Citation:
Hui Yuan, Guang-Tong Xu, Hui-Feng Li, Li-Jun Lu. Study of oxidic and sulfided selective hydrodesulfurization catalysts for gasoline using Raman spectroscopy[J]. Chinese Chemical Letters,
;2013, 24(12): 1041-1044.

-
A series of CoMo/Al2O3 catalysts for selective hydrodesulfurization (HDS) of gasoline were studied with Raman spectroscopy, a powerfulmethod that creates specific signals for the states and the distributions of oxidic precursors and sulfided active phases. The higher the Mo and Co, the lower the tetrahedrally coordinated molybdate, and the higher the polymolybdate. But the amount of polymolybdate decreased when CoMoO4 appeared. Cobalt-promoted polymolybdate was the precursor, and its relative content correlated well with the HDS selectivity. For sulfided catalysts, adding the cobalt-promoter led to local distortion-disorder of the MoS2 structure and the formation of a CoMoS phase. This method can provide important information for designing new industrial selective-HDS catalysts.
-
Keywords:
- Raman spectroscopy,
- Co-Mo/Al2O3,
- HDS selectivity
-
Spintronics is a cutting-edge field of developing new electronic devices by manipulating the electron spin and magnetic moment [1]. Traditional spintronic research mainly focuses on transition metals and inorganic semiconductors, while organic molecules have the advantage of being extremely easy to realize efficient spin control by modifying the specific external conditions for desired electronic structures and magnetic characteristics. Corrole, as a ring-contracted porphyrin, is the aromatic analog of the central macrocycle of vitamin B12. Corrole has a squeezed inner cavity and three inner NHs in its free-base form, making it easier to stabilize high-valent metal ions and thus a promising candidate in spintronics.
When coordinated to metal ions such as Cu, Co, and Fe, the electron-rich corrole ligand could be partially oxidized to exhibit radical character, making it difficult to determine the exact oxidation states of central metals and ligands. The most controversial debate was the Cu(Ⅱ)/Cu(Ⅲ) dilemma on copper corrole [2]. The compound was initially thought to be a closed-shell Cu(Ⅲ) complex in 2000, but significant experimental and theoretical evidence over the next twenty years progressively revealed its open-shell singlet state ground state comprised of a Cu(Ⅱ) core and partially oxidized radical ligand (Fig. 1a).
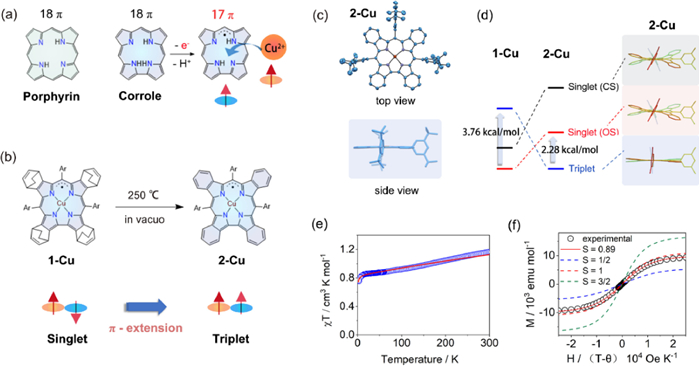
Figure 1
 Figure 1. (a) Structure of 18π porphyrin and corrole, and schematic of the 17π corrole radical formation during coordination. (b) Schematic representation of the singlet-to-triplet conversion from 1-Cu to 2-Cu. (c) Single-crystal X-ray diffraction structure of 2-Cu. (d) DFT calculated structures and relative energies of 1-Cu and 2-Cu in triplet, open-shell singlet and closed-shell singlet states. (e) Observed (circle) and simulated (line) χT–T curve of 2-Cu at H = 5000 Oe. (f) Observed (circle) and simulated (line) M-H curves of 2-Cu with different S values.
Figure 1. (a) Structure of 18π porphyrin and corrole, and schematic of the 17π corrole radical formation during coordination. (b) Schematic representation of the singlet-to-triplet conversion from 1-Cu to 2-Cu. (c) Single-crystal X-ray diffraction structure of 2-Cu. (d) DFT calculated structures and relative energies of 1-Cu and 2-Cu in triplet, open-shell singlet and closed-shell singlet states. (e) Observed (circle) and simulated (line) χT–T curve of 2-Cu at H = 5000 Oe. (f) Observed (circle) and simulated (line) M-H curves of 2-Cu with different S values.Shen Z. and Wu F. from Nanjing University have developed a series of metallocorroles with extended π-conjugation systems, which not only facilitated the formation and stabilization of radical ligands, but also allowed spin configurations of complexes to be easily controlled [3-5]. Recently, Shen, Wu, and co-workers reported that the unambiguous Cu(Ⅱ) corrole with fully oxidized [4n + 1]π radical ligand was obtained through the benzo-fusion at the β-position of corrole ligand [6]. The ground-state conversion of copper corrole radical from singlet to triplet was achieved via a retro-Diels-Alder reaction (Fig. 1b).
The authors first synthesized a bicyclo[2.2.2]octadiene (BCOD) fused corrole 1-Cu by employing the classic H2O-MeOH approach with starting materials 4, 7-dihydro-4, 7-ethano-2H-isoindole and 3, 5-di-tert-butyl-benzaldehyde. Heating solid 1-Cu at 250 ℃ in vacuo cut the C—C bond in the BCOD bridge, eliminated the ethylene, and quantitatively afforded the benzo-fused 2-Cu. The singlet ground state of 1-Cu was clearly confirmed by the peripheral BCOD protons signals that appeared in the region of 6.60~2.09 ppm, while the signals of benzo protons in 2-Cu were located in a range of −6.2~−25.6 ppm, demonstrating its enhanced paramagnetism.
The conformations of copper corroles were assumed to be "inherently saddle distorted" owing to the strong d-π interactions of antiferromagnetically coupled Cu(Ⅱ) corrole radicals. When compared to other copper corroles, 2-Cu stood out due to its highly planar macrocycle with a mean plane deviation value of only 0.024 Å (Fig. 1c). The planar structure could perfectly sustain the ferromagnetic coupling (S = 1) between Cu(Ⅱ) and corrole radical.
The theoretical analysis of 2-Cu was conducted by the authors for three different states, including a close-shell singlet Cu(Ⅲ) (CS), an open-shell singlet antiferromagnetically coupled Cu(Ⅱ) corrole radical (OS) and a triplet ferromagnetically coupled Cu(Ⅱ) corrole radical (T). A lower T state was discovered for 2-Cu than the CS and OS states with a calculated singlet-triplet energy gap of 2.28 kcal/mol, providing theoretical support for the triplet ground state (Fig. 1d). The strongest support for the triplet ground state came from temperature- and field-dependent superconducting quantum interference device (SQUID) magnetometry. The χT value of 2-Cu in 2 K was 0.77 cm3 K/mol, and it reached approximately 1 cm3 K/mol at 300 K. The singlet-triplet energy gap was estimated to be 1.66 kcal/mol by fitting the χT–T plot (Fig. 1e). The field-dependent magnetization plot of 2-Cu at 2 K was fitted to a Brillouin function with S = 0.89, which was close to the value (S = 1) corresponding to the triplet ground state (Fig. 1f). The magnetic hysteresis of 2-Cu was observed at 2 K. Moreover, 2-Cu exhibits remarkable stability in air despite its radical character. The calculated density plots of spin and SOMO both demonstrate that the density is concentrated mostly in the inner corrole ring, which is nicely protected by fused benzenes with low reactivity.
The research conducted by Shen's group introduces a new approach to the fine-tuning of interactions between metal center and corrole ligand and provides a promising strategy for the creation of stable corrole radical complexes with distinctive high-spin systems. The strategy will further trigger the development of novel functional materials based on corroles and their work will encourage an increasing amount of spintronics research for the use of innovative magnetic and electrical devices.
References
-
[1]
[1] S. Bruneta, D. Meya, G. Perot, et al., On the hydrodesulfurization of FCC gasoline: a review, Appl. Catal. A: Gen. 278 (2005) 143-172.
-
[2]
[2] M.F. Li, H.F. Li, F. Jiang, et al., The relation between morphology of (Co)MoS2 phases and selective hydrodesulfurization for CoMo catalyst, Catal. Today 149 (2010) 35- 39.
-
[3]
[3] M.F. Li, H.F. Li, F. Jiang, et al., Effect of surface characteristics of different alumina on metal-support interaction and hydrodesulfurization activity, Fuel 88 (2009) 1281-1285.
-
[4]
[4] E. Payen, J. Grimblot, S. Kasztelan, Study of oxidic and reduced alumina-supported molybdate and heptamolybdate species by in situ laser Raman spectroscopy, J. Phys. Chem. 91 (1987) 6642-6648.
-
[5]
[5] C. Li, Identifying the isolated transition metal ions/oxides in molecular sieves and on oxide supports by UV resonance Raman spectroscopy, J. Catal. 216 (2003) 203- 212.
-
[6]
[6] S. Gonzalez-Cortes, T. Xiao, P.M. Costa, et al., Urea-organic matrix method: an alternative approach to prepare Co-MoS2/γ-Al2O3 HDS catalyst, Appl. Catal. A: Gen. 270 (2004) 209-222.
-
[7]
[7] Qiherima, H. Yuan, H.F. Li, et al., Investigation on the active phase of CoMo catalyst for selective HDS by low temperature in situ FT-IR, Chin. Chem. Lett. 22 (2011) 366-369.
-
[8]
[8] Qiherima, H. Yuan, Y.H. Zhang, et al., In situ FTIR and XPS study on selective hydrodesulfurization catalyst of FCC gasoline, Spectrosc. Spectral Anal. 31 (2011) 1752-1757.
-
[9]
[9] M. Gerhard, In situ Raman spectroscopy - a valuable tool to understand operating catalysts, J. Mol. Catal. A: Chem. 158 (2000) 45-65.
-
[10]
[10] G. Xiong, C. Li, Z. Feng, et al., Surface coordination structure of molybdate with extremely low loading on g-alumina characterized by UV resonance Raman spectroscopy, J. Catal. 186 (1999) 234-237.
-
[11]
[11] Z.B. Wei, C.D. Wei, Q. Xin, Study of the reducing and sulfiding process of Mosupported catalyst by in situ LRS, Acta Physico-Chem. Sin. 10 (1994) 402-408.
-
[12]
[12] T. Xiao, A.P. York, H. Al-Megren, et al., Preparation and characterization of bimetallic cobalt and molybdenum carbides, J. Catal. 202 (2001) 100-109.
-
[13]
[13] L. Le-Bihan, P. Blanchard, M. Fournier, et al., Raman spectroscopic evidence for the existence of 6-molybdoaluminate entities on an Mo/Al2O3 oxidic precursor, J. Chem. Soc. Faraday Trans. 94 (1998) 937-940.
-
[14]
[14] E. Payen, S. Kasztelan, S. Houssenbay, et al., Genesis and characterization by laser Raman spectroscopy and high-resolution electron microscopy of supported MoS2 crystallites, J. Phys. Chem. 93 (1989) 6501-6506.
-
[1]
-
-
[1]
[1] S. Bruneta, D. Meya, G. Perot, et al., On the hydrodesulfurization of FCC gasoline: a review, Appl. Catal. A: Gen. 278 (2005) 143-172.
-
[2]
[2] M.F. Li, H.F. Li, F. Jiang, et al., The relation between morphology of (Co)MoS2 phases and selective hydrodesulfurization for CoMo catalyst, Catal. Today 149 (2010) 35- 39.
-
[3]
[3] M.F. Li, H.F. Li, F. Jiang, et al., Effect of surface characteristics of different alumina on metal-support interaction and hydrodesulfurization activity, Fuel 88 (2009) 1281-1285.
-
[4]
[4] E. Payen, J. Grimblot, S. Kasztelan, Study of oxidic and reduced alumina-supported molybdate and heptamolybdate species by in situ laser Raman spectroscopy, J. Phys. Chem. 91 (1987) 6642-6648.
-
[5]
[5] C. Li, Identifying the isolated transition metal ions/oxides in molecular sieves and on oxide supports by UV resonance Raman spectroscopy, J. Catal. 216 (2003) 203- 212.
-
[6]
[6] S. Gonzalez-Cortes, T. Xiao, P.M. Costa, et al., Urea-organic matrix method: an alternative approach to prepare Co-MoS2/γ-Al2O3 HDS catalyst, Appl. Catal. A: Gen. 270 (2004) 209-222.
-
[7]
[7] Qiherima, H. Yuan, H.F. Li, et al., Investigation on the active phase of CoMo catalyst for selective HDS by low temperature in situ FT-IR, Chin. Chem. Lett. 22 (2011) 366-369.
-
[8]
[8] Qiherima, H. Yuan, Y.H. Zhang, et al., In situ FTIR and XPS study on selective hydrodesulfurization catalyst of FCC gasoline, Spectrosc. Spectral Anal. 31 (2011) 1752-1757.
-
[9]
[9] M. Gerhard, In situ Raman spectroscopy - a valuable tool to understand operating catalysts, J. Mol. Catal. A: Chem. 158 (2000) 45-65.
-
[10]
[10] G. Xiong, C. Li, Z. Feng, et al., Surface coordination structure of molybdate with extremely low loading on g-alumina characterized by UV resonance Raman spectroscopy, J. Catal. 186 (1999) 234-237.
-
[11]
[11] Z.B. Wei, C.D. Wei, Q. Xin, Study of the reducing and sulfiding process of Mosupported catalyst by in situ LRS, Acta Physico-Chem. Sin. 10 (1994) 402-408.
-
[12]
[12] T. Xiao, A.P. York, H. Al-Megren, et al., Preparation and characterization of bimetallic cobalt and molybdenum carbides, J. Catal. 202 (2001) 100-109.
-
[13]
[13] L. Le-Bihan, P. Blanchard, M. Fournier, et al., Raman spectroscopic evidence for the existence of 6-molybdoaluminate entities on an Mo/Al2O3 oxidic precursor, J. Chem. Soc. Faraday Trans. 94 (1998) 937-940.
-
[14]
[14] E. Payen, S. Kasztelan, S. Houssenbay, et al., Genesis and characterization by laser Raman spectroscopy and high-resolution electron microscopy of supported MoS2 crystallites, J. Phys. Chem. 93 (1989) 6501-6506.
-
[1]
-

-
-
[1]
Tianlong Zhang , Rongling Zhang , Hongsheng Tang , Yan Li , Hua Li . Online Monitoring and Mechanistic Analysis of 3,5-diamino-1,2,4-triazole (DAT) Synthesis via Raman Spectroscopy: A Recommendation for a Comprehensive Instrumental Analysis Experiment. University Chemistry, 2024, 39(6): 303-311. doi: 10.3866/PKU.DXHX202312006
-
[2]
Haixia Wu , Kailu Guo . Sulfur reduction reaction mechanism elucidated with in situ Raman spectroscopy. Chinese Chemical Letters, 2025, 36(6): 110654-. doi: 10.1016/j.cclet.2024.110654
-
[3]
Kaifu Zhang , Shan Gao , Bin Yang . Application of Theoretical Calculation with Fun Practice in Raman Spectroscopy Experimental Teaching. University Chemistry, 2025, 40(3): 62-67. doi: 10.12461/PKU.DXHX202404045
-
[4]
Jiajie Li , Xiaocong Ma , Jufang Zheng , Qiang Wan , Xiaoshun Zhou , Yahao Wang . Recent Advances in In-Situ Raman Spectroscopy for Investigating Electrocatalytic Organic Reaction Mechanisms. University Chemistry, 2025, 40(4): 261-276. doi: 10.12461/PKU.DXHX202406117
-
[5]
Chengde Wang , Liping Huang , Shanshan Wang , Lihao Wu , Yi Wang , Jun Dong . A distinction of gliomas at cellular and tissue level by surface-enhanced Raman scattering spectroscopy. Chinese Chemical Letters, 2024, 35(5): 109383-. doi: 10.1016/j.cclet.2023.109383
-
[6]
Liuyun Chen , Wenju Wang , Tairong Lu , Xuan Luo , Xinling Xie , Kelin Huang , Shanli Qin , Tongming Su , Zuzeng Qin , Hongbing Ji . Soft template-induced deep pore structure of Cu/Al2O3 for promoting plasma-catalyzed CO2 hydrogenation to DME. Acta Physico-Chimica Sinica, 2025, 41(6): 100054-0. doi: 10.1016/j.actphy.2025.100054
-
[7]
Sanmei Wang , Dengxin Yan , Wenhua Zhang , Liangbing Wang . Graphene-supported isolated platinum atoms and platinum dimers for CO2 hydrogenation: Catalytic activity and selectivity variations. Chinese Chemical Letters, 2025, 36(4): 110611-. doi: 10.1016/j.cclet.2024.110611
-
[8]
Hui Li , Yanxing Qi , Jia Chen , Juanjuan Wang , Min Yang , Hongdeng Qiu . Synthesis of amine-pillar[5]arene porous adsorbent for adsorption of CO2 and selectivity over N2 and CH4. Chinese Chemical Letters, 2024, 35(11): 109659-. doi: 10.1016/j.cclet.2024.109659
-
[9]
Shiqi Xu , Zi Ye , Shuang Shang , Fengge Wang , Huan Zhang , Lianguo Chen , Hao Lin , Chen Chen , Fang Hua , Chong-Jing Zhang . Pairs of thiol-substituted 1,2,4-triazole-based isomeric covalent inhibitors with tunable reactivity and selectivity. Chinese Chemical Letters, 2024, 35(7): 109034-. doi: 10.1016/j.cclet.2023.109034
-
[10]
Xiujuan Wang , Yijie Wang , Luyun Cui , Wenqiang Gao , Xiao Li , Hong Liu , Weijia Zhou , Jingang Wang . Coordination-based synthesis of Fe single-atom anchored nitrogen-doped carbon nanofibrous membrane for CO2 electroreduction with nearly 100% CO selectivity. Chinese Chemical Letters, 2024, 35(12): 110031-. doi: 10.1016/j.cclet.2024.110031
-
[11]
Lijun Yan , Shiqi Chen , Penglu Wang , Xiangyu Liu , Lupeng Han , Tingting Yan , Yuejin Li , Dengsong Zhang . Hydrothermally stable metal oxide-zeolite composite catalysts for low-temperature NOx reduction with improved N2 selectivity. Chinese Chemical Letters, 2024, 35(6): 109132-. doi: 10.1016/j.cclet.2023.109132
-
[12]
Shanyuan Bi , Jin Zhang , Dengchao Peng , Danhong Cheng , Jianping Zhang , Lupeng Han , Dengsong Zhang . Improved N2 selectivity for low-temperature NOx reduction over etched ZSM-5 supported MnCe oxide catalysts. Chinese Chemical Letters, 2025, 36(5): 110295-. doi: 10.1016/j.cclet.2024.110295
-
[13]
Hongyi LI , Aimin WU , Liuyang ZHAO , Xinpeng LIU , Fengqin CHEN , Aikui LI , Hao HUANG . Effect of Y(PO3)3 double-coating modification on the electrochemical properties of Li[Ni0.8Co0.15Al0.05]O2. Chinese Journal of Inorganic Chemistry, 2024, 40(7): 1320-1328. doi: 10.11862/CJIC.20230480
-
[14]
Lina Guo , Ruizhe Li , Chuang Sun , Xiaoli Luo , Yiqiu Shi , Hong Yuan , Shuxin Ouyang , Tierui Zhang . 层状双金属氢氧化物的层间阴离子对衍生的Ni-Al2O3催化剂光热催化CO2甲烷化反应的影响. Acta Physico-Chimica Sinica, 2025, 41(1): 2309002-. doi: 10.3866/PKU.WHXB202309002
-
[15]
Ce Liang , Qiuhui Sun , Adel Al-Salihy , Mengxin Chen , Ping Xu . Recent advances in crystal phase induced surface-enhanced Raman scattering. Chinese Chemical Letters, 2024, 35(9): 109306-. doi: 10.1016/j.cclet.2023.109306
-
[16]
Wenlong LI , Xinyu JIA , Jie LING , Mengdan MA , Anning ZHOU . Photothermal catalytic CO2 hydrogenation over a Mg-doped In2O3-x catalyst. Chinese Journal of Inorganic Chemistry, 2024, 40(5): 919-929. doi: 10.11862/CJIC.20230421
-
[17]
Fei ZHOU , Xiaolin JIA . Co3O4/TiO2 composite photocatalyst: Preparation and synergistic degradation performance of toluene. Chinese Journal of Inorganic Chemistry, 2024, 40(11): 2232-2240. doi: 10.11862/CJIC.20240236
-
[18]
Ling-Hao Zhao , Hai-Wei Yan , Jian-Shuang Jiang , Xu Zhang , Xiang Yuan , Ya-Nan Yang , Pei-Cheng Zhang . Effective assignment of positional isomers in dimeric shikonin and its analogs by 1H NMR spectroscopy. Chinese Chemical Letters, 2024, 35(5): 108863-. doi: 10.1016/j.cclet.2023.108863
-
[19]
Manyu Zhu , Fei Liang , Lie Wu , Zihao Li , Chen Wang , Shule Liu , Xiue Jiang . Revealing the difference of Stark tuning rate between interface and bulk by surface-enhanced infrared absorption spectroscopy. Chinese Chemical Letters, 2025, 36(2): 109962-. doi: 10.1016/j.cclet.2024.109962
-
[20]
Qijun Tang , Wenguang Tu , Yong Zhou , Zhigang Zou . High efficiency and selectivity catalyst for photocatalytic oxidative coupling of methane. Chinese Journal of Structural Chemistry, 2023, 42(12): 100170-100170. doi: 10.1016/j.cjsc.2023.100170
-
[1]
Metrics
- PDF Downloads(0)
- Abstract views(897)
- HTML views(27)

 Login In
Login In




 DownLoad:
DownLoad:
 DownLoad:
DownLoad: