Citation:
Wei Ming CHEN, Pei Ling CHANG, Bin WU, Qi Tai ZHENG. STUDIES ON THE CHEMICAL CONSTITUENTS OF TAXUS YUNNANENSIS[J]. Chinese Chemical Letters,
;1991, 2(6): 441-442.

-
An extract of the barks of Taxus yunnanensis has afforded eight constituents,taxane diterpenoids and alkaloids,of which one appear to be new.The structure of the new taxane diterpene,named yunnanxane(Ⅰ) was elucidated by spectroscopic means (UV,IR,MS,1H NMR,13C NMR,1H-1H COSY,13C-1H COSY and 13C-1H COLOC) and X-ray diffraction analysis.
-
Lithium-ion batteries (LIBs) using organic electrolytes have developed rapidly in the past two decades owing to the high energy and power density. However, their further applications in large-scale energy storage systems are limited by the hidden safety hazards and high production costs [1-4]. Therefore, it is urgent to develop alternative electrochemical systems for lithium-ion batteries [5, 6]. Aqueous zinc-ion batteries (AZIBs) have attracted more and more attention in recent years owing to their environmental friendliness, safety, low cost, high theoretical specific capacity (820 mAh/g), and low electrode potential (−0.76 V vs. standard hydrogen electrode) of zinc metal [7-10]. The big progress has been made in the cathode materials of AZIBs (such as Li+ intercalated V2O5·nH2O, in-situ carbon reduced Mn3O4 and self-doped polyaniline cathode) [11-13]. However, compared with the development of cathode materials, zinc anodes suffer from inescapable issues, such as the dendrites, corrosion, and hydrogen evolution reactions [14, 15]. Among them, the capacity attenuation and short circuit problems caused by dendrites are fatal to the practical application of zinc-ion batteries [16-18]. Some modifications based on the following eq. 1 have been proposed for addressing the dendrite problem:
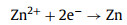
(1) As seen clearly, the key factors affecting zinc deposition include the zinc ion distribution, the interfacial electric field, and the spatial location of zinc growth. That is, a dendrite-free zinc deposition process can be achieved when there are more uniform electron-ion distribution, easier electron-ion exchange process, and more regular growth direction on the reaction interface. A suitable interfacial coating strategy can introduce the above modification effects on zinc anode [19]. For example, the porous nano-CaCO3 coating, and nano-ZnO coating can adjust the uniformity of ion transmission and electric field distribution at the electrode interface [20, 21]. The UiO-66 MOF solid electrolyte coating can optimize the zinc nucleation process by nanoscale wetting effects [22]. However, the interaction between the coating layer and zinc is insufficiently investigated in these works. Herein, the binding energy of zinc on different coating layers is proposed to differentiate their zincophobicity and zincophilicity. Generally, if the interaction is lower than that between the zinc crystal plane and zinc atoms, the zinc can be guided to deposit under the coating, indicating a huge difficulty to puncture the coating during zinc growth. If not, the zincophilic coating layer will induce the direct deposit of zinc dendrites on its surface, leading to the rapid failure of coating layer. In our previous work, (001) exposed TiO2 protective layer was specially designed by hydrofluoric acid assisted hydrothermal method to achieve dendrites-free zinc metal anode due to the zincophobic crystal orientation [23]. Note that the normal TiO2 tends to form (100) exposed plane, which is zincophilic based on the density functional theory (DFT) calculation. Therefore, it is of great significance to find some new protective coating materials with intrinsic zincophobicity to achieve more stable zinc anodes without special design.
In this work, we first evaluate the interactions between zinc and barium-titanate (BT) by density functional theory (DFT) calculation, which indicates that (100) and (110) facets of barium-titanate exhibit strong zincophobicity. These crystal facets dominantly exist in common barium-titanate, that is, it can be a perfect intrinsically zincophobic protective layer. As a result, the modified zinc anode with commercial barium-titanate can exhibit dendrite-free morphology, long cycle life, and low voltage hysteresis by adjusting the electric field and ion distribution at the zinc anode interface to restrict the uneven zinc deposition. Moreover, an aqueous Zn||MnO2 full battery assembled with barium-titanate protective anode can deliver high specific capacity and capacity retention ratio.
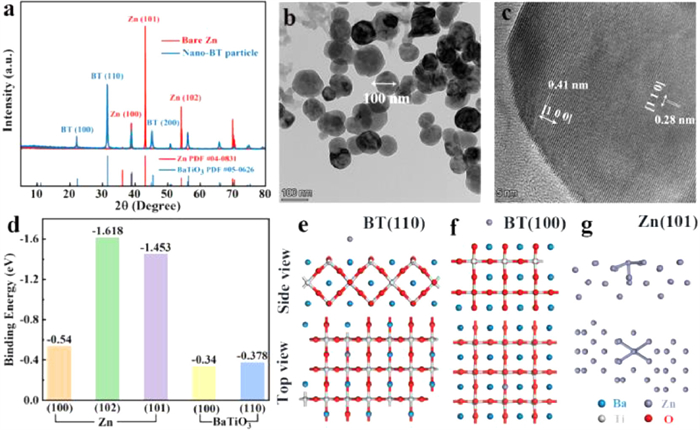
The X-ray powder diffraction (XRD) patterns (Fig. 1a) indicate that the dominant crystal facets of zinc foil are (100), (101) and (102) facets and those of commercial barium-titanate particles are (100) and (110) facets. The transmission electron microscopy (TEM) characterizations were conducted to further elucidate the crystal structure of barium-titanate. The lattice spacing of about 0.28 nm and 0.4 nm can be observed in the high-resolution transmission electron microscopy (HRTEM) image, which is well indexed to (110) and (100) crystal facet of barium-titanate, respectively (Figs. 1b and c). The well-crystallized nano barium-titanate can be verified by the corresponding selected area electron diffraction result (Fig. S1 in Supporting information). Afterward, the interactions between zinc atom and these dominant crystal facets are simulated by DFT method. The calculated binding energy and the corresponding model are shown in Figs. 1d-g.
Figure 1
 Figure 1. (a) XRD patterns of zinc foil and nano barium-titanate. (b, c) TEM images of barium-titanate. (d) Calculated binding energies of zinc atom with different facets. Calculation models of zinc absorbed on (e) BT (110) facet, (f) BT (100) facet and (g) zinc (101) facet.
Figure 1. (a) XRD patterns of zinc foil and nano barium-titanate. (b, c) TEM images of barium-titanate. (d) Calculated binding energies of zinc atom with different facets. Calculation models of zinc absorbed on (e) BT (110) facet, (f) BT (100) facet and (g) zinc (101) facet.As seen clearly, the binding energies of the binding energies of zinc atom on barium-titanate (100) and (110) crystal facets (−0.34 eV and −0.378 eV) are both lower than those of zinc atom on dominant zinc facets (−0.54 eV, −1.618 eV and −1.453 eV), which indicates that zinc prefers to deposit on zinc substrate in comparison to the barium-titanate layer, in another word, the as-prepared barium-titanate layer is intrinsically zincophobic. It should be noted that the zinc atom with the lowest binding energy is adsorbed on the apex of the octahedron composed of oxygen and barium atoms, indicating that the zincophobicity of barium-titanate is mainly due to the strong repulsive force generated by large radius Ba2+ against zinc atoms [23, 24]. In contrast, the high binding energy between zinc atom and zinc facets can be ascribed to the strong metal bond [25]. Accordingly, the commercial barium-titanate can be considered as a perfect interface protective layer for zinc anodes, which can adjust the electric field and ion distribution at the zinc anode interface and restrict the zinc deposition by strong zincophobicity.
A uniform and porous barium-titanate protective layer was coated on zinc anode by a simple blade coating method (Fig. 2a). The cross-sectional SEM image and energy dispersive X-ray (EDX) mapping image of as-prepared zinc anode illustrate that the thickness of the coating layer is about 8 µm (Fig. 2b, Figs. S3c and d in Supporting information). Brunauere-Emmette-Teller (BET) test results show that the pore volume density of the coating is 2.787 × 10−2 cm3/g in the range of 2–10 nm (Fig. S2 in Supporting information). Compared with two-dimensional surface of smooth zinc foil, the three-dimensional porous coating layer can provide more ion transport channels and regulate ion flux, uniformize the zinc deposition [21]. The contact angles of the electrolyte on the bare zinc and BT coated zinc are 99.9° and 72.4° (Fig. 2c), respectively, indicating the better wettability of coated anode, which can improve the kinetic process of zinc ions at the electrode interface and reduce the ions transmission impedance [22]. The faster ion transport may be related to the strong space charge polarization of barium-titanate [26-28]. The lower ion transmission impedance of the coated anode is also proven by electrochemical impedance spectroscopy (EIS) results (Fig. 2d, Table 1). The capacitance (C) is obtained by calculating the slope of the ic-v (current vs. scan rate) curves, and the ic is the half value of current difference between positive and negative scanning at 0 V (Fig. S4 in Supporting information) [29]. As seen in Fig. 2e, the interface capacitance of BT coated zinc (110.2 µF/cm2) is much higher than that of the bare zinc (38.57 µF/cm2), indicating the stronger adsorption ability of zinc ions on the coated zinc anode (The adsorption site is at the junction of coating and zinc foil). The enrichment of zinc ions on the electrode interface can provide more nucleation sites for zinc deposition, thereby optimizing the uniformity of deposition [20]. Its lower corrosion current effectively demonstrates the better anti-corrosion ability that can inhibit the generation of the by-product, thereby maintaining uniformity of ion transmission and improving the zinc anode utilization [14, 30].
Figure 2
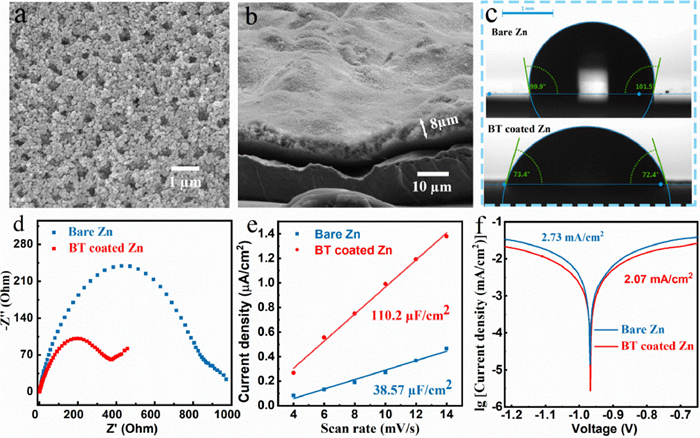 Figure 2. SEM images of coating (a) surface and (b) cross section. (c) Contact angles of electrolyte on bare zinc and BT coated zinc anodes. (d) EIS curve and fitting circuit diagram of Zn||Zn symmetric batteries. (e) Capacitance fitting curves of Zn||Zn symmetric batteries. (f) Linear polarization curves displaying the corrosion on bare zinc and BT coated zinc anodes.
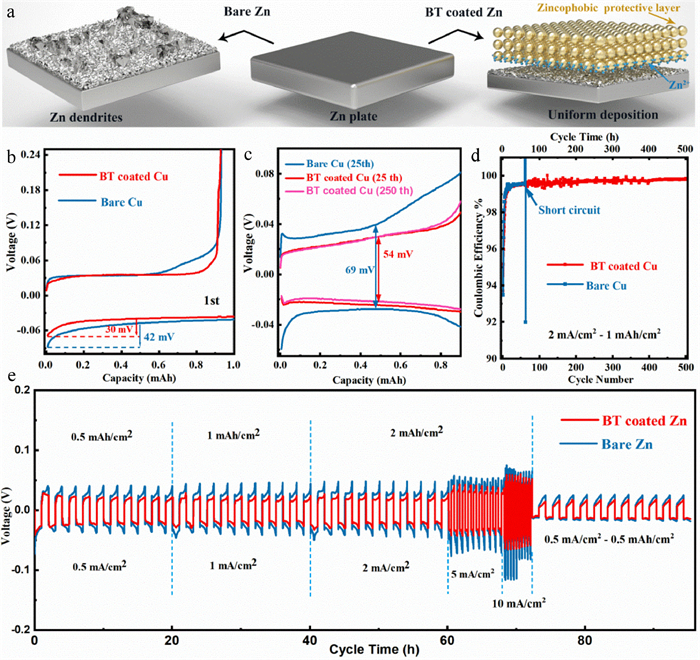
Figure 2. SEM images of coating (a) surface and (b) cross section. (c) Contact angles of electrolyte on bare zinc and BT coated zinc anodes. (d) EIS curve and fitting circuit diagram of Zn||Zn symmetric batteries. (e) Capacitance fitting curves of Zn||Zn symmetric batteries. (f) Linear polarization curves displaying the corrosion on bare zinc and BT coated zinc anodes.The zinc deposition processes on different anodes are illustrated in Fig. 3a. Zinc prefers to deposit at the tip sites and the sites with high ion concentration on bare zinc electrode. These sites distributed unevenly and sparsely on the electrode interface can induce the zinc dendrites formation during the repeated cycles. When using barium-titanate as the protective layer, zinc ions can be enriched on the electrode surface to provide more nucleation sites for zinc deposition, so that the ion flux and electric field strength on the electrode surface are evenly divided. The layer with strong zincophobicity (such as "gold armor") can also homogenize zinc deposition by guiding zinc to grow beneath the layer. The Zn||Cu half batteries were employed to further test the reversibility and Coulombic efficiency of zinc deposition/stripping. As shown in Fig. 3b, the zinc nucleation overpotentials on bare Cu and BT coated Cu in the first deposition are calculated to be 42 mV and 30 mV, respectively. The lower overpotential of BT coated Cu indicates that the coating layer can provide more activated nucleation sites for zinc deposition [31]. The Cu electrode coated by BT exhibits much longer cycle life (500 h vs. 60 h) and lower voltage polarization (54 mV vs. 69 mV at 25th cycle) compared with bare Cu at a current density of 2 mA/cm2 (Figs. 3c and d, Fig. S5 in Supporting information). When decreasing the current density to 0.5 mA/cm2 and the specific capacity to 0.5 mAh/cm2, the modified anode can still deliver excellent reversibility (Fig. S6 in Supporting information). The Cu electrode was extracted from the cell after 10 cycles for further morphology observation. As seen, the zinc deposition under the coating is more homogeneous than that on bare Cu foil because there are only few active sites on Cu foil, which may lead to the formation of a large amount of "dead zinc" and cause the battery to short circuit (Fig. S7 in Supporting information). Meanwhile, the binding energies of the (100) and (110) crystal facets of Cu with zinc are calculated by DFT (Fig. S8 in Supporting information), indicating the zincophilicity of copper host. However, zinc dendrites can be still induced on the zincophilic Cu due to its uneven ion distribution and disordered growth direction [32]. For comparison, it is interesting to note that the zinc can be guided by the porous and zincophobic coating layer to uniformly nucleate and grow on the electrode surface, thereby obtaining a dendritic-free electrode.
Figure 3
In Fig. 3e, the superior rate performance of the BT coated zinc anode with a lower voltage hysteresis and more stable voltage plateau can be observed when the current density is increased from 0.5 mA/cm2 to 1, 2, 5, 10 mA/cm2, whereas the bare zinc breaks down at 10 mA/cm2, indicating that the easier electron-ion exchange process and more uniform stripping/deposition of the BT coated zinc anode. The surface morphologies of the cycled anodes after 10 cycles are shown in optical pictures and SEM images (Fig. 4a, Figs. S9a and b in Supporting information). The coated anode is intact without the generation of "island-like dendrites" and the well-defined distribution of Ti, Ba and Zn in the EDX mapping image also proves that the coating layer can protect the separator from being pierced by restricting the zinc growth (Figs. 4b and c). Furthermore, the barium-titanate coating layer on the cycled anodes was removed by using methyl-2-pyrrolidinone (NMP) to dissolve polyvinylidene difluoride (PVDF) in the coating layer. Fig. 4d and Fig. S8d show the morphology of zinc deposition under the coating. No dendrite is observed and the surface remains flat, which is in sharp contrast with the dendritic deposition on bare zinc (Fig. S9c in Supporting information). In the long-cycle symmetric batteries test, profited from the adjustment of zinc deposition by the protective layer, the BT coated zinc can be operated steadily for more than 840 h at 0.5 mA/cm2 for 0.5 mAh/cm2, which is much superior to the bare zinc (60 h), and it also exhibits a low voltage hysteresis (36 mV at 20th cycle) and stable voltage plateau (Fig. 4e). When increasing the current density to 2 mA/cm2 and the specific capacity to 1 mAh/cm2, the coated anode can still deposit and strip for 400 h while the bare one shows the quick failure of 60 h (Fig. S10). The positive role of this intrinsically zincophobic barium-titanate protective layer in improving anode stability has been well proven through the comparison of the symmetrical battery electrochemical behaviors.
Figure 4
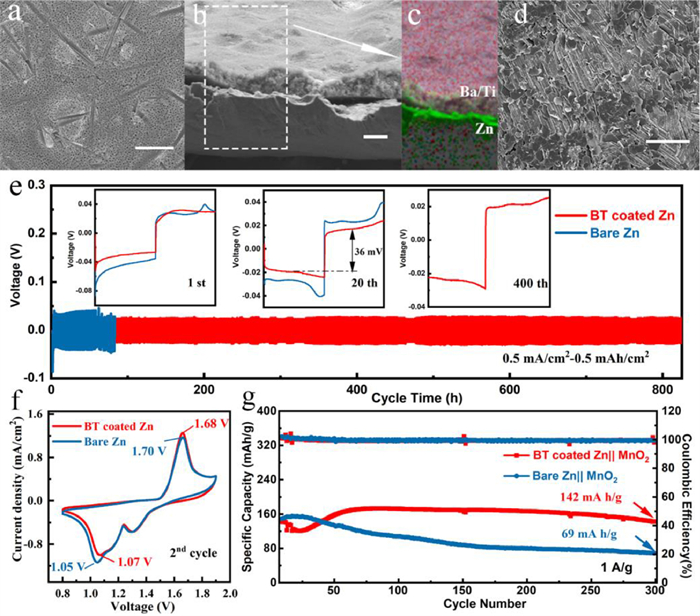 Figure 4. The SEM images of BT coated zinc anode (a) surface, (b) cross section, (c) corresponding EDX mapping image, (d) without coating layer, cycled at 0.5 mA/cm2 with a capacity of 0.5 mAh/cm2 after 10 cycles. (e) Cycling performance of Zn||Zn symmetric batteries at 0.5 mA/cm2 for 0.5 mAh/cm2. Cyclic voltammetry curve (f) and cycling performance (g) of the full batteries at a current density of 1 A/g. Scale bar: 10 μm.
Figure 4. The SEM images of BT coated zinc anode (a) surface, (b) cross section, (c) corresponding EDX mapping image, (d) without coating layer, cycled at 0.5 mA/cm2 with a capacity of 0.5 mAh/cm2 after 10 cycles. (e) Cycling performance of Zn||Zn symmetric batteries at 0.5 mA/cm2 for 0.5 mAh/cm2. Cyclic voltammetry curve (f) and cycling performance (g) of the full batteries at a current density of 1 A/g. Scale bar: 10 μm.The full battery was assembled with BT coated zinc anode and the fibrous β-MnO2 cathode synthesized by previous (Fig. S11 in Supporting information) [7]. As shown in cyclic voltammetry (CV) curves (Fig. 4f), the BT coated Zn||MnO2 battery exhibits higher reduction peak (1.07 V vs. 1.05 V) and lower oxidation peak (1.68 V vs. 1.70 V), indicating the faster Zn2+ kinetics and the higher deposition/stripping efficiency, which is in consistent with the EIS results (Fig. S12a in Supporting information). The long-term cycling performance of the batteries cycled at 1 A/g are depicted in Fig. 4g and Fig. S12b (Supporting information), from which the battery with BT coated zinc anode exhibits much better cycling stability with a specific discharge capacity of 142 mAh/g remaining after 300 cycles compared with that of bare one (69 mAh/g). The excellent performance of the full battery certifies the practical value of this protective layer.
In summary, a porous and intrinsically zincophobic barium-titanate protective layer was proposed to stabilize zinc anode. Benefitting from the strong repulsive force generated by large radius Ba2+ against zinc atoms, the commercial barium-titanate exhibited strong zincophobicity and the zinc ions showed strong adsorption at the zinc anode interface. Accordingly, this coating layer played an important role in regulating ion transport, zinc nucleation and zinc crystal growth. Based on these synergistic effects, the as-prepared zinc anode showed superior reversibility of zinc stripping/deposition with a long lifespan (840 h) and a low voltage hysteresis (36 mV) at 0.5 mA/cm2 for 0.5 mAh/cm2. This work provides a novel guiding direction for discovering naturally zincophobic protective layer materials to modify zinc anode interface, which can be also extended to other metal anodes.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowldgments
This research was financially supported by National Nature Science Foundation of China (Nos. U19A2019, U22109181), Hunan Provincial Science and Technology Plan Project of China (Nos. 2017TP1001 and 2020JJ2042), and the Open Research Fund of School of Chemistry and Chemical Engineering, Henan Normal University.
Supplementary materials
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.cclet.2021.09.083.
-

-
-
[1]
Weichen Zhu , Wei Zuo , Pu Wang , Wei Zhan , Jun Zhang , Lipin Li , Yu Tian , Hong Qi , Rui Huang . Fe-N-C heterogeneous Fenton-like catalyst for the degradation of tetracycline: Fe-N coordination and mechanism studies. Chinese Chemical Letters, 2024, 35(9): 109341-. doi: 10.1016/j.cclet.2023.109341
-
[2]
Xueling Yu , Lixing Fu , Tong Wang , Zhixin Liu , Na Niu , Ligang Chen . Multivariate chemical analysis: From sensors to sensor arrays. Chinese Chemical Letters, 2024, 35(7): 109167-. doi: 10.1016/j.cclet.2023.109167
-
[3]
Tiantian Long , Hongmei Luo , Jingbo Sun , Fengniu Lu , Yi Chen , Dong Xu , Zhiqin Yuan . Carbonization-engineered ultrafast chemical reaction on nanointerface. Chinese Chemical Letters, 2025, 36(3): 109728-. doi: 10.1016/j.cclet.2024.109728
-
[4]
Min Fu , Pan He , Sen Zhou , Wenqiang Liu , Bo Ma , Shiying Shang , Yaohao Li , Ruihan Wang , Zhongping Tan . An unexpected stereochemical effect of thio-substituted Asp in native chemical ligation. Chinese Chemical Letters, 2024, 35(8): 109434-. doi: 10.1016/j.cclet.2023.109434
-
[5]
Xianxu Chu , Lu Wang , Junru Li , Hui Xu . Surface chemical microenvironment engineering of catalysts by organic molecules for boosting electrocatalytic reaction. Chinese Chemical Letters, 2024, 35(8): 109105-. doi: 10.1016/j.cclet.2023.109105
-
[6]
Xu-Hui Yue , Xiang-Wen Zhang , Hui-Min He , Lei Qiao , Zhong-Ming Sun . Synthesis, chemical bonding and reactivity of new medium-sized polyarsenides. Chinese Chemical Letters, 2024, 35(7): 108907-. doi: 10.1016/j.cclet.2023.108907
-
[7]
Ali Dai , Zhiguo Zheng , Liusheng Duan , Jian Wu , Weiming Tan . Small molecule chemical scaffolds in plant growth regulators for the development of agrochemicals. Chinese Chemical Letters, 2025, 36(4): 110462-. doi: 10.1016/j.cclet.2024.110462
-
[8]
Ying Li , Long-Jie Wang , Yong-Kang Zhou , Jun Liang , Bin Xiao , Ji-Shen Zheng . An improved installation of 2-hydroxy-4-methoxybenzyl (iHmb) method for chemical protein synthesis. Chinese Chemical Letters, 2024, 35(5): 109033-. doi: 10.1016/j.cclet.2023.109033
-
[9]
Min Huang , Ru Cheng , Shuai Wen , Liangtong Li , Jie Gao , Xiaohui Zhao , Chunmei Li , Hongyan Zou , Jian Wang . Ultrasensitive detection of microRNA-21 in human serum based on the confinement effect enhanced chemical etching of gold nanorods. Chinese Chemical Letters, 2024, 35(9): 109379-. doi: 10.1016/j.cclet.2023.109379
-
[10]
Lian Sun , Honglei Wang , Ming Ma , Tingting Cao , Leilei Zhang , Xingui Zhou . Shape and composition evolution of Pt and Pt3M nanocrystals under HCl chemical etching. Chinese Chemical Letters, 2024, 35(9): 109188-. doi: 10.1016/j.cclet.2023.109188
-
[11]
Yongjian Li , Xinyu Zhu , Chenxi Wei , Youyou Fang , Xinyu Wang , Yizhi Zhai , Wenlong Kang , Lai Chen , Duanyun Cao , Meng Wang , Yun Lu , Qing Huang , Yuefeng Su , Hong Yuan , Ning Li , Feng Wu . Unraveling the chemical and structural evolution of novel Li-rich layered/rocksalt intergrown cathode for Li-ion batteries. Chinese Chemical Letters, 2024, 35(12): 109536-. doi: 10.1016/j.cclet.2024.109536
-
[12]
Xue Zheng , Jizhen Xie , Xing Zhang , Weiting Sun , Heyang Zhao , Yantuan Li , Cheng Wang . Corrigendum to "An overview of polymeric nanomicelles in clinical trials and on the market" [Chinese Chemical Letters 32 (2021) 243-257]. Chinese Chemical Letters, 2025, 36(2): 110545-. doi: 10.1016/j.cclet.2024.110545
-
[13]
Jingyu Shi , Xiaofeng Wu , Yutong Chen , Yi Zhang , Xiangyan Hou , Ruike Lv , Junwei Liu , Mengpei Jiang , Keke Huang , Shouhua Feng . Structure factors dictate the ionic conductivity and chemical stability for cubic garnet-based solid-state electrolyte. Chinese Chemical Letters, 2025, 36(5): 109938-. doi: 10.1016/j.cclet.2024.109938
-
[14]
Jian Li , Jinjin Chen , Qi-Long Hu , Zhen Wang , Xiao-Feng Xiong . Recent progress of chemical methods for lysine site-selective modification of peptides and proteins. Chinese Chemical Letters, 2025, 36(5): 110126-. doi: 10.1016/j.cclet.2024.110126
-
[15]
Shuai Guo , Kening Li , Bin Li . Parallel on-tissue chemical derivatization for enhanced molecular annotation of spatial carbonyl submetabolome. Chinese Chemical Letters, 2025, 36(6): 110366-. doi: 10.1016/j.cclet.2024.110366
-
[16]
Jinjin Yang , Chuanhui Zhu , Shuang Zhao , Tao Xia , Pengfei Tan , Yutian Zhang , Mei-Huan Zhao , Yijie Zeng , Man-Rong Li . Spin-orbit-controlled metal-insulator transition in metastable SrIrO3 stabilized by physical and chemical pressures. Chinese Chemical Letters, 2025, 36(6): 109891-. doi: 10.1016/j.cclet.2024.109891
-
[17]
Chunshi He , Linqing Li , Yuanrong Sun , Xuefang Wang , Jie Ren , Jianbo Li . Enhanced durability of a novel thiol-epoxy network thermosets with excellent hygrothermal and chemical resistance. Chinese Chemical Letters, 2025, 36(6): 110905-. doi: 10.1016/j.cclet.2025.110905
-
[18]
Yan Wang , Huixin Chen , Fuda Yu , Shanyue Wei , Jinhui Song , Qianfeng He , Yiming Xie , Miaoliang Huang , Canzhong Lu . Oxygen self-doping pyrolyzed polyacrylic acid as sulfur host with physical/chemical adsorption dual function for lithium-sulfur batteries. Chinese Chemical Letters, 2024, 35(7): 109001-. doi: 10.1016/j.cclet.2023.109001
-
[19]
Feng-Qing Huang , Yu Wang , Ji-Wen Wang , Dai Yang , Shi-Lei Wang , Yuan-Ming Fan , Raphael N. Alolga , Lian-Wen Qi . Chemical isotope labeling-assisted liquid chromatography-mass spectrometry enables sensitive and accurate determination of dipeptides and tripeptides in complex biological samples. Chinese Chemical Letters, 2024, 35(11): 109670-. doi: 10.1016/j.cclet.2024.109670
-
[20]
Jia-Qi Feng , Xiang Tian , Rui-Ge Cao , Yong-Xiu Li , Wen-Long Liu , Rong Huang , Si-Yong Qin , Ai-Qing Zhang , Yin-Jia Cheng . An AIE-based theranostic nanoplatform for enhanced colorectal cancer therapy: Real-time tumor-tracking and chemical-enhanced photodynamic therapy. Chinese Chemical Letters, 2024, 35(12): 109657-. doi: 10.1016/j.cclet.2024.109657
-
[1]
Metrics
- PDF Downloads(4)
- Abstract views(828)
- HTML views(3)

 Login In
Login In




 DownLoad:
DownLoad:
 DownLoad:
DownLoad: