Citation:
Ping Da REN, Shi Feng PAN, Ting Wei DONG, Shi Hui WU. FACILE AND SELECTIVE REDUCTION OF NTIROARENES IN THE PRESENCE OF NITRILES WITH NaBH4-BiCl3 SYSTEM[J]. Chinese Chemical Letters,
;1995, 6(7): 553-554.

-
Nitroarenes were selectively reduced to primary amines by treatment with sodium borohydride bismuth chloride in the presence of nitrites.The selectivity was always excellent.
-
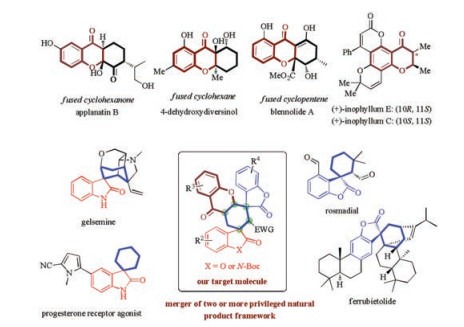
The rich structural diversity and complexity of molecules has always captured the attention of chemists to design cost-effective and sustainable synthetic ways [1], especially diversity-oriented synthesis of known natural and synthetic bioactive compounds [2]. In this context, spirocyclic benzofuranones have attracted considerable interest due to their privileged structural motifs found in natural and unnatural compounds with diverse and important biological activities (Fig. 1) [3]. There are some examples, concerning the catalytic asymmetric construction of such typically spirocyclic benzofuranones, have been disclosed [4, 5]. In spite of these elegant progresses, catalytic asymmetric methods for catalytic asymmetric assembly of spirocyclohexane-based benzofuranones have been less studied (Scheme 1a).
Figure 1
Scheme 1
Spirocyclic oxindoles [6] with carbocyclic six-membered rings are also important substructures that are widely present in numerous bioactive natural products (Fig. 1) [7]. Consequently, considerable effort has been devoted to asymmetric approaches to access enantioenriched spirocyclohexane oxindoles [7, 8]. However, highly efficient, diastereoselective, and enantioselective reactions generating complex and structurally diverse chiral spirocyclohexane oxindoles in one step remain a challenging but highly desirable goal in organic synthesis (Scheme 1a).
Chromanones are an important class of heterocycles containing the core structure of dihydrobenzopyranone. Even though there exist many strategies for the asymmetric synthesis of chiral chromanones with only one chiral center [9], attempts toward the direct asymmetric synthesis of enantioenriched fused chromanone systems bearing two consecutive stereocenters are limited [10]. Especially, fused ring chromanone skeletons are also abundant in natural products like cyclopentene, cyclohexanone or cyclohexane fused chromanones (Fig. 1) [11]. The use of readily available chromones as a class of two-carbon building blocks to participate Michael cycloaddition reactions presents a promising one [12]. However the activation of the chemically inert chromones retards the development of this methold. Therefore, the stereoselective construction of fused ring chromanones is also a highly desirable but challenging goal.
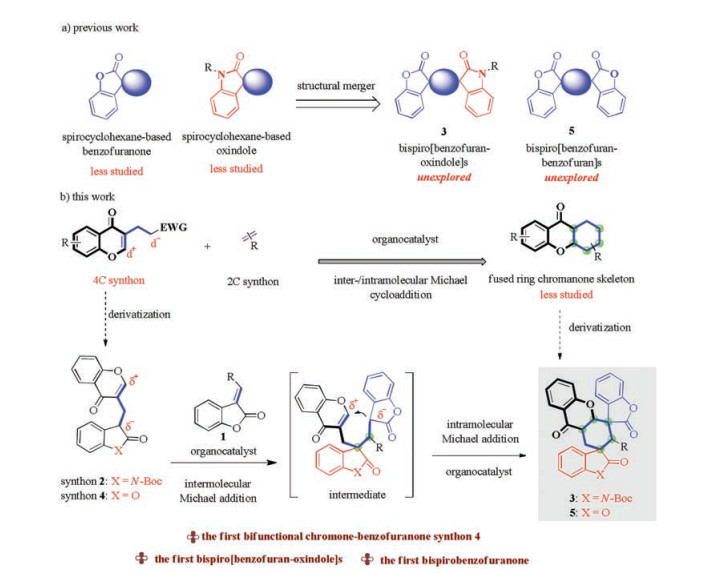
Considering the wide occurrence of chromanones, spirocyclohexanebenzofuranones and spirocyclohexaneoxindoles in natural products and drugs, integrating these medicinally relevant motifs to generate unprecedented polycyclic skeletons would be highly desirable [1, 2]. The intimating challenge lies in the scarcity of efficient pathways to rapidly install these structural units simultaneously. Herein, we hypothesized that highly functionalized chiral bispiro[benzofuran-oxindole/benzofuran-chromanone]s could be constructed via an organocatalytic domino inter-/intramolecular Michael cycloaddition of 3-substituted methylenebenzofuranone and bifunctional chromone-oxindole/benzofuran synthon. To the best of our knowledge, if successful, this is the first example of bifunctional chromone-benzofuranone synthon directed organocatalytic tandem reaction, and also the first example of the bispiro [benzofuran-oxindole]s and bispirobenzofuranone (Scheme 1b).
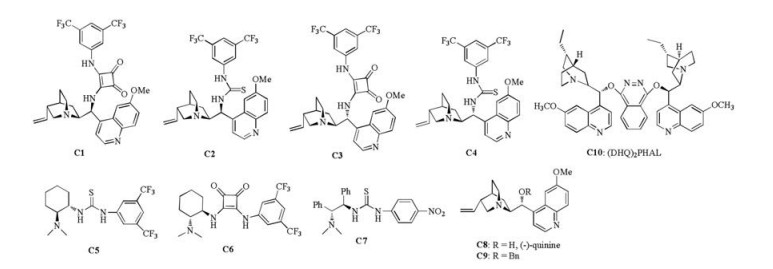
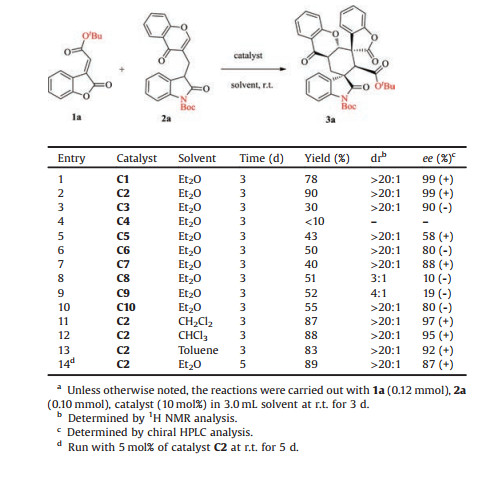
Initially, the reaction of tert-butyl ester substituted methylenebenzofuranone 1a with chromone-oxindole synthon 2a [13] was investigated under the catalysis of quinine-derived squaramidetertiary amine C1 (Fig. 2) (Table 1, entry 1). Gratifyingly, the designed inter-/intramolecular Michael cycloaddition reaction indeed afforded the desired product 3a in a 78% yield albeit with 99% ee and >20:1 dr. This preliminary result demonstrated the feasibility of our design. In order to improve the yield, other chiral bifunctional tertiaryamine catalysts C2-C10 (Fig. 2) were utilized as catalysts in the reaction (entries 2–10). It was found that 10 mol% of quinine-derived thiourea-tertiary amine catalyst C2 fully converted chromone-oxindole synthon 1a into the desired product 3a with good control over the stereochemistry (90% yield, 99% ee, >20:1 dr) (entry 2). The subsequent evaluation of solvents discovered that Et2O could deliver the cycloaddition with better enantioselectivity than other solvents (entry 2 vs. entries 11–13). Thus, the optimal reaction conditions were chosen as those illustrated in entry 2.
Figure 2
Table 1
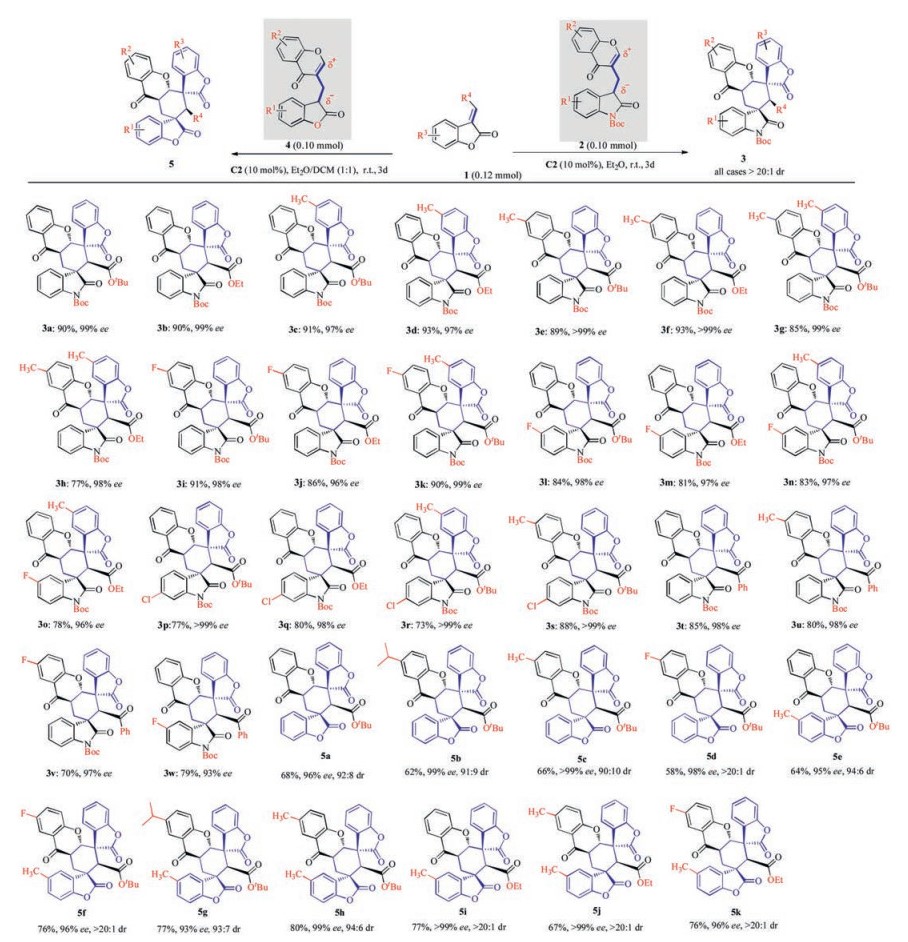
After establishing the optimal reaction conditions, the substrate scope and limitations were explored. Initially, the possibility to employ various 3-substituted methylenebenzofuranones 1 was evaluated using chromone-oxindole synthon 2a as a model substrate (Scheme 2, products 3a-d). The reaction proved unbiased towards the substituents of substrates 1. Notably, in all of the cases high yields and high stereoselectivities were observed. Replacement of a tert-butyl ester substituent with an ethyl ester group in 2 was also well tolerated, could deliver the desired products 3 with excellent dr and ee (products 3b, 3d, 3f, 3h, 3j, 3m, 3o and 3q). Moreover, the reaction was shown to work well with benzoyl substituted substrates 1 to give the desired products 3t-w with very good yields (70%–85%), diastereoselectivities (all cases >20:1 dr), and good enantioselectivities (93%–98% ee).
Scheme 2
Then, various chromone-oxindole synthons 2 were reacted with 3-substituted methylenebenzofuranones 1 (Scheme 2). To our delight, a highly efficient synthesis was observed under the reaction conditions in highly enantioselective fashions (products 3e-s with 96% to >99% ee). The reactions were shown to work well with a range of chromone-oxindole synthons 2 bearing either electronwithdrawing or electron-donating groups in the chromone ring to give the desired products 3e-k and 3s with very good yields (up to 93%), excellent diastereoselectivities (all cases >20:1 dr) and enantioselectivities (96% to >99% ee).
Inspired by the success in the chromone-oxindole synthons 2, and based on diverse structural library design, considering that bispiro[bibenzofuran-chromanone]s 5 would be medicinally important hybrids for the development of new biological molecules, subsequently, we further designed and synthesized a novel bifunctional benzofuran-chromone 4 as a 4C synthon and was then subjected to methylene benzofuranone 1 in 3.0 mL of Et2O/DCM (1:1, v/v) at room temperature for 3 day. The reaction proceeded smoothly, affording the medicinally important bispiro [benzofuran-benzofuran-chromanone]s 5a in 68% yield with 98% ee, 92:8 dr. The reaction scope generally proved to be broad with respect to a wide variety of electron-rich and -poor reagents 1 and 4 to give diversely substituted bispiro[bibenzofuran-chromanone]s 5a-k in 58%–80% yields with 93% to >99% ee and up to >20:1 dr.
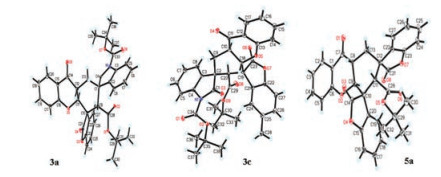
The absolute configuration was unambiguously assigned on the basis of the X-ray analysis of the products 3a, 3c and 5a (Fig. 3). The absolute configurations of all remaining products 3 and 5 were given by analogy.
Figure 3
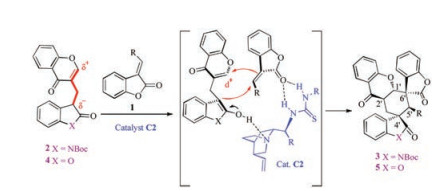
Based on the experimental results, we suggested a possible bifunctional catalytic mode to account for the observed stereoselectivities (Scheme 3), The tertiary amine group of the catalyst C2 acted as a base to deprotonate the OH group of the enolized substrate 2 or 4. Meanwhile, the carbonyl group of substrate 1 was activated by catalyst C2 via forming a hydrogen bond. Subsequently, the Re-face of the 1 was preferably attacked by the Si-face of enolated substrate 2 or 4, delivering the transient Michael adduct intermediate under this dual activating mode of chiral catalyst C2. Finally, the α-position of the resulting prochiral nucleophile intermediate initiates an intermolecular annulation process through the attack to the Si-face of the electron-defficient chromone moiety to accomplish the desired (C1'S, C2'R, C4'S, C5'S, C6'S)-configured 3 or 5.
Scheme 3
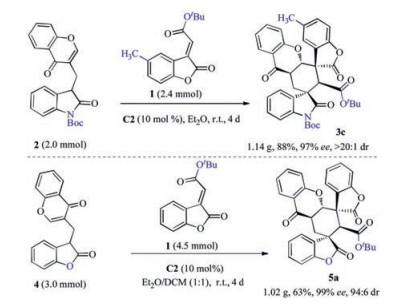
Subsequently, we carried out two gram-scale inter-/intramolecular Michael cycloadditions to examine the applicability of the reaction. As shown in Scheme 4, the gram-scale reaction could smoothly take place to give products 3c and 5a with excellent enantio-and, diastereoselectivities, which was similar to the results of the small-scale reaction (for details, see the Supporting infomation).
Scheme 4
In summary, we have demonstrated that a wide range of complex bispiro[benzofuran-oxindole/benzofuran-chromanone]s 3 and 5 can be efficiently assembled in high stereoselectivity in a single step from bifunctional chromone-oxindole/benzofuran synthons 2 and 4 servring as starting materials. The transformation provides products 3 and 5 comprised of five consecutive stereocenters including two spiro quaternary carton centers, in high yields and stereoselectivities (up to 93% yield, >20:1 dr and >99% ee). Moreover, this is the first example of the bifunctional chromonebenzofuranone synthon 4 directed organocatalytic tandem reaction, and also the first example of the bispiro[benzofuran-oxindole] and bispirobenzofuranone, potentially useful in medicinal chemistry. We believe this strategy would broaden the synthetic utility of bifunctional chromone synthon in the assembly of biologically valuable compounds.
Acknowledgments
This work was received financial support from the National Nature Science Foundation of China (Nos. 81760625, 81660576 and 81560563) and Projects of Guizhou Province (Nos. [2016]5623, JG [2016]06, [2019]1402, [2017]5609 and [2018]5781).
Appendix A. Supplementary data
Supplementary material related to this article can befound, inthe online version, at doi: https://doi.org/10.1016/j.cclet.2019.06.015.
-

-
-
[1]
Yu BAI , Jijiang WANG , Long TANG , Erlin YUE , Chao BAI , Xiao WANG , Yuqi ZHANG . A cadmium(Ⅱ) coordination polymer based on a semirigid tetracarboxylate ligand for highly selective detection of Fe3+ and 4-nitrophenol. Chinese Journal of Inorganic Chemistry, 2025, 41(6): 1217-1226. doi: 10.11862/CJIC.20240457
-
[2]
Liangfeng Yang , Liang Zeng , Yanping Zhu , Qiuan Wang , Jinheng Li . Copper-catalyzed photoredox 1,4-amidocyanation of 1,3-enynes with N-amidopyridin-1-ium salts and TMSCN: Facile access to α-amido allenyl nitriles. Chinese Chemical Letters, 2024, 35(11): 109685-. doi: 10.1016/j.cclet.2024.109685
-
[3]
Liang Ma , Zhou Li , Zhiqiang Jiang , Xiaofeng Wu , Shixin Chang , Sónia A. C. Carabineiro , Kangle Lv . Effect of precursors on the structure and photocatalytic performance of g-C3N4 for NO oxidation and CO2 reduction. Chinese Journal of Structural Chemistry, 2024, 43(11): 100416-100416. doi: 10.1016/j.cjsc.2024.100416
-
[4]
Xiuzheng Deng , Changhai Liu , Xiaotong Yan , Jingshan Fan , Qian Liang , Zhongyu Li . Carbon dots anchored NiAl-LDH@In2O3 hierarchical nanotubes for promoting selective CO2 photoreduction into CH4. Chinese Chemical Letters, 2024, 35(6): 108942-. doi: 10.1016/j.cclet.2023.108942
-
[5]
Yuwei Liu , Yihui Zhu , Weijian Duan , Yizhuo Yang , Haorui Tuo , Chunhua Feng . Electrocatalytic nitrate reduction on Fe, Fe3O4, and Fe@Fe3O4 cathodes: Elucidating structure-sensitive mechanisms of direct electron versus hydrogen atom transfer. Chinese Chemical Letters, 2025, 36(6): 110347-. doi: 10.1016/j.cclet.2024.110347
-
[6]
Guixu Pan , Zhiling Xia , Ning Wang , Hejia Sun , Zhaoqi Guo , Yunfeng Li , Xin Li . Preparation of high-efficient donor-π-acceptor system with crystalline g-C3N4 as charge transfer module for enhanced photocatalytic hydrogen evolution. Chinese Journal of Structural Chemistry, 2024, 43(12): 100463-100463. doi: 10.1016/j.cjsc.2024.100463
-
[7]
Rui PAN , Yuting MENG , Ruigang XIE , Daixiang CHEN , Jiefa SHEN , Shenghu YAN , Jianwu LIU , Yue ZHANG . Selective electrocatalytic reduction of Sn(Ⅳ) by carbon nitrogen materials prepared with different precursors. Chinese Journal of Inorganic Chemistry, 2024, 40(5): 1015-1024. doi: 10.11862/CJIC.20230433
-
[8]
Yusong Bi , Rongzhen Zhang , Kaikai Niu , Shengsheng Yu , Hui Liu , Lingbao Xing . Construction of a three-step sequential energy transfer system with selective enhancement of superoxide anion radicals for photocatalysis. Chinese Chemical Letters, 2025, 36(5): 110311-. doi: 10.1016/j.cclet.2024.110311
-
[9]
Xiuzheng Deng , Yi Ke , Jiawen Ding , Yingtang Zhou , Hui Huang , Qian Liang , Zhenhui Kang . Construction of ZnO@CDs@Co3O4 sandwich heterostructure with multi-interfacial electron-transfer toward enhanced photocatalytic CO2 reduction. Chinese Chemical Letters, 2024, 35(4): 109064-. doi: 10.1016/j.cclet.2023.109064
-
[10]
Tianbo Jia , Lili Wang , Zhouhao Zhu , Baikang Zhu , Yingtang Zhou , Guoxing Zhu , Mingshan Zhu , Hengcong Tao . Modulating the degree of O vacancy defects to achieve selective control of electrochemical CO2 reduction products. Chinese Chemical Letters, 2024, 35(5): 108692-. doi: 10.1016/j.cclet.2023.108692
-
[11]
Shuqi Yu , Yu Yang , Keisuke Kuroda , Jian Pu , Rui Guo , Li-An Hou . Selective removal of Cr(Ⅵ) using polyvinylpyrrolidone and polyacrylamide co-modified MoS2 composites by adsorption combined with reduction. Chinese Chemical Letters, 2024, 35(6): 109130-. doi: 10.1016/j.cclet.2023.109130
-
[12]
Kun Zou , Yihang Xiao , Jinyu Yang , Mingxuan Wu . Facile semisynthesis of histone H3 enables nucleosome probes for investigation of histone H3K79 modifications. Chinese Chemical Letters, 2024, 35(10): 109497-. doi: 10.1016/j.cclet.2024.109497
-
[13]
Jie ZHANG , Xin LIU , Zhixin LI , Yuting PEI , Yuqi YANG , Huimin LI , Zhiqiang LIU . Assembling a luminescence silencing system based on post-synthetic modification strategy: A highly sensitive and selective turn-on metal-organic framework probe for ascorbic acid detection. Chinese Journal of Inorganic Chemistry, 2024, 40(4): 823-833. doi: 10.11862/CJIC.20230310
-
[14]
Jingtai Bi , Yupeng Cheng , Mengmeng Sun , Xiaofu Guo , Shizhao Wang , Yingying Zhao . Efficient and selective photocatalytic nitrite reduction to N2 through CO2 anion radical by eco-friendly tartaric acid activation. Chinese Chemical Letters, 2024, 35(11): 109639-. doi: 10.1016/j.cclet.2024.109639
-
[15]
Gang Hu , Chun Wang , Qinqin Wang , Mingyuan Zhu , Lihua Kang . The controlled oxidation states of the H4PMo11VO40 catalyst induced by plasma for the selective oxidation of methacrolein. Chinese Chemical Letters, 2025, 36(2): 110298-. doi: 10.1016/j.cclet.2024.110298
-
[16]
Xianchen Hu , Junli Yang , Fang Gao , Zhiyong Zhao , Simin Liu . Highly selective [4+4] cross-photodimerization of (4a-azonia)anthracenes driven by confinement of D-A hetero-guest pair in cucurbit[10]uril host. Chinese Chemical Letters, 2025, 36(3): 109967-. doi: 10.1016/j.cclet.2024.109967
-
[17]
Haoran Shi , Jiaxin Wang , Yuqin Zhu , Hongyang Li , Guodong Ju , Lanlan Zhang , Chao Wang . Highly selective α-C(sp3)-H arylation of alkenyl amides via nickel chain-walking catalysis. Chinese Chemical Letters, 2024, 35(7): 109333-. doi: 10.1016/j.cclet.2023.109333
-
[18]
Zimo Yang , Yan Tong , Yongbo Liu , Qianlong Liu , Zhihao Ni , Yuna He , Yu Rao . Developing selective PI3K degraders to modulate both kinase and non-kinase functions. Chinese Chemical Letters, 2024, 35(11): 109577-. doi: 10.1016/j.cclet.2024.109577
-
[19]
Xiangyu Chen , Aihao Xu , Dong Wei , Fang Huang , Junjie Ma , Huibing He , Jing Xu . Atomic cerium-doped CuOx catalysts for efficient electrocatalytic CO2 reduction to CH4. Chinese Chemical Letters, 2025, 36(1): 110175-. doi: 10.1016/j.cclet.2024.110175
-
[20]
Xiaobo Li , Qunyan Wu , Congzhi Wang , Jianhui Lan , Meng Zhang , Weiqun Shi . Theoretical perspectives on the reduction of Pu(Ⅳ) and Np(Ⅵ) by methylhydrazine in HNO3 solution: Implications for Np/Pu separation. Chinese Chemical Letters, 2024, 35(7): 109359-. doi: 10.1016/j.cclet.2023.109359
-
[1]
Metrics
- PDF Downloads(3)
- Abstract views(710)
- HTML views(2)

 Login In
Login In




 DownLoad:
DownLoad:
 DownLoad:
DownLoad: