Citation:
Bao Lin SONG, Zai Sheng LU, De Zhong NIU, Yang CAO, Cheng ZENG. THE SYNTHESIS AND CHARACTERISTICS OF THE COORDINATION COMPOUNDS OF 2-MERCAPTOPYRIDINE-1-OXIDE WITH THE FIPST TRANSITION SERIES METALS[J]. Chinese Chemical Letters,
;1990, 1(2): 117-118.

-
The stability of the coordination compounds of the first transition series metal ions(Mn(Ⅱ),Co(Ⅱ),Ni(Ⅱ),Cu(Ⅱ) and Zn(Ⅱ)) with 2-mercaptopyridine-1-oxide is reported.A coordination compound CoL2 is synthesized and characterized for the first time.
-
1. INTRODUCTION
Polynuclear transition-metal clusters have attracted increasing interest due to their aesthetically pleasing architectures[1-5] and diverse applications in magnetism[6-8], luminescence[9-11], and catalysis[12, 13]. Particularly, polynuclear cobalt clusters have gathered tremendous attention in recent years especially for the following two important reasons: (ⅰ) some cobalt clusters behave as single-molecule magnets (SMMs)[14-17], and could be potentially utilized in high-density information storage devices[18, 19]; (ⅱ) these clusters are also successfully used in many catalysis reactions, such as dioxygen reduction[20-22] and epoxidation of alkenes[23].
A fertile route for the preparation of polynuclear cobalt clusters involves employing polydentate ligands containing several oxygen donors that could incorporate many cobalt ions into one molecular entity. In this work, we employed polydentate Schiff base H2L (H2L = 2-((2-hydroxy-4-methoxy-benzylideneamino)methyl)phenol)[24] as a ligand to assemble polynuclear cobalt compound. The existence of two -OH groups renders H2L as a good ligand to generate polynuclear clusters. A hexanuclear cobalt compound with formula [Co2ⅢCo4Ⅱ(L)4(CH3COO)2(MeO)4]·MeOH (1) was prepared. Herein, we report the synthesis, structure and magnetic properties of complex 1.
2. EXPERIMENTAL
2.1 Materials and physical measurements
All manipulations were performed under aerobic and solvothermal conditions using reagents and solvents as received. The H2L ligand (H2L = 2-((2-hydroxy-4-methoxy-benzylideneamino)methyl)phenol) was prepared according to the literature procedure[24].
The C, H and N microanalyses were carried out with a Carlo-Erba EA1110 CHNO-S elemental analyser. FT-IR spectrum was recorded from KBr pellets in the range of 400~4000 cm–1 on a Nicolet MagNa-IR 500 spectrometer. Variable-temperature dc magnetic susceptibility data were collected using a Quantum Design MPMS-7 SQUID magnetometer.
2.2 Synthesis of complex 1
A mixture of H2L (0.0257 g, 0.1 mmol), Co(OAc)2·4H2O (0.0249 g, 0.1 mmol) and MeOH (1.5 mL) was sealed in a Pyrex-tube (10 mL). The tube was heated at 80 ℃ for 2 days under autogenous pressure. Cooling of the resultant solution to room temperature gave dark-red needle-like crystals. The crystals were collected by filtration, washed with MeOH (2 mL) and dried in air. Yield: 0.019 g (45% based on cobalt). Anal. Calcd. (%) for C70H78N4O22Co6 (Mr = 1672.94): C, 50.02; H, 4.68; N, 3.33. Found (%): C, 49.97; H, 4.65; N, 3.49. Selected IR data for 1 (cm−1): 1606 (s), 1529 (m), 1479 (m), 1446 (m), 1299 (w), 1251 (s), 1219 (s), 1165 (m), 1144 (m), 1122 (m), 1031 (m), 979 (m), 874 (w), 851 (w), 766 (w).
2.3 Structure determination
The data collection for 1 were carried out on a Bruker Smart ApexⅡ diffractometer equipped with a graphite monochromator utilizing MoKα radiation (λ = 0.71073 Å); the ω-2θ scan technique was applied. The crystal structure of complex 1 was solved with the Olex2 solve solution program[25] using Intrinsic Phasing and refined by full-matrix least-squares minimization with the ShelXL refinement package[26]. All non-hydrogen atoms were refined anisotropically. The collected crystal data for 1 are shown in Table S1. Selected bond lengths and bond angles of 1 are listed in Table 1 and Table S2.
Table 1
1 Bond Dist. Bond Dist. Bond Dist. Co(1)–O(2) 1.905(4) Co(2)–O(2) 2.139(4) Co(4)–O(19) 2.199(4) Co(1)–O(13) 1.911(4) Co(3)–O(4) 1.933(4) Co(5)–O(9) 1.905(4) Co(1)–O(1) 1.912(4) Co(3)–O(5) 2.008(4) Co(5)–O(8) 1.906(4) Co(1)–N(4) 1.916(5) Co(3)–N(1) 2.011(5) Co(5)–N(3) 1.911(5) Co(1)–O(19) 1.925(4) Co(3)–O(1) 2.013(4) Co(5)–O(20) 1.925(4) Co(1)–O(17) 1.946(4) Co(3)–O(19) 2.212(4) Co(5)–O(16) 1.927(4) Co(2)–O(15) 2.049(4) Co(4)–O(10) 2.050(4) Co(5)–O(18) 1.941(4) Co(2)–O(14) 2.053(4) Co(4)–O(5) 2.058(4) Co(6)–O(11) 1.921(5) Co(2)–O(18) 2.068(4) Co(4)–O(18) 2.130(4) Co(6)–O(10) 1.995(4) Co(2)–O(17) 2.074(4) Co(4)–O(17) 2.138(4) Co(6)–N(2) 2.011(5) Co(2)–O(8) 2.132(4) Co(4)–O(20) 2.196(4) Co(6)–O(9) 2.025(4) Co(6)–O(20) 2.198(4) Angle (°) Angle (°) Angle (°) O(2)–Co(1)–O(13) 89.07(2) O(15)–Co(2)–O(17) 171.08(2) O(4)–Co(3)–O(19) 93.26(2) O(2)–Co(1)–O(1) 174.59(2) O(14)–Co(2)–O(17) 89.83(2) O(5)–Co(3)–O(19) 81.65(2) O(13)–Co(1)–O(1) 92.04(2) O(18)–Co(2)–O(17) 82.06(2) O(10)–Co(4)–O(5) 100.73(2) O(2)–Co(1)–N(4) 93.94(2) O(15)–Co(2)–O(8) 84.43(2) O(10)–Co(4)–O(18) 91.02(2) O(13)–Co(1)–N(4) 87.72(2) O(14)–Co(2)–O(8) 108.12(2) O(5)–Co(4)–O(18) 167.25(2) O(1)–Co(1)–N(4) 91.40(2) O(4)–Co(3)–O(5) 135.69(2) O(10)–Co(4)–O(17) 168.70(2) O(2)–Co(1)–O(19) 92.43(2) O(4)–Co(3)–N(1) 92.2(2) O(5)–Co(4)–O(17) 89.58(2) O(13)–Co(1)–O(19) 176.07(2) O(5)–Co(3)–N(1) 91.46(2) O(18)–Co(4)–O(17) 79.16(2) O(15)–Co(2)–O(14) 97.96(2) O(4)–Co(3)–O(1) 115.95(2) O(10)–Co(4)–O(20) 80.62(2) O(15)–Co(2)–O(18) 90.58(2) O(5)–Co(3)–O(1) 105.60(2) O(5)–Co(4)–O(20) 104.88(2) O(14)–Co(2)–O(18) 170.12(2) N(1)–Co(3)–O(1) 104.95(2) O(18)–Co(4)–O(20) 71.95(2) 3. RESULTS AND DISCUSSION
3.1 Synthesis and IR spectrum of 1
Complex 1 is prepared by mixing H2L and Co(OAc)2·4H2O in 1:1 ratio in MeOH under solvothermal conditions. This solvothermal reaction mode allows to crystallize complex 1 directly from the reaction solution.
Several bands appear in the 1605~1445 cm–1 range (Fig. S1). Contributions from the carboxylic νas(CO2) and νs(CO2) vibrations would be expected in this region. The vibration of the C=N bonds is at 1445 cm–1. Several bands appear in the 1299~1121 cm–1 range, whilst the contributions from the vibrations of aromatic rings would be expected in this region. The overlap of the signals of aromatic rings with the vibrations of -CH3 and -CH2 groups makes assignments difficult. Several peaks in the 979~766 cm–1 range are found. They can be ascribed to the vibrations of C–H bonds.
3.2 Structural description of 1
X-ray single-crystal analysis reveals that complex 1 crystallizes in the triclinic space group P
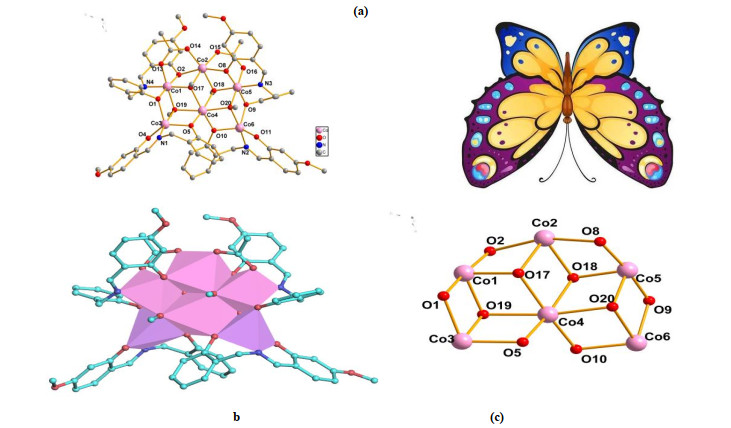
¯1 . The structure consists of six cobalt atoms, four L2– ligands, two acetate ions, two MeO– ions and two solvent molecules (Fig. 1). Bond valence calculation[27, 28] indicated that two cobalt atoms (Co(1), Co(5) are in 3+ valence states while the other metal centers (Co(2), Co(3), Co(4) and Co(6)) are in 2+ oxidation states (Table 2). The presence of two CoⅢ ions is possibly due to the aerial oxidation of CoⅡ to CoⅢ. This phenomenon was observed in many polynuclear CoⅡ/Ⅲ clusters[29, 30]. The six cobalt atoms are located at six corners of a defective tetracubane. Two cubanes share one face and each misses one vertex (Fig. 1), and the Co(4) atom is shared by four cubanes. Thus, complex 1 looks like a butterfly (Fig. 1). In this hexanuclear compound, Co(1), Co(2), Co(4) and Co(5) atoms are hexa-coordinated, while Co(3) and Co(6) atoms are penta-coordinated. The six cobalt atoms are held together by six phenolate oxygen atoms from four L2– ligands, four oxygen atoms from two chelating acetates and four μ3-O atoms from four MeO– groups. The coordination environments of Co(3) and Co(6) atoms are identical (NO4), and they are coordinated by two oxygen atoms and one nitrogen atom from one L2– ligand, one oxygen atom from a MeO- unit and one phenolate oxygen atom from another L2– ligand. The coordination environments of Co(1) and Co(5) atoms are the same (NO(5)). The six coordination atoms are from one L2– ligand (NO(2)), two MeO– units and one acetate. The coordination spheres of Co(2) and Co(4) atoms are distinct. Co(4) atom is coordinated by four oxygen atoms of four MeO– units and two oxygen atoms from two L2– ligands. Co(2) atom is coordinated by two oxygen atoms of two acetates, two oxygen atoms of two MeO– ions and two oxygen atoms from two L2– ligands. The L2– ligand displays μ2: ɳ2, ɳ1, ɳ1, ɳ0 and μ3: ɳ0, ɳ2, ɳ1, ɳ2 chelating modes.Figure 1
Table 2
Atom Valence Atom Valence Co(1) 3.57 Co(4) 1.88 Co(2) 2.06 Co(5) 3.29 Co(3) 2.24 Co(6) 2.28 Complex 1 is a member of a big family of hexanuclear cobalt clusters. These complexes display various structures including cage[31], hexameric ring[2, 21], and giant wheel[22]. The examples of hexanuclear CoⅡ/Ⅲ clusters which exhibit defect tetracubane core are very rare[29].
There are many Co7 clusters that show disc-like configuration, in which the seven cobalt centers are almost coplanar and occupy the vertexes of the six defect cubanes[32-41]. Compared the structure of complex 1 with those of Co7 clusters, complex 1 can be regarded as obtained by removing one of seven vertexes of the six cubanes.
3.3 Magnetic properties of 1
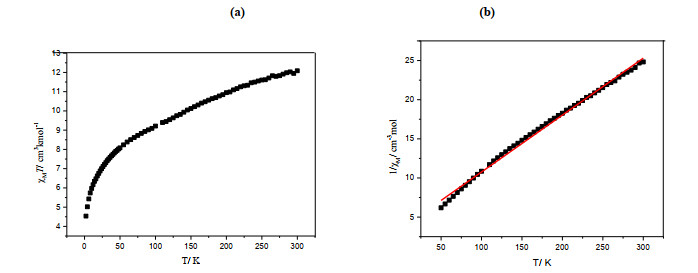
Direct current (dc) magnetic susceptibilities for complex 1 were determined at an applied magnetic field of 1000 Oe in the temperature range of 2~300 K. The χMT value of 1 at 300 K is 12.08 cm3·mol–1·K (Fig. 2), which is much larger than the spin-only value of 7.50 cm3·mol–1·K expected for four S = 3/2 uncoupled spins, probably due to the orbital contributions of the metal ions[42, 43]. As the temperature is lowered, the χMT value decreases gradually to a minimum value of 4.53 cm3·mol–1·K at 2 K. This behavior is indicative of the presence of antiferromagnetic exchange interactions between the metal ions. The relationship between 1/χM and temperature of 2~300 K obeys the Curie-Weiss Law of 1/χM = (T – θ)/C. The Curie constant C = 14.28 cm3·mol–1·K and Weiss constant θ = –49.84 K were obtained. The negative θ value confirms the antiferromagnetic exchange interactions.
Figure 2
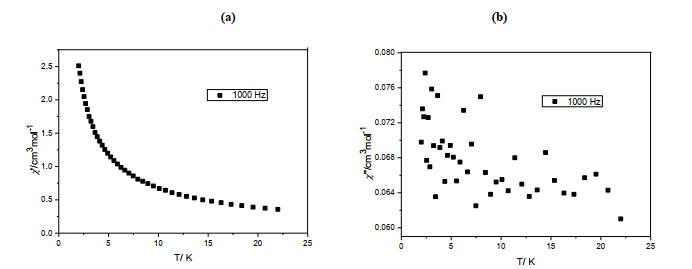
In order to study the magnetic dynamic behavior of 1, the ac magnetic susceptibilities for complex 1 at 1000 Hz under a zero dc field were determined (Fig. 3). The χ ''susceptibilities do not increase with the decrease of temperature or no peaks were observed, which indicate that complex 1 is not a single-molecule magnet.
Figure 3
4. CONCLUSION
A mixed-valence hexanuclear cobalt complex [Co2ⅢCo4Ⅱ(L)4(CH3COO)2(MeO)4]·2MeOH (1) supported by a Schiff base ligand H2L was synthesized. Complex 1 exhibits defect tetracubane-type architecture. Four cobalt atoms are hexa-coordinated and two cobalt atoms are penta-coordinated. The dc magnetic property measurements reveal the existence of antiferromagnetic interactions.
-

-
-
[1]
Peipei CUI , Xin LI , Yilin CHEN , Zhilin CHENG , Feiyan GAO , Xu GUO , Wenning YAN , Yuchen DENG . Transition metal coordination polymers with flexible dicarboxylate ligand: Synthesis, characterization, and photoluminescence property. Chinese Journal of Inorganic Chemistry, 2024, 40(11): 2221-2231. doi: 10.11862/CJIC.20240234
-
[2]
Zhenyang Lin . A classification scheme for inorganic cluster compounds based on their electronic structures and bonding characteristics. Chinese Journal of Structural Chemistry, 2024, 43(5): 100254-100254. doi: 10.1016/j.cjsc.2024.100254
-
[3]
Yatian Deng , Dao Wang , Jinglan Cheng , Yunkun Zhao , Zongbao Li , Chunyan Zang , Jian Li , Lichao Jia . A new popular transition metal-based catalyst: SmMn2O5 mullite-type oxide. Chinese Chemical Letters, 2024, 35(8): 109141-. doi: 10.1016/j.cclet.2023.109141
-
[4]
Caihong Mao , Yanfeng He , Xiaohan Wang , Yan Cai , Xiaobo Hu . Synthesis and molecular recognition characteristics of a tetrapodal benzene cage. Chinese Chemical Letters, 2024, 35(8): 109362-. doi: 10.1016/j.cclet.2023.109362
-
[5]
Mei Peng , Wei-Min He . Photochemical synthesis and group transfer reactions of azoxy compounds. Chinese Chemical Letters, 2024, 35(8): 109899-. doi: 10.1016/j.cclet.2024.109899
-
[6]
Shuwen SUN , Gaofeng WANG . Design and synthesis of a Zn(Ⅱ)-based coordination polymer as a fluorescent probe for trace monitoring 2, 4, 6-trinitrophenol. Chinese Journal of Inorganic Chemistry, 2025, 41(4): 753-760. doi: 10.11862/CJIC.20240399
-
[7]
Ze-Yuan Ma , Mei Xiao , Cheng-Kun Li , Adedamola Shoberu , Jian-Ping Zou . S-(1,3-Dioxoisoindolin-2-yl)O,O-diethyl phosphorothioate (SDDP): A practical electrophilic reagent for the phosphorothiolation of electron-rich compounds. Chinese Chemical Letters, 2024, 35(5): 109076-. doi: 10.1016/j.cclet.2023.109076
-
[8]
Shuai Tang , Zian Wang , Mengyi Zhu , Xinyun Zhao , Xiaoyun Hu , Hua Zhang . Synthesis of organoboron compounds via heterogeneous C–H and C–X borylation. Chinese Chemical Letters, 2025, 36(5): 110503-. doi: 10.1016/j.cclet.2024.110503
-
[9]
Gangsheng Li , Xiang Yuan , Fu Liu , Zhihua Liu , Xujie Wang , Yuanyuan Liu , Yanmin Chen , Tingting Wang , Yanan Yang , Peicheng Zhang . Three-step synthesis of flavanostilbenes with a 2-cyclohepten-1-one core by Cu-mediated [5 + 2] cycloaddition/decarboxylation cascade. Chinese Chemical Letters, 2025, 36(2): 109880-. doi: 10.1016/j.cclet.2024.109880
-
[10]
Shengyong Liu , Hui Li , Wei Zhang , Yan Zhang , Yan Dong , Wei Tian . Multiple host-guest and metal coordination interactions induce supramolecular assembly and structural transition. Chinese Chemical Letters, 2025, 36(6): 110465-. doi: 10.1016/j.cclet.2024.110465
-
[11]
Bairu Meng , Zongji Zhuo , Han Yu , Sining Tao , Zixuan Chen , Erik De Clercq , Christophe Pannecouque , Dongwei Kang , Peng Zhan , Xinyong Liu . Design, synthesis, and biological evaluation of benzo[4,5]thieno[2,3-d]pyrimidine derivatives as novel HIV-1 NNRTIs. Chinese Chemical Letters, 2024, 35(6): 108827-. doi: 10.1016/j.cclet.2023.108827
-
[12]
Qiang Feng , Jindong Hao , Ya Hu , Rong Fu , Wei Wei , Dong Yi . Photocatalytic multi-component synthesis of ester-containing quinoxalin-2(1H)-ones using water as the hydrogen donor. Chinese Chemical Letters, 2025, 36(6): 110582-. doi: 10.1016/j.cclet.2024.110582
-
[13]
Liping GUO . Synthesis and crystal structure characterization of yttrium imido complex: The reactivity of 2-substituted-1-amino-o-carborane with yttrium dialkyl complex. Chinese Journal of Inorganic Chemistry, 2025, 41(7): 1409-1415. doi: 10.11862/CJIC.20250065
-
[14]
Xiujuan Wang , Yijie Wang , Luyun Cui , Wenqiang Gao , Xiao Li , Hong Liu , Weijia Zhou , Jingang Wang . Coordination-based synthesis of Fe single-atom anchored nitrogen-doped carbon nanofibrous membrane for CO2 electroreduction with nearly 100% CO selectivity. Chinese Chemical Letters, 2024, 35(12): 110031-. doi: 10.1016/j.cclet.2024.110031
-
[15]
Shunyu Wang , Yanan Zhu , Yang Zhao , Wanli Nie , Hong Meng . Steric effects and electronic manipulation of multiple donors on S0/S1 transition of Dn-A emitters. Chinese Chemical Letters, 2025, 36(4): 110555-. doi: 10.1016/j.cclet.2024.110555
-
[16]
Genxiang Wang , Linfeng Fan , Peng Wang , Junfeng Wang , Fen Qiao , Zhenhai Wen . Efficient synthesis of nano high-entropy compounds for advanced oxygen evolution reaction. Chinese Chemical Letters, 2025, 36(4): 110498-. doi: 10.1016/j.cclet.2024.110498
-
[17]
Gaofeng WANG , Shuwen SUN , Yanfei ZHAO , Lixin MENG , Bohui WEI . Structural diversity and luminescence properties of three zinc coordination polymers based on bis(4-(1H-imidazol-1-yl)phenyl)methanone. Chinese Journal of Inorganic Chemistry, 2024, 40(5): 849-856. doi: 10.11862/CJIC.20230479
-
[18]
Hongren RONG , Gexiang GAO , Zhiwei LIU , Ke ZHOU , Lixin SU , Hao HUANG , Wenlong LIU , Qi LIU . High-performance supercapacitor based on 1D cobalt-based coordination polymer. Chinese Journal of Inorganic Chemistry, 2025, 41(6): 1183-1195. doi: 10.11862/CJIC.20250034
-
[19]
Yihong Li , Zhong Qiu , Lei Huang , Shenghui Shen , Ping Liu , Haomiao Zhang , Feng Cao , Xinping He , Jun Zhang , Yang Xia , Xinqi Liang , Chen Wang , Wangjun Wan , Yongqi Zhang , Minghua Chen , Wenkui Zhang , Hui Huang , Yongping Gan , Xinhui Xia . Plasma enhanced reduction method for synthesis of reduced graphene oxide fiber/Si anode with improved performance. Chinese Chemical Letters, 2024, 35(11): 109510-. doi: 10.1016/j.cclet.2024.109510
-
[20]
Ting Pan , Dinghu Zhang , Guomei You , Xiaoxia Wu , Chenguang Zhang , Xinyu Miao , Wenzhi Ren , Yiwei He , Lulu He , Yuanchuan Gong , Jie Lin , Aiguo Wu , Guoliang Shao . PD-L1 targeted iron oxide SERS bioprobe for accurately detecting circulating tumor cells and delineating tumor boundary. Chinese Chemical Letters, 2025, 36(1): 109857-. doi: 10.1016/j.cclet.2024.109857
-
[1]
Metrics
- PDF Downloads(7)
- Abstract views(873)
- HTML views(11)

 Login In
Login In




 DownLoad:
DownLoad:
 DownLoad:
DownLoad: