Citation:
Peng GAO, Qun LI, Shi Yu WANG, Pang ZHANG. A NOVEL SCISSION OF ALKYL-CARBONYL C-C BOND BY ALKYL p-HYDROXYPHENYL KETONES[J]. Chinese Chemical Letters,
;1992, 3(7): 489-492.

-
Methyl, ethyl, n-propyl, and benzyl p-hydroxyphenyl ketones and 6-hydroxy-1-tetralone are shown under the condition of ethylene ketal formation to undergo alkyl-carbonyl C-C bondscission, but not with p-hydroxybenzophenone, p-hydroxyisobutyrophenone, and 5-hydroxy-1-indanone. It is suggested that the scissiou is preceded by an aldol condensation.
-
1. Introduction
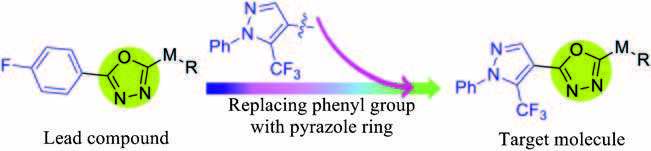
Heterocyclic substructures have been extensively studied for their powerful applications in construction of bioactive compounds [1-4]. Among them, pyrazole ring as an important functional group has already been used in the development of pharmaceuticals and agrochemicals due to its derivatives bearing multitudinous bioactivities, including anti-inflammatory, antitumor, herbicidal, insecticidal, antifungal, and antibacterial activities [5-13]. Furthermore, some pyrazole compounds have already been commercialized as fungicides, like sedaxane (Syngenta, 2005), isopyrazam (Syngenta, 2006), bixafen (Bayer, 2005), and fluxapyroxad (BASF, 2008) [14-17]. As another crucial scaffold, 1, 3, 4-oxadiazole, has exerted promising applications in creating new agrochemicals on account of the diverse bioactivities of its derivatives [18-21]. In our previous work, we had found a series of new 1, 3, 4-oxadiazole sulfone compounds (structure depicted in Fig. 1, lead compound) with high antibacterial/fungicidal bioactivities [22-24]. In order to find new structures with antibacterial/antifungal bioactivities, the two functional moieties of pyrazole and 1, 3, 4-oxadiazole were combined into one molecule by replacing the phenyl group to pyrazole moiety at the 5-position of the lead compound, as shown in Fig. 1. All the title compounds were bioassayed against pathogenic bacteria Xanthomonas oryzae pv. oryzae (Xoo) and five phytopathogenic fungi.
图 1
2. Experimental
All the chemicals were purchased from Aladdin and used as received. The organic solvents were distilled before used. NMR spectra were obtained by using a JEOL-ECX-500 apparatus. Chemical shifts were reported in parts per million (ppm) down field from TMS with the solvent resonance as the internal standard. Coupling constants (J) were reported in Hz and referred to apparent peak multiplications. MS data were recorded on an Agilent ESI-MSD Trap (VL) mass instrument.
2.1 General synthetic procedures for the target compounds (6a-6o) and (7a-7i)
A solution of carbon disulfide (0.015 mol) in ethanol (10 mL) was added dropwise to the mixture of compound 4 (0.01 mol) and potassium hydroxide (0.012 mol) in ethanol (40 mL) at room temperature. Then, the reaction mixture was heated under reflux with stirring for 8 h. After the reaction was completed, ethanol was evaporated to give unpurified intermediate 5. An appropriate halohydrocarbon (0.01 mol) was added to the solution of unpurified intermediate 5 in water (20 mL) and the mixture was stirred for 1 h at room temperature. The solid was filtered, purified by column chromatography using a mixture of petroleum ether and ethyl acetate (10:1) as the eluent, and then the pure target compounds (6a-6o) were obtained.
The compound (6a-6i) (5 mmol) and acetic acid (15 mL) were added to a 50 mL three-neck round-bottom flask equipped with a magnetic stirrer. The resulting solution was stirred for 10 min when a clear solution was obtained, and then 7% KMnO4 solution (5 mmol) was added dropwise at room temperature and the progress of the reaction was monitored by thin layer chromatography (TLC) using petroleum ether:ethyl acetate (3:1). After the reaction was completed, 10% NaHSO3 solution was added to deoxidize the unreacted KMnO4. The resulted solid was filtered, washed with water, from which the pure compounds (7a-7i) can be obtained by column chromatography using a mixture of petroleum ether and ethyl acetate (15:1) as the eluent.
2.2 in vitro antibacterial bioassay (turbidimeter test)
In our study, all the synthesized target compounds were evaluated for their antibacterial activities against Xoo by the turbidimeter test in vitro. Dimethylsulfoxide in sterile distilled water served as a blank control, Bismerthiazol and Thiodiazole Copper served as the positive controls. Approximately 40 μL of solvent NB (1.5 g beef extract, 2.5 g peptone, 0.5 g yeast powder, 5.0 g glucose, and 500 mL distilled water; pH 7.0-7.2) containing Xoo, incubated on the phase of logarithmic growth, was added to 5 mL of solvent NB containing the test compounds and positive control. The inoculated test tubes were incubated at 28±1 ℃ and continuously shaken at 180 rpm for 24-48 h until the bacteria were incubated on the logarithmic growth phase. The growth of the cultures was monitored on a microplate reader by measuring the optical density at 595 nm (OD595) given by turbidity corrected values=ODbacterial wilt-ODno bacterial wilt, and the inhibition rate I was calculated by I=(C -T)/C × 100%. C is the corrected turbidity values of bacterial growth on untreated NB (blank control), and T is the corrected turbidity values of bacterial growth on treated NB. The experiment was repeated three times.
3. Results and discussion
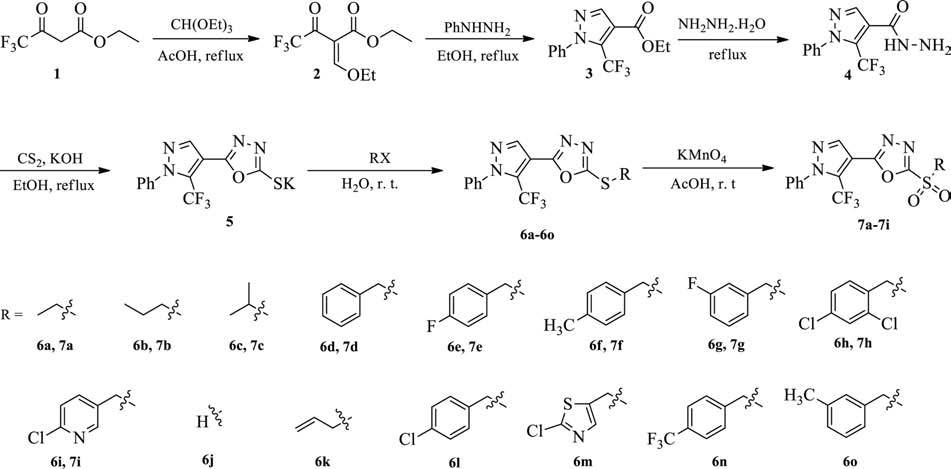
The synthesis and structures of (6a-6o), and (7a-7i) are shown in Scheme 1. Briefly, ethyltrifluoroacetoacetate (1) was treated with triethoxymethane to give intermediate (E)-2-trifluoroacetyl-3-ethoxy-2-propenoate (2), followed by the cyclocondensation reaction to provide an important intermediate ethyl 1-phenyl-5-(trifluoromethyl)-1H-pyrazole-4-carboxylate (3) containing pyrazole group in 82% yield. Next, the hydrazide 4 was obtained through refluxing 3 in hydrazine hydrate with the yield of 94%. A subsequent reaction with carbon disulfide in the presence of potassium hydroxide leaded to the formation of the crucial intermediate 5 containing 1, 3, 4-oxadiazole. Finally, the corresponding target thioethers (6a-6o) were achieved via thioetherification with halogenated agents in good yields ranging from 76% to 85%, and subsequently converted into the corresponding sulfones (7a-7i) by oxidizing the related thioether at room temperature. All the structures were confirmed by 1H NMR, 13C NMR, and MS (detailed information see Supplementary data).
Scheme 1
In our study, we first evaluated the antibacterial activity of all the title compounds via turbidmeter test [25-27] against pathogenic bacteria Xanthomonas oryzae pv. oryzae (Xoo), which was considered as one of devastative bacteria against rice in ricegrowing countries. Meanwhile, the commercial agricultural antibacterial bismerthiazol (BT) and thiodiazole copper (TC) were employed for the comparison of bioactivity in vitro. Preliminary bioassays revealed that most of the target compounds exerted appreciable inhibition bioactivity against Xoo in the dosage of 200 or 100 μg/mL (Table 1). Among them, compounds 6c, 6e, 6f, 6j, 7a, 7b, and 7c gives the inhibition rate above 72.3% against Xoo in the dosage of 200 μg/mL, which were better than that of BT (72.1%) and TC (64.2%); while compounds 6c, 6f, 7a, 7b, and 7c offersbetter inhibition rate above 66.2% against Xoo than that of BT (53.7%) and TC (43.1%) in the dosage of 100 μg/mL. The half-maximal effective concentration (EC50) values of 6c, 7a, 7b, and 7c were detected as 47.5, 31.6, 65.7, and 16.6 μg/mL, respectively, which were obviously better than that of commercial bactericides (92.6 or 121.8 μg/mL). Based on the above results, among all the thioether compounds (6a-6o), the isopropyl group compound (6c) exhibited the best bioactivity against Xoo than the other groups, while for benzyl thioether compounds, 4-methylbenzyl thioether (6f) gives superior activity than the other substituted benzyl in the dosage of 200 μg/mL or 100 μg/mL. For sulfone compounds, the antibacterial activity of alkyl sulfone compounds (such as 7a-7c) was dramatically better than the benzyl derivatives.
表 1
 表 1 Inhibition effect of sulfides/sulfones against Xoo.Table 1. Inhibition effect of sulfides/sulfones against Xoo.
表 1 Inhibition effect of sulfides/sulfones against Xoo.Table 1. Inhibition effect of sulfides/sulfones against Xoo.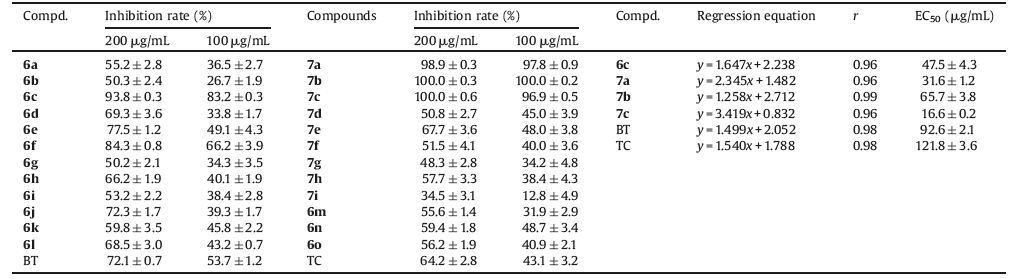
The antifungal activity of (6a-6o) and (7a-7i) was examined via the poisonplate technique [28] against fivephytopathogenic fungi, Gibberella zeae (G. z.), Fusarium oxysporum (F. o.), Cytospora mandshurica (C. m.), Sclertinia sclerotiorum (S. s.), and Rhizoctonia solani (R. s.) at the concentrate of 100 μg/mL, Meanwhile, the commercial agricultural antifungal Hymexazol (HM) and Carbendazim (CB) were employed for the comparison of bioactivity. As shown in Table 2, compounds 7a and 7c were observed having comprehensive antifungal activity with the inhibition rate ranging from 53.8% to 75.5% against the five kinds of fungi, which were comparable to the commercial fungicide HM. It is worth pointing out that compound 6j exerted good antifungal activity with the inhibition rate of 86.4% against S. sclerotiorum. In comparison of 6a and 7a, 6b and 7b, 6c and 7c, 6d and 7d, 6f and 7f, the antifungal activity was improved after oxidizing the thioether into the sulfone, further suggested sulfonyl group as a crucial functional group may improve the bioactivity of the target compound. It can be seen that compound 7a showed the strongest antifungi activity against the five phytopathogenic fungi.
表 2
 表 2 Inhibition effect of sulfides/sulfones at 100 μg/mL against five phytopathogenic fungi.Table 2. Inhibition effect of sulfides/sulfones at 100 μg/mL against five phytopathogenic fungi.
表 2 Inhibition effect of sulfides/sulfones at 100 μg/mL against five phytopathogenic fungi.Table 2. Inhibition effect of sulfides/sulfones at 100 μg/mL against five phytopathogenic fungi.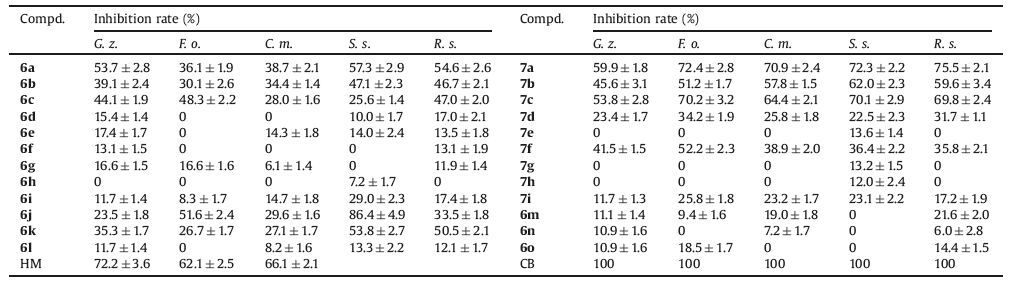
4. Conclusion
In summary, a series of 2-(thioether/sulfone)-5-pyrazolyl-1, 3, 4-oxadiazole derivatives containing both pyrazole moiety and 1, 3, 4-oxadiazole moiety were designed and synthesized, and which antibacterial activity and antifungal activity were evaluated via turbidmeter test or the poison plate technique in vitro. Compounds 6c, 7a, 7b and 7c showed good inhibition effects against Xoo with the EC50 values ranging from 16.6 μg/mL to 65.7 μg/mL, which were better than those of commercial agricultural antibacterial bismerthiazol (92.6 μg/mL) and thiediazole copper (121.8 μg/mL). Meanwhile, compounds 7a, 7b, and 7c exerted good antifungal activities against fiveplant fungi, which were comparable tothatof HM. The results demonstrated that this kind of compounds can be further studied and developed as promising antifungal and antibacterial agents.
Acknowledgments
We acknowledge the financial support of the Key Technologies R & D Program (No. 2014BAD23B01), National Natural Science Foundation of China (No. 21372052), the Research Project of Chinese Ministry of Education (Nos. 213033A, 20135201110005), and Scientific Research Foundation for the Introduced Talents of Guizhou University (2015-34).
Appendix A. Supplementary data
Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.cclet.2016.06.055
-

-
-
[1]
Qinghong Zhang , Qiao Zhao , Xiaodi Wu , Li Wang , Kairui Shen , Yuchen Hua , Cheng Gao , Yu Zhang , Mei Peng , Kai Zhao . Visible-light-induced ring-opening cross-coupling of cycloalcohols with vinylazaarenes and enones via β-C-C scission enabled by proton-coupled electron transfer. Chinese Chemical Letters, 2025, 36(2): 110167-. doi: 10.1016/j.cclet.2024.110167
-
[2]
Junmeng Luo , Qiongqiong Wan , Suming Chen . Chemistry-driven mass spectrometry for structural lipidomics at the C=C bond isomer level. Chinese Chemical Letters, 2025, 36(1): 109836-. doi: 10.1016/j.cclet.2024.109836
-
[3]
Qihang Wu , Hui Wen , Wenhai Lin , Tingting Sun , Zhigang Xie . Alkyl chain engineering of boron dipyrromethenes for efficient photodynamic antibacterial treatment. Chinese Chemical Letters, 2024, 35(12): 109692-. doi: 10.1016/j.cclet.2024.109692
-
[4]
Qiongqiong Wan , Yanan Xiao , Guifang Feng , Xin Dong , Wenjing Nie , Ming Gao , Qingtao Meng , Suming Chen . Visible-light-activated aziridination reaction enables simultaneous resolving of C=C bond location and the sn-position isomers in lipids. Chinese Chemical Letters, 2024, 35(4): 108775-. doi: 10.1016/j.cclet.2023.108775
-
[5]
Guoju Guo , Xufeng Li , Jie Ma , Yongjia Shi , Jian Lv , Daoshan Yang . Photocatalyst/metal-free sequential C–N/C–S bond formation: Synthesis of S-arylisothioureas via photoinduced EDA complex activation. Chinese Chemical Letters, 2024, 35(11): 110024-. doi: 10.1016/j.cclet.2024.110024
-
[6]
Shan-Shan Li , Juan Luo , Shu-Nuo Liang , Dan-Na Chen , Li-Ning Chen , Cheng-Xue Pan , Peng-Ju Xia . Efficient and regioselective C=S bond difunctionalization through a three-component radical relay strategy. Chinese Chemical Letters, 2025, 36(6): 110424-. doi: 10.1016/j.cclet.2024.110424
-
[7]
Yunkang Tong , Haiqiao Huang , Haolan Li , Mingle Li , Wen Sun , Jianjun Du , Jiangli Fan , Lei Wang , Bin Liu , Xiaoqiang Chen , Xiaojun Peng . Cooperative bond scission by HRP/H2O2 for targeted prodrug activation. Chinese Chemical Letters, 2024, 35(12): 109663-. doi: 10.1016/j.cclet.2024.109663
-
[8]
Yi Luo , Lin Dong . Multicomponent remote C(sp2)-H bond addition by Ru catalysis: An efficient access to the alkylarylation of 2H-imidazoles. Chinese Chemical Letters, 2024, 35(10): 109648-. doi: 10.1016/j.cclet.2024.109648
-
[9]
Yang Li , Yanan Dong , Zhihong Wei , Changzeng Yan , Zhen Li , Lin He , Yuehui Li . Fluoride-promoted Ni-catalyzed cyanation of C–O bond using CO2 and NH3. Chinese Chemical Letters, 2025, 36(5): 110206-. doi: 10.1016/j.cclet.2024.110206
-
[10]
Xiangyang Ji , Yishuang Chen , Peng Zhang , Shaojia Song , Jian Liu , Weiyu Song . Boosting the first C–H bond activation of propane on rod-like V/CeO2 catalyst by photo-assisted thermal catalysis. Chinese Chemical Letters, 2025, 36(5): 110719-. doi: 10.1016/j.cclet.2024.110719
-
[11]
Li Li , Zhi-Xin Yan , Chuan-Kun Ran , Yi Liu , Shuo Zhang , Tian-Yu Gao , Long-Fei Dai , Li-Li Liao , Jian-Heng Ye , Da-Gang Yu . Electro-reductive carboxylation of CCl bonds in unactivated alkyl chlorides and polyvinyl chloride with CO2. Chinese Chemical Letters, 2024, 35(12): 110104-. doi: 10.1016/j.cclet.2024.110104
-
[12]
Yan-Bo Li , Yi Li , Liang Yin . Copper(Ⅰ)-catalyzed diastereodivergent construction of vicinal P-chiral and C-chiral centers facilitated by dual "soft-soft" interaction. Chinese Chemical Letters, 2024, 35(7): 109294-. doi: 10.1016/j.cclet.2023.109294
-
[13]
Yikun Wang , Qiaomei Chen , Shijie Liang , Dongdong Xia , Chaowei Zhao , Christopher R. McNeill , Weiwei Li . Near-infrared double-cable conjugated polymers based on alkyl linkers with tunable length for single-component organic solar cells. Chinese Chemical Letters, 2024, 35(4): 109164-. doi: 10.1016/j.cclet.2023.109164
-
[14]
Rong-Nan Yi , Wei-Min He . Photocatalytic Minisci-type multicomponent reaction for the synthesis of 1-(halo)alkyl-3-heteroaryl bicyclo[1.1.1]pentanes. Chinese Chemical Letters, 2024, 35(10): 110115-. doi: 10.1016/j.cclet.2024.110115
-
[15]
Jiangqi Ning , Junhan Huang , Yuhang Liu , Yanlei Chen , Qing Niu , Qingqing Lin , Yajun He , Zheyuan Liu , Yan Yu , Liuyi Li . Alkyl-linked TiO2@COF heterostructure facilitating photocatalytic CO2 reduction by targeted electron transport. Chinese Journal of Structural Chemistry, 2024, 43(12): 100453-100453. doi: 10.1016/j.cjsc.2024.100453
-
[16]
Peng Wang , Jianjun Wang , Ni Song , Xin Zhou , Ming Li . Radical dehydroxymethylative fluorination of aliphatic primary alcohols and diverse functionalization of α-fluoroimides via BF3·OEt2-catalyzed C‒F bond activation. Chinese Chemical Letters, 2025, 36(1): 109748-. doi: 10.1016/j.cclet.2024.109748
-
[17]
Ao Sun , Zipeng Li , Shuchun Li , Xiangbao Meng , Zhongtang Li , Zhongjun Li . Stereoselective synthesis of α-3-deoxy-D-manno-oct-2-ulosonic acid (α-Kdo) derivatives using a C3-p-tolylthio-substituted Kdo fluoride donor. Chinese Chemical Letters, 2025, 36(3): 109972-. doi: 10.1016/j.cclet.2024.109972
-
[18]
Bowen Xu , Jiahao Chen , Lulu Cui , Xinyue Li , Yuan Xue , Sheng Han . Terpolymers of alkyl methacrylate-trans anethole-1,2,3,6-tetrahydrophthalic anhydride copolymers: A low dosage and high-efficiency cold flow improver for diesel fuel. Chinese Chemical Letters, 2025, 36(5): 110196-. doi: 10.1016/j.cclet.2024.110196
-
[19]
Shuai Tang , Zian Wang , Mengyi Zhu , Xinyun Zhao , Xiaoyun Hu , Hua Zhang . Synthesis of organoboron compounds via heterogeneous C–H and C–X borylation. Chinese Chemical Letters, 2025, 36(5): 110503-. doi: 10.1016/j.cclet.2024.110503
-
[20]
Lei Wan , Yizhou Tong , Xi Lu , Yao Fu . Cobalt-catalyzed reductive alkynylation to construct C(sp)-C(sp3) and C(sp)-C(sp2) bonds. Chinese Chemical Letters, 2024, 35(7): 109283-. doi: 10.1016/j.cclet.2023.109283
-
[1]
Metrics
- PDF Downloads(2)
- Abstract views(737)
- HTML views(2)

 Login In
Login In




 下载:
下载:
 DownLoad:
DownLoad: