Citation:
Yuan GUO, Xi XIA. THE CONSTANT PHASE ELEMENT (CPE) ON MnO2 ELECTRODE[J]. Chinese Chemical Letters,
;1996, 7(5): 457-458.

-
Ac impedance responses of electrolytic manganese dioxide (EMD) electrode are measured with EIS.It was found that the impedance responses are consistent with the constant phase element (CPE) form and calculated the fractal dimension of MnO2.
-
1. INTRODUCTION
Recently, the design and assembly of Zn(Ⅱ) coordination polymers (CPs) have received remarkable attention because of their intriguing topological structures and potential applications in many fields[1-4]. In order to generate novel CPs, the important step is to choose suitable organic ligands. As we know, bidentate μ2-N, N΄ ligands bearing an appropriate auxiliary group, such as 4, 4΄-bipyridine, 1, 2-bis(4-pyridyl)-ethylene, 1, 3-bis(4-pyridyl)propane, 4-bis(2-methylimidazolyl)butane and related species, have been widely used as bridging ligands with carboxylic acids in crystal engineering[5-9]. However, the presently known cases of CPs with carboxylic acids and bis(imidazol-1-yl)methane (bimm) or N-(4-pyridylmethyl)imidazole (pyim) organic ligand are still very rare[10-13]. In the crystal self-assembly of CPs, these μ2-bridging bridging ligands are expected to construct multi-dimensional compounds. Among them, the flexible bimm ligand with an alkyl spacer between two imidazole unities may coordinate with central metal ions via two imidazole-type nitrogen atoms (Scheme 1). Compared with bimm ligand, the rigid pyim ligand may coordinate with central metal ions using pyridine-type and imidazole-type nitrogen atoms, yielding intriguing structures.
Scheme1
On the other hand, 5-nitro-1, 2, 3-benzenetricarboxylic acid (H3nbta) containing rich coordination sites has been proved to be an excellent organic ligand, which can exhibit versatile coordination modes in the assembly of CPs[14-16]. In this study, we have successfully prepared two auxiliary N-donor ligand-mediated Zn(Ⅱ)-containing CPs incorporating H3nbta, {[Zn(Hnbta)(bimm)](3H2O)}n (1) with a 1D chain structure and {[Zn1.5(nbta)(pyim)(H2O)](2H2O)}n (2) with a 2D layer structure. Their single-crystal structures, spectral properties and thermal stabilities were investigated. Moreover, luminescence properties of compounds 1 and 2 have been studied and discussed.
2. EXPERIMENTAL
All analytical grade chemicals and solvents were purchased and used as received without further purification. The IR spectra were recorded as KBr pellets on a Nicolet Avatar-360 spectrometer in the range of 4000~400 cm−1. Elemental analyses for C, H and N were carried out on a Flash 2000 elemental analyzer. Thermogravimetric analyses (TGA) were carried out on a SDTQ600 thermogravimetric analyzer. A platinum pan was used for heating the sample at a heating rate of 10 ℃/min under air atmosphere. Fluorescence measurements were recorded with a Hitachi F4500 fluorescence spectrophotometer.
2.1 Synthesis of {[Zn(Hnbta)(bimm)](3H2O)}n (1)
A mixture of H3nbta (25.5 mg, 0.1 mmol), Zn(OAc)2·2H2O (22.0 mg, 0.1 mmol), bimm (0.029 g, 0.2 mmol), and 6 mL deionized water was sealed in a 25 mL Teflon-lined autoclave and was kept under autogenous pressure at 140 ℃ for 4 days, followed by cooling to room temperature at a rate of 5 ℃·h-1. Block colourless crystals were collected (yield: 35% based on Zn). Elemental analysis calculated for C16H17N5O11Zn (%): C, 36.90; H, 3.29; N, 13.45. Found (%): C, 36.97; H, 3.25; N, 13.31. Selected IR peaks (cm-1): 3106 (m), 1597 (m), 1560 (s), 1523 (s), 1445 (s), 1340 (s), 1276 (s), 1229 (s), 1085 (m), 1023 (m), 945 (m), 828 (m), 764 (m), 739 (m), 705 (m), 649 (m).
2.2 Synthesis of {[Zn1.5(nbta)(pyim)(H2O)](2H2O)}n (2)
An identical procedure with 2 was followed to prepare 1 except that bis(imidazol-1-yl)methane was replaced by N-(4-pyridylmethyl)imidazole (14.61 mg, 0.1 mmol). Block yellow crystals were collected (yield: 56% based on Zn). Elemental analysis calculated for C17H15N4O11Zn1.5 (%): C, 37.13; H, 2.73; N, 10.19. Found (%): C, 37.16; H, 2.76; N, 10.21. Selected IR peaks (cm−1): 3135 (w), 1635 (m), 1612 (m), 1568 (s), 1553 (w), 1514 (s), 1428 (s), 1347 (s), 1316 (s), 1232 (m), 1124 (m), 1062 (s), 1022 (m), 974 (m), 949 (m), 922 (m), 837 (m), 750 (m), 729 (m), 709 (m), 705 (m).
2.3 X-ray structure determination
The structures of 1 and 2 were determined by single-crystal X-ray diffraction technique. Diffraction data were collected on an Oxford Diffraction Gemini with MoKα radiation (λ = 0.71073 Å) at 293 K. The structures were solved by direct methods using the Olex2 program as an interface together with the SHELXT and SHELXL programs, in order to solve and refine the structure respectively[17-19]. All non-hydrogen atoms were refined anisotropically. The hydrogen atoms on water molecules were located from difference Fourier maps and were refined using a riding model. Other hydrogen atoms were placed at the calculation positions. In compounds 1 and 2, the diffused electron densities resulting from highly disordered water molecules were removed using the SQUEEZE option in PLATON. The final chemical formulas of compounds 1 and 2 were estimated from the SQUEEZE result combined with the TGA result. For compound 1: triclinic system, space group P
¯1 with a = 9.3712(9), b = 9.4045(9), c = 12.9642(13) Å, α = 69.757(9)°, β = 72.145(9)°, γ = 86.916(8)º, V = 1018.60(2) Å3, Dc = 1.580 g/cm3, Mr = 520.68, F(000) = 492, μ = 1.026 mm-1, the final R = 0.0469 and wR = 0.0961, S = 1.026; for 2: monoclinic system, space group P2/n, with a = 10.2183(3), b = 13.2364(5), c = 15.0772(5) Å, β = 90.649(3)°, V = 2039.11(1) Å3, Dc = 1.672 g/m3, Mr = 513.35, F(000) = 1032, μ = 1.837 mm-1, the final R = 0.0302 and wR = 0.0820, S = 1.092. Selected bond lengths and bond angles are listed in Tables 1 and 2, respectively.Table 1
Bond Dist. Bond Dist. Bond Dist. Zn(1)–O(2) 1.953(2) Zn(1)–O(4)#1 1.979(2) Zn(1)–N(2) 2.000(3) Zn(1)–N(5)#2 2.008(3) Angle (°) Angle (°) Angle (°) O(2)−Zn(1)−O(4)#1 105.19(9) N(2)−Zn(1)−O(2) 120.04(1) O(2)−Zn(1)−N(5)#2 113.42(1) N(2)−Zn(1)−O(4)#1 96.43(1) N(5)#2−Zn(1)−O(4)#1 115.33(1) N(2)−Zn(1)−N(5)#2 105.64(1) Symmetry transformation: #1: 2 − x, 1 − y, –z; #2: 1 − x, 2 − y, –z Table 2
Bond Dist. Bond Dist. Bond Dist. Zn(1)−N(1) 1.991(2) Zn(1)−O(1) 1.9415(2) Zn(1)−O(6)#1 1.9899(2) Zn(1)−O(9) 2.025(2) Zn(2)−O(3) 2.0770(2) Zn(2)−O(5) 2.1222(2) Zn(2)−N(3)#3 2.156(2) Angle (°) Angle (°) Angle (°) O(1)−Zn(1)−O(6)#1 105.51(8) O(1)−Zn(1)−O(9) 100.49(9) O(1)−Zn(1)−N(1) 123.78(9) O(6)#1−Zn(1)−O(9) 117.89(8) O(6)#1−Zn(1)−N(1) 104.47(9) N(1)−Zn(1)−O(9) 105.74(1) O(3)−Zn(2)−O(3)#2 168.91 O(3)−Zn(2)−O(5) 86.07(7) O(3)−Zn(2)−O(5)#2 86.96(7) O(3)−Zn(2)−N(3)#3 88.23(8) O(3)#2−Zn(2)−N(3)#3 100.02(8) O(3)−Zn(2)−N(3)#4 100.03(8) O(5)#2−Zn(2)−O(5) 102.01(1) O(5)−Zn(2)−N(3)#3 168.84(8) O(5)−Zn(2)−N(3)#4 87.24(8) N(3)#3−Zn(2)−N(3)#4 84.31(1) Symmetry transformation: #1: x −1, y, z; #2: 1.5 − x, y, 1.5 −z; #3: x − 1, 2 − y, 1 − z; #4: x + 0.5, 2 − y, 0.5 + z 3. RESULTS AND DISCUSSION
Compounds 1 and 2 were obtained as block colourless crystalline materials via the reaction of H3nbta and zinc acetate with auxiliary N-donor ligand in aqueous medium at 140 ℃ for 4 days, respectively. Compound 1 crystallizes in the triclinic P
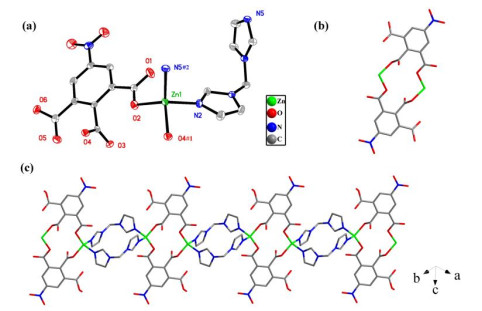
¯1 space group and exhibits a 1D chain. In the asymmetric unit, there are one independent Zn2+ ion, one protonated Hnbta2− anion, one neutral bimm ligand and one free water molecule (Fig. 1a). The Zn(1) atom is located in a distorted tetrahedral geometry, completed by two O atoms from two different Hnbta2− anions and two N atoms from two different bimm ligands. The maximum and minimum bond angles for Zn2+ ion are 120.04(1) and 96.43(1)°, respectively, with an average value of 109.34(2)°, which slightly deviates from the angle of 109.34° in a perfect tetrahedron. The bond lengths vary from 1.953(2) to 2.008(3) Å, which are well-matched to those observed in similar compounds[4, 16]. In compound 1, a pair of oppositely arranged Hnbta2− anions bind two central Zn2+ ions to generate a [Zn2(Hnbta)2] ring subunit, in which Hnbta2− anion exhibits the μ2-(μ1-η1: η0/μ1-η1: η0/μ0-η0: η0) coordination mode. These subunits are connected by μ2-bimm ligands to form a resultant 1D chain structure (Fig. 1b). Noted, the flexible μ2-bimm ligand shows a relative rotation, in which the dihedral angle between two imidazole rings is 89.054° (Fig. 1c).Figure 1
Compound 2 crystallizes in a monoclinic space group P2/n and features a 2D layered structure. There exist one and a half crystallographically independent Zn2+ ions, one nbta3− anion, one pyim ligand, one coordinated water molecule and two lattice water molecules in the asymmetric unit. As shown in Fig. 2a, the two Zn2+ ions exhibit two different coordination manners. The Zn(1) atom is also located in a distorted tetrahedral geometry, completed by two O atoms from two different nbta3− anions and one imidazole N atom from one pyim ligand. The maximum and minimum bond angles for Zn2+ ion are 123.78(9)° and 104.47(9)°, respectively, with an average value of 109.65 °, which also slightly deviates from the angle of 109.47° in a perfect tetrahedron. The central Zn(2) atom resides at a crystallographic inversion left and assumes an octahedral coordination environment with four carboxyl atoms from three nbta3- anions, two pyridine nitrogen atoms of two pyim ligands and one coordinated water molecule. The bond lengths of Zn–N are 1.991(2) and 2.156(2) Å, while the Zn–O bond lengths range from 1.9415(19) to 2.1221(18) Å, which fall in the normal range[4, 16]. In compound 2, the nbta3− anion adopts the μ3-(μ1-η1: η0/μ1-η1: η0/μ2-η1: η1) coordination mode and acts as a tridentate bridging ligand, extending the structure into an infinite 1D structure. Moreover, the 1D structure is further linked by μ2-pyim ligand into a 2D architecture, in which the rigid μ2-pyim ligand shows a slight rotation, and the dihedral angle between the imidazole and pyridine rings is 23.609°. Topologically, if the nbta3− anion and Zn(1) atoms are simplified as three-connected nodes, the Zn(2) atoms could be considered as four-connected nodes, with the pyim ligand serving as the linear linkers. As a result, the structure of compound 2 represents an unprecedented (3, 4)-connected network with the {62.84}{62.8}4 topology (Fig. 2d).
Figure 2
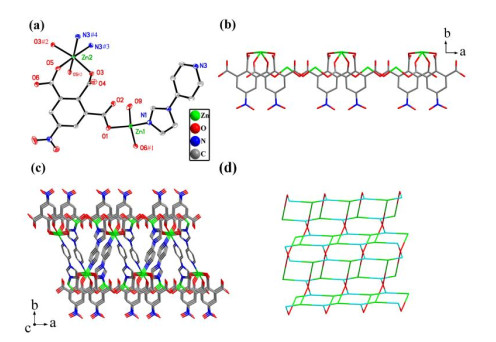 Figure 2. (a) Coordination environment of the Zn(Ⅱ) lefts. Displacement ellipsoids are drawn at the 30% probability level. Hydrogen atoms are omitted for clarity. Symmetry codes: #1: x − 1, y, z; #2: 1.5 − x, y, 1.5 – z; #3: 0.5 + x, 2 – y, 0.5 + z; #4: 1 – x, 2 – y, 1 – z. (b) 1D double structure chain constructed from nbta3− anions. (c) 2D architecture of compound 2. (d) Schematic representation of the 2D (3, 4)-connected network
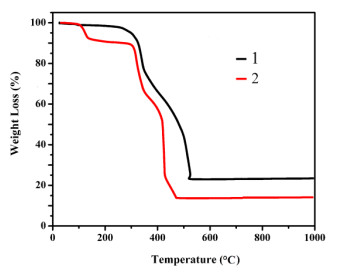
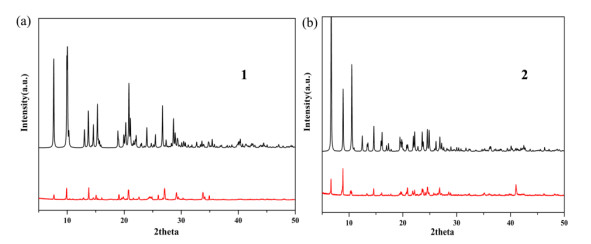
Figure 2. (a) Coordination environment of the Zn(Ⅱ) lefts. Displacement ellipsoids are drawn at the 30% probability level. Hydrogen atoms are omitted for clarity. Symmetry codes: #1: x − 1, y, z; #2: 1.5 − x, y, 1.5 – z; #3: 0.5 + x, 2 – y, 0.5 + z; #4: 1 – x, 2 – y, 1 – z. (b) 1D double structure chain constructed from nbta3− anions. (c) 2D architecture of compound 2. (d) Schematic representation of the 2D (3, 4)-connected networkTo confirm the phase purity of bulk samples, the X-ray powder diffraction pattern was recorded. As seen in Fig. 3, the peak positions of experimental and simulated patterns are in good agreement with each other, demonstrating the phase purity of 1 and 2. The dissimilarities in intensity may be owing to the preferred orientation of the samples. In addition, thermal behaviors of 1 and 2 were examined by thermal gravimetric analysis (TGA) in a dry air atmosphere from 30 to 700 ℃. As shown in Fig. 4, compound 1 undergoes two steps of weight loss, with the first one of 9.66% corresponding to the removal of water molecules in the temperature range of 97~107 ℃ (calcd. 9.59%). From then on, almost no weight loss is observed until 253 ℃, beyond which the intense weight loss is attributed to the decomposition and collapse of the structure. Compound 2 also undergoes two steps of weight loss. The weight loss of 9.79% from 97 to 116 ℃ corresponds to the release of water molecules (calcd. 9.83%) and that from 273 ºC results from the decomposition and collapse of the structure.
Figure 3
Figure 4
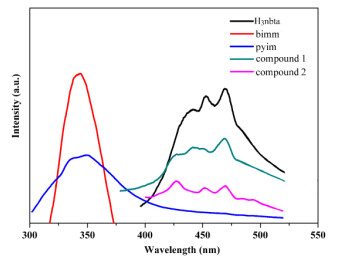
CPs with Zn lefts usually present photoluminescent properties with potential applications such as chemical sensors and photochemistry fields[20, 21]. Here, the solid-state emission spectra of 1, 2 and free ligands were explored at room temperature. As shown in Fig. 5, compound 1 shows a main peak at 471 nm with two shoulders at 453, 440 nm upon excitation at 290 nm and compound 2 shows a main peak at 420 nm with two shoulders at 452 and 469 nm under 374 nm excitation. The free H3nbta shows a main peak at 469 nm with a shoulder at 453 nm upon excitation at 250 nm and the free bimm and pyim ligands were observed with wavelengths at 344 and 350 nm, respectively. Considering the Zn2+ ion is difficultly oxidized or reduced, the peaks of compounds 1 and 2 should be attributed to the transitions of Hnbta2−/nbta3− anions because similar peaks also appear for the free H3nbta ligand. The peaks for 1 and 2 exhibit a blue-shift with respect to the free H3nbta, which may be tentatively assigned to the intraligand charge transfer of Hnbta2−/nbta3− anions and/or metal-ligand coordination interactions.
Figure 5
4. CONCLUSION
In summary, we have successfully synthesized and characterized two new CPs based on 5-nitro-1, 2, 3-benzenetricarboxylic acid and bimm/pyim N-donor auxiliary ligands. Compound 1 is a 1D chain structure and compound 2 features a 2D network with a 4-connected sql topology. The results indicate the bimm and pyim ligands may act as additional metal linkers to mediate the structures of CPs with 5-nitro-1, 2, 3-benzenetricarboxylic acid in crystal engineering. What is more, both compounds show photoluminescence and could be good candidates for potential luminescence materials.
-

-
-
[1]
Yiqian Jiang , Zihan Yang , Xiuru Bi , Nan Yao , Peiqing Zhao , Xu Meng . Mediated electron transfer process in α-MnO2 catalyzed Fenton-like reaction for oxytetracycline degradation. Chinese Chemical Letters, 2024, 35(8): 109331-. doi: 10.1016/j.cclet.2023.109331
-
[2]
Shilong Li , Ming Zhao , Yefei Xu , Zhanyi Liu , Mian Li , Qing Huang , Xiang Wu . Performance optimization of aqueous Zn/MnO2 batteries through the synergistic effect of PVP intercalation and GO coating. Chinese Chemical Letters, 2025, 36(3): 110701-. doi: 10.1016/j.cclet.2024.110701
-
[3]
Kun Chen , Huimin Lin , Xin Peng , Ziying Wu , Jingyue Dai , Yi Sun , Yaxuan Feng , Ziyi Huang , Zhiqiang Yu , Meng Yu , Guangyu Yao , Jigang Wang . In situ synthesis of MnO2 micro/nano-adjuvants for enhanced immunotherapy of breast tumors. Chinese Chemical Letters, 2025, 36(5): 110045-. doi: 10.1016/j.cclet.2024.110045
-
[4]
Haoting Wang , Mengfan Luo , Yuzhong Wang , Jialong Yin , Heng Zhang , Jia Zhao , Bo Lai . Mn(Ⅱ) enhanced permanganate oxidation of trace organic pollutants in water: Critical role of in situ formation of colloidal MnO2. Chinese Chemical Letters, 2025, 36(6): 110348-. doi: 10.1016/j.cclet.2024.110348
-
[5]
Qinjin DAI , Shan FAN , Pengyang FAN , Xiaoying ZHENG , Wei DONG , Mengxue WANG , Yong ZHANG . Performance of oxygen vacancy-rich V-doped MnO2 for high-performance aqueous zinc ion battery. Chinese Journal of Inorganic Chemistry, 2025, 41(3): 453-460. doi: 10.11862/CJIC.20240326
-
[6]
Jiahong ZHENG , Jingyun YANG . Preparation and electrochemical properties of hollow dodecahedral CoNi2S4 supported by MnO2 nanowires. Chinese Journal of Inorganic Chemistry, 2024, 40(10): 1881-1891. doi: 10.11862/CJIC.20240170
-
[7]
Lumin Zheng , Ying Bai , Chuan Wu . Multi-electron reaction and fast Al ion diffusion of δ-MnO2 cathode materials in rechargeable aluminum batteries via first-principle calculations. Chinese Chemical Letters, 2024, 35(4): 108589-. doi: 10.1016/j.cclet.2023.108589
-
[8]
Sanmei Wang , Yong Zhou , Hengxin Fang , Chunyang Nie , Chang Q Sun , Biao Wang . Constant-potential simulation of electrocatalytic N2 reduction over atomic metal-N-graphene catalysts. Chinese Chemical Letters, 2025, 36(3): 110476-. doi: 10.1016/j.cclet.2024.110476
-
[9]
Liang Lou , Xuncheng Liu , Yuanyu Wang , Tao Hu , Zhongjie Wang , Houqiang Shi , Junkai Xiong , Siqi Jing , Liankang Ye , Qihui Guo , Xiang Ge . Achieving reusability of leachate for multi-element recovery of the discarded LiNixCoyMn1-x-yO2 cathode by regulating the co-precipitation coefficient. Chinese Chemical Letters, 2025, 36(5): 109726-. doi: 10.1016/j.cclet.2024.109726
-
[10]
Chuanming GUO , Kaiyang ZHANG , Yun WU , Rui YAO , Qiang ZHAO , Jinping LI , Guang LIU . Performance of MnO2-0.39IrOx composite oxides for water oxidation reaction in acidic media. Chinese Journal of Inorganic Chemistry, 2024, 40(6): 1135-1142. doi: 10.11862/CJIC.20230459
-
[11]
Min LUO , Xiaonan WANG , Yaqin ZHANG , Tian PANG , Fuzhi LI , Pu SHI . Porous spherical MnCo2S4 as high-performance electrode material for hybrid supercapacitors. Chinese Journal of Inorganic Chemistry, 2025, 41(2): 413-424. doi: 10.11862/CJIC.20240205
-
[12]
Huakang Zong , Xinyue Li , Yanlin Zhang , Faxun Wang , Xingxing Yu , Guotao Duan , Yuanyuan Luo . Pt/Ti3C2 electrode material used for H2S sensor with low detection limit and high stability. Chinese Chemical Letters, 2025, 36(5): 110195-. doi: 10.1016/j.cclet.2024.110195
-
[13]
Lili Zhang , Hui Gao , Gong Zhang , Yuning Dong , Kai Huang , Zifan Pang , Tuo Wang , Chunlei Pei , Peng Zhang , Jinlong Gong . Cross-section design of the flow channels in membrane electrode assembly electrolyzer for CO2 reduction reaction through numerical simulations. Chinese Chemical Letters, 2025, 36(1): 110204-. doi: 10.1016/j.cclet.2024.110204
-
[14]
Tsegaye Tadesse Tsega , Jiantao Zai , Chin Wei Lai , Xin-Hao Li , Xuefeng Qian . Earth-abundant CuFeS2 nanocrystals@graphite felt electrode for high performance aqueous polysulfide/iodide redox flow batteries. Chinese Journal of Structural Chemistry, 2024, 43(1): 100192-100192. doi: 10.1016/j.cjsc.2023.100192
-
[15]
Le Ye , Wei-Xiong Zhang . Structural phase transition in a new organic-inorganic hybrid post-perovskite: (N,N-dimethylpyrrolidinium)[Mn(N(CN)2)3]. Chinese Journal of Structural Chemistry, 2024, 43(6): 100257-100257. doi: 10.1016/j.cjsc.2024.100257
-
[16]
Xinyu Guo , Chang Li , Wenjun Deng , Yi Zhou , Yan Chen , Yushuang Xu , Rui Li . Phase engineering and heteroatom incorporation enable defect-rich MoS2 for long life aqueous iron-ion batteries. Chinese Chemical Letters, 2025, 36(3): 109715-. doi: 10.1016/j.cclet.2024.109715
-
[17]
Dan Shao , Yujing Lyu , Chengyuan Liu , Hao Wang , Ning Ma , Hao Xu , Wei Yan , Xiaohua Jia , Haojie Song . Attracting magnetic BDD particles onto Ti/RuO2-IrO2 by using a magnet: A novel 2.5-dimensional electrode for electrochemical oxidation wastewater treatment. Chinese Chemical Letters, 2025, 36(6): 110641-. doi: 10.1016/j.cclet.2024.110641
-
[18]
Mingjiao Lu , Zhixing Wang , Gui Luo , Huajun Guo , Xinhai Li , Guochun Yan , Qihou Li , Xianglin Li , Ding Wang , Jiexi Wang . Boosting the performance of LiNi0.90Co0.06Mn0.04O2 electrode by uniform Li3PO4 coating via atomic layer deposition. Chinese Chemical Letters, 2024, 35(5): 108638-. doi: 10.1016/j.cclet.2023.108638
-
[19]
Xi Tang , Chunlei Zhu , Yulu Yang , Shihan Qi , Mengqiu Cai , Abdullah N. Alodhayb , Jianmin Ma . Additive regulating Li+ solvation structure to construct dual LiF−rich electrode electrolyte interphases for sustaining 4.6 V Li||LiCoO2 batteries. Chinese Chemical Letters, 2024, 35(12): 110014-. doi: 10.1016/j.cclet.2024.110014
-
[20]
Yi ZHANG , Guang LI , Wenxuan FAN , Qingfeng YI . Influence of bismuth trisulfide on the electrochemical performance of iron electrode. Chinese Journal of Inorganic Chemistry, 2025, 41(6): 1196-1206. doi: 10.11862/CJIC.20240445
-
[1]
Metrics
- PDF Downloads(0)
- Abstract views(890)
- HTML views(79)

 Login In
Login In




 DownLoad:
DownLoad:
 DownLoad:
DownLoad: