bond breakage and hydroxyl addition. The intermediates P1 and P2 are further degraded by the loss of N-methyl and hydroxyl groups to generate intermediate P4 (m/z 432), which is then degraded by removing hydroxyl groups to produce intermediate P7 (m/z 415). The intermediate P5 (m/z 432) is produced by removing N-methyl and hydroxyl groups from P2 to produce both amino and carbonyl groups, and then P5 is further degraded by losing hydroxyl and methyl groups to produce methylene groups to form the intermediate P8 (m/z 415). The intermediate P6 is generated from P3 by removing n-methyl and producing amino group at the same time. In addition, the intermediate P9 (m/z 388) is produced through a multi-step degradation process of intermediates P6 and P8. The obtained P7, P8 and P9 are further converted to low molecular weight organic compounds (P10-P13) and eventually degraded to CO2 and H2O.
Figure 7
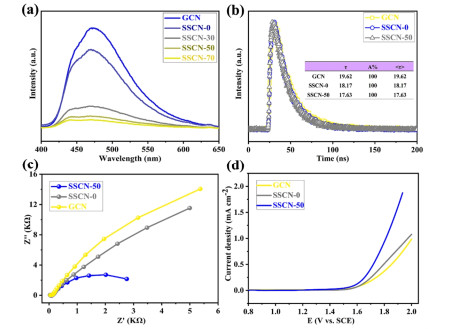
Possible Photocatalytic Mechanism. The PL spectra are carried out to investigate the separation and migration of photogenerated carriers.[60-63] As shown in Figure 8a, the SSCN-50 sample shows an evident quenching of PL intensity compared with GCN, revealing that the recombination rate of photo-induced electrons and holes for SSCN-50 is effectively suppressed. Meanwhile, the time-resolved PL spectra of GCN, SSCN-0 and SSCN-50 are recorded (Figure 8b). The photoluminescence intensity of SSCN-50 decays is much faster than that of GCN. The emission decay data could be fitted by double-exponential formula with one component of life time. It is found that the photo-generated carrier lifetime of SSCN-50 (17.63 ns) is less than that of GCN (19.62 ns) due to the fact that the introduction of selenium and sulfur can shorten the migration path of photo-generated electrons and promote the electron transfer of g-C3N4.[64-66] As shown in Figure 8c, by comparison with the GCN and SSCN-0, SSCN-50 shows a significantly decreased arc radius, suggesting a low charge transfer resistance. The LSV curves of the samples show that the over potential of SSCN-50 sample is significantly reduced compared to GCN and SSCN-0 during the whole voltage range (Figure 8d), thus demonstrating a fast charge transfer and separation in SSCN-50.
Figure 8
CONCLUSIONS
In summary, sulfur and selenium co-doped graphitic carbon nitride (SSCN) has been synthesized successfully by synchronously introducing sulfur and selenium atoms into the melon structure of g-C3N4. The as-prepared SSCN possesses a larger specific surface area with a richer pore structure that provides more active centers for catalytic reaction. More importantly, the asymmetric structure of SSCN due to introducing sulfur and selenium not only maintains the easier activation of π-π* electron transition but also awakens the n-π* electron transition in g-C3N4. The experimental results indicate that SSCN-50 shows the best photocatalytic performance, achieving 78.0% and 99.4% degradation of antibiotics (TC) and organic dyes (RhB), which is 2.5 and 16.8 times higher than GCN, respectively. This work demonstrates a simple, low-cost and sustainable strategy for the preparation of high-performance non-metallic g-C3N4 photocatalyst.
EXPERIMENTAL
Preparation of Sulfur and Selenium Co-doped g-C3N4 (SSCN). All chemicals in this experiment were analytical grade and used without further purification. The sulfur and selenium co-doped g-C3N4 was fabricated via a facile solid-state thermal treatment of g-C3N4 and SeS2. In a typical synthesis, 5 g of melamine was added into an alumina crucible with a cover and then heated at 550 ℃ for 2 h by using a ramp rate of 5 ℃ min-1. The sample was fabricated and grounded into powder. Afterwards, 300 mg of g-C3N4 was mixed with a certain amount of SeS2 evenly, and then put into an alumina crucible with a cover. Subsequently, the mixture was heated at 400 ℃ for 1 h at a heating rate of 5 ℃ min-1. The as-prepared samples were labeled as SSCN-0, SSCN-30, SSCN-50 and SSCN-70, when the mass of SeS2 in the mixture is 0, 30, 50 and 70 mg, respectively. The pure g-C3N4 (GCN) was prepared by direct calcination of melamine at 550 ℃ for 2 h.
Characterization. X-ray diffraction (XRD) pattern of the photocatalyst was analyzed by a Dmax Rapid II diffractometer. Fourier transform infrared spectroscopy (FT-IR) was measured by a Spotlight 400 spectrometer. X-ray photoelectron spectroscopy (XPS) was examined by a Thermo ESCALAB 250 XPS equipment with standard monochromatic light source. Scanning electron microscopy (SEM) image and energy dispersive X-ray (EDX) elemental mapping were tested by a FEI Quanta 450 FEG microscope. Electrochemical impedance spectroscopy (EIS), Mott-Schottky plot and linear sweep voltammetry (LSV) were conducted by using a standard three-electrode cell with probe solution as the electrolyte, platinum as a counter, saturated calomel electrode as a reference electrode and ITO glass deposited with sample as a working electrode. Photoluminescence (PL) spectrum was determined by a FLSP920 Edinburgh Fluorescence Spectrometer. UV-vis diffuse reflectance spectrum (UV-vis DRS) was performed on a Cary 500 spectrometer.
Photocatalytic Test. To evaluate the photocatalytic efficiency of as-prepared samples, 30 mg photocatalysts for the degradation of typical antibiotic TC (30 mg/L, 80 mL) and 60 mg photocatalysts for degradation of RhB (10 mg/L, 80 mL) were studied by using 500 W Xe lamp as light source (λ > 420 nm). To ensure the adsorption-desorption equilibrium between the target contaminants and photocatalysts, the suspension was treated by ultrasonic for 5 min and stirred in the dark for 30 min. During the irradiation process, 3 mL suspension was extracted at given time intervals, then centrifuged at 10000 rpm for 5 min to remove the solid photocatalysts. The residual concentration of antibiotic TC and RhB was analyzed by a UV-vis-NIR spectrophotometer.
ACKNOWLEDGEMENTS: This work is supported by the National Natural Science Foundation of China (22008185), Shaanxi Provincial Key Research and Development Program (2022GY-166), and Scientific Research Program Funded by Shaanxi Provincial Education Department (19JK0376). The authors declare no competing interests.
COMPETING INTERESTS
For submission: https://mc03.manuscriptcentral.com/cjsc
Supplementary information is available for this paper at http://manu30.magtech.com.cn/jghx/EN/10.14102/j.cnki.0254-5861.2022-0152
ADDITIONAL INFORMATION
-

-
-
[1]
Yu-Yu Tan , Lin-Heng He , Wei-Min He . Copper-mediated assembly of SO2F group via radical fluorine-atom transfer strategy. Chinese Chemical Letters, 2024, 35(9): 109986-. doi: 10.1016/j.cclet.2024.109986
-
[2]
Xiang Li , Beibei Zhang , Zhixiang Wang , Xiangyu Chen . Organocatalyzed iodine-mediated reversible-deactivation radical polymerization via photoinduced charge transfer complex catalysis. Chinese Chemical Letters, 2025, 36(6): 110383-. doi: 10.1016/j.cclet.2024.110383
-
[3]
Mengyuan Li , Xitong Ren , Yanmei Gao , Mengyao Mu , Shiping Zhu , Shufang Tian , Minghua Lu . Constructing bifunctional magnetic porous poly(divinylbenzene) polymer for high-efficient removal and sensitive detection of bisphenols. Chinese Chemical Letters, 2024, 35(12): 109699-. doi: 10.1016/j.cclet.2024.109699
-
[4]
Yaxuan Jin , Chao Zhang , Guigang Zhang . Atomically dispersed low-valent Au on poly(heptazine imide) boosts photocatalytic hydroxyl radical production. Chinese Journal of Structural Chemistry, 2024, 43(12): 100414-100414. doi: 10.1016/j.cjsc.2024.100414
-
[5]
Bing Niu , Honggao Huang , Liwei Luo , Li Zhang , Jianbo Tan . Coating colloidal particles with a well-defined polymer layer by surface-initiated photoinduced polymerization-induced self-assembly and the subsequent seeded polymerization. Chinese Chemical Letters, 2025, 36(2): 110431-. doi: 10.1016/j.cclet.2024.110431
-
[6]
Yuan Teng , Zichun Zhou , Jinghua Chen , Siying Huang , Hongyan Chen , Daibin Kuang . Dual atom-bridge effect promoting interfacial charge transfer in 2D/2D Cs3Bi2Br9/BiOBr epitaxial heterojunction for efficient photocatalysis. Chinese Chemical Letters, 2025, 36(2): 110430-. doi: 10.1016/j.cclet.2024.110430
-
[7]
Bowen Xu , Jiahao Chen , Lulu Cui , Xinyue Li , Yuan Xue , Sheng Han . Terpolymers of alkyl methacrylate-trans anethole-1,2,3,6-tetrahydrophthalic anhydride copolymers: A low dosage and high-efficiency cold flow improver for diesel fuel. Chinese Chemical Letters, 2025, 36(5): 110196-. doi: 10.1016/j.cclet.2024.110196
-
[8]
Shuwen SUN , Gaofeng WANG . Design and synthesis of a Zn(Ⅱ)-based coordination polymer as a fluorescent probe for trace monitoring 2, 4, 6-trinitrophenol. Chinese Journal of Inorganic Chemistry, 2025, 41(4): 753-760. doi: 10.11862/CJIC.20240399
-
[9]
Zhenchun Yang , Bixiao Guo , Zhenyu Hu , Kun Wang , Jiahao Cui , Lina Li , Chun Hu , Yubao Zhao . Molecular engineering towards dual surface local polarization sites on poly(heptazine imide) framework for boosting H2O2 photo-production. Chinese Chemical Letters, 2024, 35(8): 109251-. doi: 10.1016/j.cclet.2023.109251
-
[10]
Haojie Song , Laiyu Luo , Siyu Wang , Guo Zhang , Baojiang Jiang . Advances in poly(heptazine imide)/poly(triazine imide) photocatalyst. Chinese Chemical Letters, 2024, 35(10): 109347-. doi: 10.1016/j.cclet.2023.109347
-
[11]
Xiao-Ya Yuan , Cong-Cong Wang , Bing Yu . Recent advances in FeCl3-photocatalyzed organic reactions via hydrogen-atom transfer. Chinese Chemical Letters, 2024, 35(9): 109517-. doi: 10.1016/j.cclet.2024.109517
-
[12]
Yuan Liu , Zhu Yin , Xintuo Yang , Jiajia Cheng . Advances in photocatalytic deracemization of sp3-hybridized chiral centers via hydrogen atom transfer. Chinese Chemical Letters, 2025, 36(5): 110521-. doi: 10.1016/j.cclet.2024.110521
-
[13]
Fan Chen , Xiaoyu Zhao , Weihang Miao , Yingying Li , Ye Yuan , Lingling Chu . Regio- and enantioselective hydrofluorination of internal alkenes via nickel-catalyzed hydrogen atom transfer. Chinese Chemical Letters, 2025, 36(5): 110239-. doi: 10.1016/j.cclet.2024.110239
-
[14]
Muhammad Humayun , Mohamed Bououdina , Abbas Khan , Sajjad Ali , Chundong Wang . Designing single atom catalysts for exceptional electrochemical CO2 reduction. Chinese Journal of Structural Chemistry, 2024, 43(1): 100193-100193. doi: 10.1016/j.cjsc.2023.100193
-
[15]
Xiaoman Dang , Zhiying Wu , Tangxin Xiao , Zhouyu Wang , Leyong Wang . Highly robust supramolecular polymer networks crosslinked by metallacycles. Chinese Chemical Letters, 2024, 35(12): 110208-. doi: 10.1016/j.cclet.2024.110208
-
[16]
Yaohua Li , Qi Cao , Xuanhua Li . Tailoring the configuration of polymer passivators in perovskite solar cells. Chinese Journal of Structural Chemistry, 2025, 44(2): 100413-100413. doi: 10.1016/j.cjsc.2024.100413
-
[17]
Yuxin Wang , Zhengxuan Song , Yutao Liu , Yang Chen , Jinping Li , Libo Li , Jia Yao . Methyl functionalization of trimesic acid in copper-based metal-organic framework for ammonia colorimetric sensing at high relative humidity. Chinese Chemical Letters, 2024, 35(6): 108779-. doi: 10.1016/j.cclet.2023.108779
-
[18]
Tiankai Sun , Hui Min , Zongsu Han , Liang Wang , Peng Cheng , Wei Shi . Rapid detection of nanoplastic particles by a luminescent Tb-based coordination polymer. Chinese Chemical Letters, 2024, 35(5): 108718-. doi: 10.1016/j.cclet.2023.108718
-
[19]
Mengjun Sun , Zhi Wang , Jvhui Jiang , Xiaobing Wang , Chuang Yu . Gelation mechanisms of gel polymer electrolytes for zinc-based batteries. Chinese Chemical Letters, 2024, 35(5): 109393-. doi: 10.1016/j.cclet.2023.109393
-
[20]
Huimin Gao , Zhuochen Yu , Xuze Zhang , Xiangkun Yu , Jiyuan Xing , Youliang Zhu , Hu-Jun Qian , Zhong-Yuan Lu . A mini review of the recent progress in coarse-grained simulation of polymer systems. Chinese Journal of Structural Chemistry, 2024, 43(5): 100266-100266. doi: 10.1016/j.cjsc.2024.100266
-
[1]
Metrics
- PDF Downloads(0)
- Abstract views(731)
- HTML views(2)

 Login In
Login In




 DownLoad:
DownLoad:
 DownLoad:
DownLoad: