Citation:
Jun LING, Yi Feng ZHANG, Zhi Quan SHEN. Rare Earth Complex Initiated Ring-Opening Polymerization of 2,2-Dimethyltrimethylene Carbonate[J]. Chinese Chemical Letters,
;2001, 12(1): 41-42.

-
The ring opening polymerization of 2,2-dimethyltrimethylene carbonate (DTC) initiated by single component of tris(2,6-di-tert-butyl-4-methylphenoxo) lanthanide (Ln(OAr)3) is reported. The initiators are highly active to the polymerization and give high molecular weight polymers. 1HNMR spectra and DSC measurement suggest the polymerization is free of decarboxylation. Mechanism study shows that the monomer insertion is via the break of acyl-oxygen bond of DTC.
-
1. Introduction
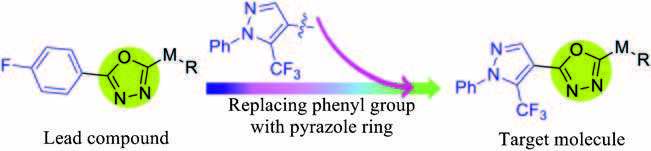
Heterocyclic substructures have been extensively studied for their powerful applications in construction of bioactive compounds [1-4]. Among them, pyrazole ring as an important functional group has already been used in the development of pharmaceuticals and agrochemicals due to its derivatives bearing multitudinous bioactivities, including anti-inflammatory, antitumor, herbicidal, insecticidal, antifungal, and antibacterial activities [5-13]. Furthermore, some pyrazole compounds have already been commercialized as fungicides, like sedaxane (Syngenta, 2005), isopyrazam (Syngenta, 2006), bixafen (Bayer, 2005), and fluxapyroxad (BASF, 2008) [14-17]. As another crucial scaffold, 1, 3, 4-oxadiazole, has exerted promising applications in creating new agrochemicals on account of the diverse bioactivities of its derivatives [18-21]. In our previous work, we had found a series of new 1, 3, 4-oxadiazole sulfone compounds (structure depicted in Fig. 1, lead compound) with high antibacterial/fungicidal bioactivities [22-24]. In order to find new structures with antibacterial/antifungal bioactivities, the two functional moieties of pyrazole and 1, 3, 4-oxadiazole were combined into one molecule by replacing the phenyl group to pyrazole moiety at the 5-position of the lead compound, as shown in Fig. 1. All the title compounds were bioassayed against pathogenic bacteria Xanthomonas oryzae pv. oryzae (Xoo) and five phytopathogenic fungi.
图 1
2. Experimental
All the chemicals were purchased from Aladdin and used as received. The organic solvents were distilled before used. NMR spectra were obtained by using a JEOL-ECX-500 apparatus. Chemical shifts were reported in parts per million (ppm) down field from TMS with the solvent resonance as the internal standard. Coupling constants (J) were reported in Hz and referred to apparent peak multiplications. MS data were recorded on an Agilent ESI-MSD Trap (VL) mass instrument.
2.1 General synthetic procedures for the target compounds (6a-6o) and (7a-7i)
A solution of carbon disulfide (0.015 mol) in ethanol (10 mL) was added dropwise to the mixture of compound 4 (0.01 mol) and potassium hydroxide (0.012 mol) in ethanol (40 mL) at room temperature. Then, the reaction mixture was heated under reflux with stirring for 8 h. After the reaction was completed, ethanol was evaporated to give unpurified intermediate 5. An appropriate halohydrocarbon (0.01 mol) was added to the solution of unpurified intermediate 5 in water (20 mL) and the mixture was stirred for 1 h at room temperature. The solid was filtered, purified by column chromatography using a mixture of petroleum ether and ethyl acetate (10:1) as the eluent, and then the pure target compounds (6a-6o) were obtained.
The compound (6a-6i) (5 mmol) and acetic acid (15 mL) were added to a 50 mL three-neck round-bottom flask equipped with a magnetic stirrer. The resulting solution was stirred for 10 min when a clear solution was obtained, and then 7% KMnO4 solution (5 mmol) was added dropwise at room temperature and the progress of the reaction was monitored by thin layer chromatography (TLC) using petroleum ether:ethyl acetate (3:1). After the reaction was completed, 10% NaHSO3 solution was added to deoxidize the unreacted KMnO4. The resulted solid was filtered, washed with water, from which the pure compounds (7a-7i) can be obtained by column chromatography using a mixture of petroleum ether and ethyl acetate (15:1) as the eluent.
2.2 in vitro antibacterial bioassay (turbidimeter test)
In our study, all the synthesized target compounds were evaluated for their antibacterial activities against Xoo by the turbidimeter test in vitro. Dimethylsulfoxide in sterile distilled water served as a blank control, Bismerthiazol and Thiodiazole Copper served as the positive controls. Approximately 40 μL of solvent NB (1.5 g beef extract, 2.5 g peptone, 0.5 g yeast powder, 5.0 g glucose, and 500 mL distilled water; pH 7.0-7.2) containing Xoo, incubated on the phase of logarithmic growth, was added to 5 mL of solvent NB containing the test compounds and positive control. The inoculated test tubes were incubated at 28±1 ℃ and continuously shaken at 180 rpm for 24-48 h until the bacteria were incubated on the logarithmic growth phase. The growth of the cultures was monitored on a microplate reader by measuring the optical density at 595 nm (OD595) given by turbidity corrected values=ODbacterial wilt-ODno bacterial wilt, and the inhibition rate I was calculated by I=(C -T)/C × 100%. C is the corrected turbidity values of bacterial growth on untreated NB (blank control), and T is the corrected turbidity values of bacterial growth on treated NB. The experiment was repeated three times.
3. Results and discussion
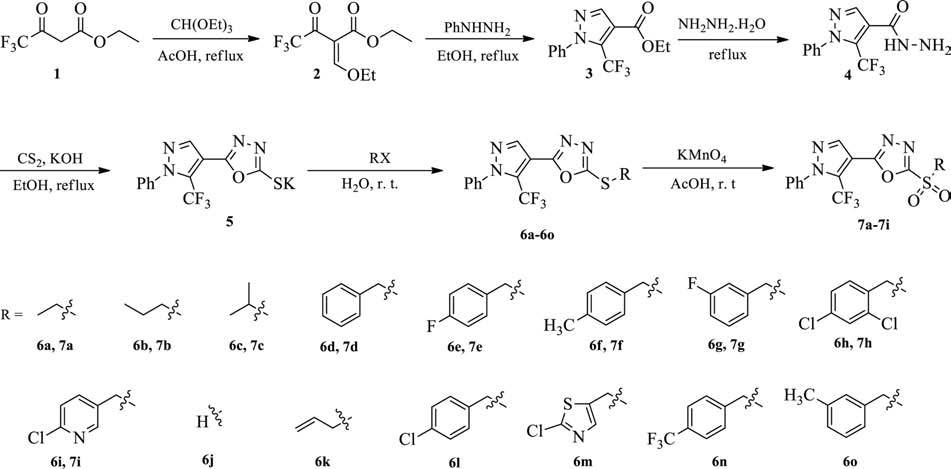
The synthesis and structures of (6a-6o), and (7a-7i) are shown in Scheme 1. Briefly, ethyltrifluoroacetoacetate (1) was treated with triethoxymethane to give intermediate (E)-2-trifluoroacetyl-3-ethoxy-2-propenoate (2), followed by the cyclocondensation reaction to provide an important intermediate ethyl 1-phenyl-5-(trifluoromethyl)-1H-pyrazole-4-carboxylate (3) containing pyrazole group in 82% yield. Next, the hydrazide 4 was obtained through refluxing 3 in hydrazine hydrate with the yield of 94%. A subsequent reaction with carbon disulfide in the presence of potassium hydroxide leaded to the formation of the crucial intermediate 5 containing 1, 3, 4-oxadiazole. Finally, the corresponding target thioethers (6a-6o) were achieved via thioetherification with halogenated agents in good yields ranging from 76% to 85%, and subsequently converted into the corresponding sulfones (7a-7i) by oxidizing the related thioether at room temperature. All the structures were confirmed by 1H NMR, 13C NMR, and MS (detailed information see Supplementary data).
Scheme 1
In our study, we first evaluated the antibacterial activity of all the title compounds via turbidmeter test [25-27] against pathogenic bacteria Xanthomonas oryzae pv. oryzae (Xoo), which was considered as one of devastative bacteria against rice in ricegrowing countries. Meanwhile, the commercial agricultural antibacterial bismerthiazol (BT) and thiodiazole copper (TC) were employed for the comparison of bioactivity in vitro. Preliminary bioassays revealed that most of the target compounds exerted appreciable inhibition bioactivity against Xoo in the dosage of 200 or 100 μg/mL (Table 1). Among them, compounds 6c, 6e, 6f, 6j, 7a, 7b, and 7c gives the inhibition rate above 72.3% against Xoo in the dosage of 200 μg/mL, which were better than that of BT (72.1%) and TC (64.2%); while compounds 6c, 6f, 7a, 7b, and 7c offersbetter inhibition rate above 66.2% against Xoo than that of BT (53.7%) and TC (43.1%) in the dosage of 100 μg/mL. The half-maximal effective concentration (EC50) values of 6c, 7a, 7b, and 7c were detected as 47.5, 31.6, 65.7, and 16.6 μg/mL, respectively, which were obviously better than that of commercial bactericides (92.6 or 121.8 μg/mL). Based on the above results, among all the thioether compounds (6a-6o), the isopropyl group compound (6c) exhibited the best bioactivity against Xoo than the other groups, while for benzyl thioether compounds, 4-methylbenzyl thioether (6f) gives superior activity than the other substituted benzyl in the dosage of 200 μg/mL or 100 μg/mL. For sulfone compounds, the antibacterial activity of alkyl sulfone compounds (such as 7a-7c) was dramatically better than the benzyl derivatives.
表 1
 表 1 Inhibition effect of sulfides/sulfones against Xoo.Table 1. Inhibition effect of sulfides/sulfones against Xoo.
表 1 Inhibition effect of sulfides/sulfones against Xoo.Table 1. Inhibition effect of sulfides/sulfones against Xoo.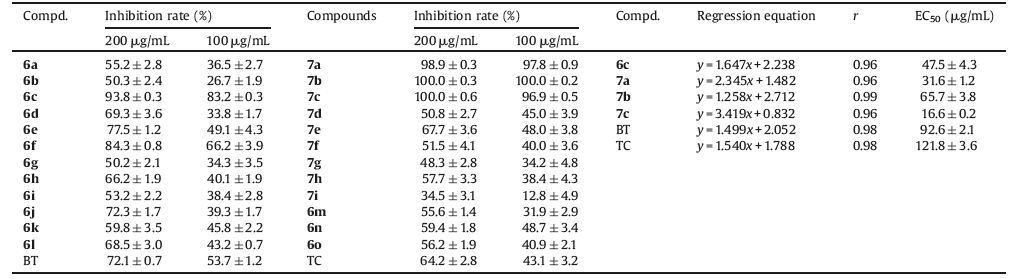
The antifungal activity of (6a-6o) and (7a-7i) was examined via the poisonplate technique [28] against fivephytopathogenic fungi, Gibberella zeae (G. z.), Fusarium oxysporum (F. o.), Cytospora mandshurica (C. m.), Sclertinia sclerotiorum (S. s.), and Rhizoctonia solani (R. s.) at the concentrate of 100 μg/mL, Meanwhile, the commercial agricultural antifungal Hymexazol (HM) and Carbendazim (CB) were employed for the comparison of bioactivity. As shown in Table 2, compounds 7a and 7c were observed having comprehensive antifungal activity with the inhibition rate ranging from 53.8% to 75.5% against the five kinds of fungi, which were comparable to the commercial fungicide HM. It is worth pointing out that compound 6j exerted good antifungal activity with the inhibition rate of 86.4% against S. sclerotiorum. In comparison of 6a and 7a, 6b and 7b, 6c and 7c, 6d and 7d, 6f and 7f, the antifungal activity was improved after oxidizing the thioether into the sulfone, further suggested sulfonyl group as a crucial functional group may improve the bioactivity of the target compound. It can be seen that compound 7a showed the strongest antifungi activity against the five phytopathogenic fungi.
表 2
 表 2 Inhibition effect of sulfides/sulfones at 100 μg/mL against five phytopathogenic fungi.Table 2. Inhibition effect of sulfides/sulfones at 100 μg/mL against five phytopathogenic fungi.
表 2 Inhibition effect of sulfides/sulfones at 100 μg/mL against five phytopathogenic fungi.Table 2. Inhibition effect of sulfides/sulfones at 100 μg/mL against five phytopathogenic fungi.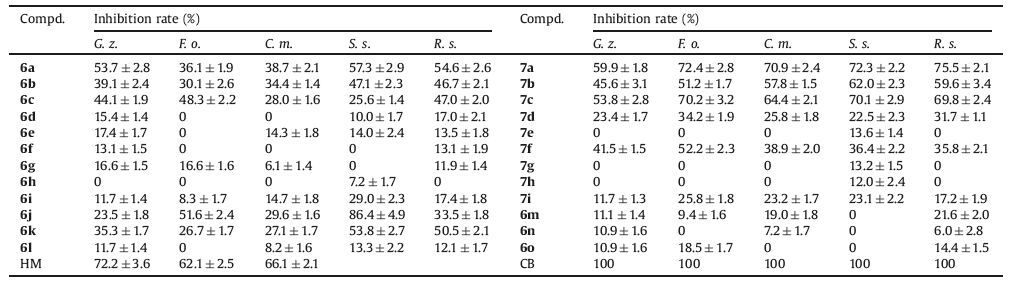
4. Conclusion
In summary, a series of 2-(thioether/sulfone)-5-pyrazolyl-1, 3, 4-oxadiazole derivatives containing both pyrazole moiety and 1, 3, 4-oxadiazole moiety were designed and synthesized, and which antibacterial activity and antifungal activity were evaluated via turbidmeter test or the poison plate technique in vitro. Compounds 6c, 7a, 7b and 7c showed good inhibition effects against Xoo with the EC50 values ranging from 16.6 μg/mL to 65.7 μg/mL, which were better than those of commercial agricultural antibacterial bismerthiazol (92.6 μg/mL) and thiediazole copper (121.8 μg/mL). Meanwhile, compounds 7a, 7b, and 7c exerted good antifungal activities against fiveplant fungi, which were comparable tothatof HM. The results demonstrated that this kind of compounds can be further studied and developed as promising antifungal and antibacterial agents.
Acknowledgments
We acknowledge the financial support of the Key Technologies R & D Program (No. 2014BAD23B01), National Natural Science Foundation of China (No. 21372052), the Research Project of Chinese Ministry of Education (Nos. 213033A, 20135201110005), and Scientific Research Foundation for the Introduced Talents of Guizhou University (2015-34).
Appendix A. Supplementary data
Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.cclet.2016.06.055
-

-
-
[1]
Yue Sun , Liming Yang , Yaohang Cheng , Guanghui An , Guangming Li . Pd(I)-catalyzed ring-opening arylation of cyclopropyl-α-aminoamides: Access to α-ketoamide peptidomimetics. Chinese Chemical Letters, 2024, 35(6): 109250-. doi: 10.1016/j.cclet.2023.109250
-
[2]
Rong-Nan Yi , Wei-Min He . Visible light/copper catalysis enabled radial type ring-opening of sulfonium salts. Chinese Chemical Letters, 2025, 36(4): 110787-. doi: 10.1016/j.cclet.2024.110787
-
[3]
Peiyan Zhu , Yanyan Yang , Hui Li , Jinhua Wang , Shiqing Li . Rh(Ⅲ)‐Catalyzed sequential ring‐retentive/‐opening [4 + 2] annulations of 2H‐imidazoles towards full‐color emissive imidazo[5,1‐a]isoquinolinium salts and AIE‐active non‐symmetric 1,1′‐biisoquinolines. Chinese Chemical Letters, 2024, 35(10): 109533-. doi: 10.1016/j.cclet.2024.109533
-
[4]
Qinghong Zhang , Qiao Zhao , Xiaodi Wu , Li Wang , Kairui Shen , Yuchen Hua , Cheng Gao , Yu Zhang , Mei Peng , Kai Zhao . Visible-light-induced ring-opening cross-coupling of cycloalcohols with vinylazaarenes and enones via β-C-C scission enabled by proton-coupled electron transfer. Chinese Chemical Letters, 2025, 36(2): 110167-. doi: 10.1016/j.cclet.2024.110167
-
[5]
Shengwen Guan , Zhaotong Wei , Ningxu Han , Yude Wei , Bin Xu , Ming Wang , Junjuan Shi . Construction of metallo-complexes with 2,2′:6′,2″-terpyridine substituted triphenylamine in different modified positions and their photophysical properties. Chinese Chemical Letters, 2024, 35(7): 109348-. doi: 10.1016/j.cclet.2023.109348
-
[6]
Shuai Liu , Wen Wu , Peili Zhang , Yunxuan Ding , Chang Liu , Yu Shan , Ke Fan , Fusheng Li . Mechanistic insights into acidic water oxidation by Mn(2,2′-bipyridine-6,6′-dicarboxylate)-based hydrogen-bonded organic frameworks. Chinese Journal of Structural Chemistry, 2025, 44(3): 100535-100535. doi: 10.1016/j.cjsc.2025.100535
-
[7]
Zhenjie Yang , Chenyang Hu , Xuan Pang , Xuesi Chen . Sequence design in terpolymerization of ε-caprolactone, CO2 and cyclohexane oxide: Random ester-carbonate distributions lead to large-span tunability. Chinese Chemical Letters, 2024, 35(5): 109340-. doi: 10.1016/j.cclet.2023.109340
-
[8]
Yahui HAN , Jinjin ZHAO , Ning REN , Jianjun ZHANG . Synthesis, crystal structure, thermal decomposition mechanism, and fluorescence properties of benzoic acid and 4-hydroxy-2, 2′: 6′, 2″-terpyridine lanthanide complexes. Chinese Journal of Inorganic Chemistry, 2025, 41(5): 969-982. doi: 10.11862/CJIC.20240395
-
[9]
Yingchun ZHANG , Yiwei SHI , Ruijie YANG , Xin WANG , Zhiguo SONG , Min WANG . Dual ligands manganese complexes based on benzene sulfonic acid and 2, 2′-bipyridine: Structure and catalytic properties and mechanism in Mannich reaction. Chinese Journal of Inorganic Chemistry, 2024, 40(8): 1501-1510. doi: 10.11862/CJIC.20240078
-
[10]
Kunyao Peng , Xianbin Wang , Xingbin Yan . Converting LiNO3 additive to single nitrogenous component Li2N2O2 SEI layer on Li metal anode in carbonate-based electrolyte. Chinese Chemical Letters, 2024, 35(9): 109274-. doi: 10.1016/j.cclet.2023.109274
-
[11]
Anqiu LIU , Long LIN , Dezhi ZHANG , Junyu LEI , Kefeng WANG , Wei ZHANG , Junpeng ZHUANG , Haijun HAO . Synthesis, structures, and catalytic activity of aluminum and zinc complexes chelated by 2-((2,6-dimethylphenyl)amino)ethanolate. Chinese Journal of Inorganic Chemistry, 2024, 40(4): 791-798. doi: 10.11862/CJIC.20230424
-
[12]
Huaixiang Yang , Miao-Miao Li , Aijun Zhang , Jiefei Guo , Yongqi Yu , Wei Ding . Visible-light-induced photocatalyst- and metal-free radical phosphinoyloximation of alkenes with tert-butyl nitrite as bifunctional reagent. Chinese Chemical Letters, 2025, 36(3): 110425-. doi: 10.1016/j.cclet.2024.110425
-
[13]
Guihuang Fang , Wei Chen , Hongwei Yang , Haisheng Fang , Chuang Yu , Maoxiang Wu . Improved performance of LiMn0.8Fe0.2PO4 by addition of fluoroethylene carbonate electrolyte additive. Chinese Chemical Letters, 2024, 35(6): 108799-. doi: 10.1016/j.cclet.2023.108799
-
[14]
Hongzhi Zhang , Hong Li , Asif Ali Haider , Junpeng Li , Zhi Xie , Hongming Jiang , Conglin Liu , Rui Wang , Jing Zhu . An unexpected role of lanthanide substitution in thermally responsive phosphors NaLnTe2O7: Eu3+ (Ln = Y and Gd). Chinese Journal of Structural Chemistry, 2025, 44(2): 100509-100509. doi: 10.1016/j.cjsc.2024.100509
-
[15]
Zhen Shi , Wei Jin , Yuhang Sun , Xu Li , Liang Mao , Xiaoyan Cai , Zaizhu Lou . Interface charge separation in Cu2CoSnS4/ZnIn2S4 heterojunction for boosting photocatalytic hydrogen production. Chinese Journal of Structural Chemistry, 2023, 42(12): 100201-100201. doi: 10.1016/j.cjsc.2023.100201
-
[16]
Xiuzheng Deng , Changhai Liu , Xiaotong Yan , Jingshan Fan , Qian Liang , Zhongyu Li . Carbon dots anchored NiAl-LDH@In2O3 hierarchical nanotubes for promoting selective CO2 photoreduction into CH4. Chinese Chemical Letters, 2024, 35(6): 108942-. doi: 10.1016/j.cclet.2023.108942
-
[17]
Jiajun Wang , Guolin Yi , Shengling Guo , Jianing Wang , Shujuan Li , Ke Xu , Weiyi Wang , Shulai Lei . Computational design of bimetallic TM2@g-C9N4 electrocatalysts for enhanced CO reduction toward C2 products. Chinese Chemical Letters, 2024, 35(7): 109050-. doi: 10.1016/j.cclet.2023.109050
-
[18]
Yan Cheng , Hua-Peng Ruan , Yan Peng , Longhe Li , Zhenqiang Xie , Lang Liu , Shiyong Zhang , Hengyun Ye , Zhao-Bo Hu . Magnetic, dielectric and luminescence synergetic switchable effects in molecular material [Et3NCH2Cl]2[MnBr4]. Chinese Chemical Letters, 2024, 35(4): 108554-. doi: 10.1016/j.cclet.2023.108554
-
[19]
Hui Li , Yanxing Qi , Jia Chen , Juanjuan Wang , Min Yang , Hongdeng Qiu . Synthesis of amine-pillar[5]arene porous adsorbent for adsorption of CO2 and selectivity over N2 and CH4. Chinese Chemical Letters, 2024, 35(11): 109659-. doi: 10.1016/j.cclet.2024.109659
-
[20]
Sikai Wu , Xuefei Wang , Huogen Yu . Hydroxyl-enriched hydrous tin dioxide-coated BiVO4 with boosted photocatalytic H2O2 production. Chinese Journal of Structural Chemistry, 2024, 43(12): 100457-100457. doi: 10.1016/j.cjsc.2024.100457
-
[1]
Metrics
- PDF Downloads(1)
- Abstract views(755)
- HTML views(9)

 Login In
Login In




 下载:
下载:
 DownLoad:
DownLoad: