Citation:
Bang-Tun Zhao, Xiao-Min Zhu, Xiu-Hua Chen, Zhen-Ning Yan, Wei-Min Zhu. Novel clicked tetrathiafulvalene-calix[4]arene assemblies:Synthesis and intermolecular electron transfer toward p-chloranil[J]. Chinese Chemical Letters,
;2013, 24(07): 573-577.

-
Two tetrathiafulvalene-calix[4]arene assemblies (TTF-calix-1 and TTF-calix-2) have been synthesized by the click reaction. Both their cyclic voltammograms show, as expected, two one-electron quasi-reversible redox behavior. The UV-vis absorption spectra studies show that these two assemblies undergo progressive oxidation at the TTF moiety in presence of increasing amounts of Cu2+ or Hg2+. Moreover, the absorption studies show intermolecular electron transfer between compounds TTF-calix-1 or TTF-calix-2 and p-chloranil may be promoted by specific metal ions such as Pb2+, Sc3+ etc.
-
Spintronics is a cutting-edge field of developing new electronic devices by manipulating the electron spin and magnetic moment [1]. Traditional spintronic research mainly focuses on transition metals and inorganic semiconductors, while organic molecules have the advantage of being extremely easy to realize efficient spin control by modifying the specific external conditions for desired electronic structures and magnetic characteristics. Corrole, as a ring-contracted porphyrin, is the aromatic analog of the central macrocycle of vitamin B12. Corrole has a squeezed inner cavity and three inner NHs in its free-base form, making it easier to stabilize high-valent metal ions and thus a promising candidate in spintronics.
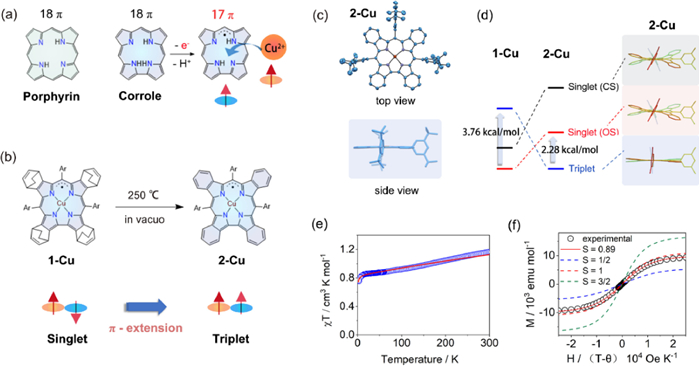
When coordinated to metal ions such as Cu, Co, and Fe, the electron-rich corrole ligand could be partially oxidized to exhibit radical character, making it difficult to determine the exact oxidation states of central metals and ligands. The most controversial debate was the Cu(Ⅱ)/Cu(Ⅲ) dilemma on copper corrole [2]. The compound was initially thought to be a closed-shell Cu(Ⅲ) complex in 2000, but significant experimental and theoretical evidence over the next twenty years progressively revealed its open-shell singlet state ground state comprised of a Cu(Ⅱ) core and partially oxidized radical ligand (Fig. 1a).
Figure 1
 Figure 1. (a) Structure of 18π porphyrin and corrole, and schematic of the 17π corrole radical formation during coordination. (b) Schematic representation of the singlet-to-triplet conversion from 1-Cu to 2-Cu. (c) Single-crystal X-ray diffraction structure of 2-Cu. (d) DFT calculated structures and relative energies of 1-Cu and 2-Cu in triplet, open-shell singlet and closed-shell singlet states. (e) Observed (circle) and simulated (line) χT–T curve of 2-Cu at H = 5000 Oe. (f) Observed (circle) and simulated (line) M-H curves of 2-Cu with different S values.
Figure 1. (a) Structure of 18π porphyrin and corrole, and schematic of the 17π corrole radical formation during coordination. (b) Schematic representation of the singlet-to-triplet conversion from 1-Cu to 2-Cu. (c) Single-crystal X-ray diffraction structure of 2-Cu. (d) DFT calculated structures and relative energies of 1-Cu and 2-Cu in triplet, open-shell singlet and closed-shell singlet states. (e) Observed (circle) and simulated (line) χT–T curve of 2-Cu at H = 5000 Oe. (f) Observed (circle) and simulated (line) M-H curves of 2-Cu with different S values.Shen Z. and Wu F. from Nanjing University have developed a series of metallocorroles with extended π-conjugation systems, which not only facilitated the formation and stabilization of radical ligands, but also allowed spin configurations of complexes to be easily controlled [3-5]. Recently, Shen, Wu, and co-workers reported that the unambiguous Cu(Ⅱ) corrole with fully oxidized [4n + 1]π radical ligand was obtained through the benzo-fusion at the β-position of corrole ligand [6]. The ground-state conversion of copper corrole radical from singlet to triplet was achieved via a retro-Diels-Alder reaction (Fig. 1b).
The authors first synthesized a bicyclo[2.2.2]octadiene (BCOD) fused corrole 1-Cu by employing the classic H2O-MeOH approach with starting materials 4, 7-dihydro-4, 7-ethano-2H-isoindole and 3, 5-di-tert-butyl-benzaldehyde. Heating solid 1-Cu at 250 ℃ in vacuo cut the C—C bond in the BCOD bridge, eliminated the ethylene, and quantitatively afforded the benzo-fused 2-Cu. The singlet ground state of 1-Cu was clearly confirmed by the peripheral BCOD protons signals that appeared in the region of 6.60~2.09 ppm, while the signals of benzo protons in 2-Cu were located in a range of −6.2~−25.6 ppm, demonstrating its enhanced paramagnetism.
The conformations of copper corroles were assumed to be "inherently saddle distorted" owing to the strong d-π interactions of antiferromagnetically coupled Cu(Ⅱ) corrole radicals. When compared to other copper corroles, 2-Cu stood out due to its highly planar macrocycle with a mean plane deviation value of only 0.024 Å (Fig. 1c). The planar structure could perfectly sustain the ferromagnetic coupling (S = 1) between Cu(Ⅱ) and corrole radical.
The theoretical analysis of 2-Cu was conducted by the authors for three different states, including a close-shell singlet Cu(Ⅲ) (CS), an open-shell singlet antiferromagnetically coupled Cu(Ⅱ) corrole radical (OS) and a triplet ferromagnetically coupled Cu(Ⅱ) corrole radical (T). A lower T state was discovered for 2-Cu than the CS and OS states with a calculated singlet-triplet energy gap of 2.28 kcal/mol, providing theoretical support for the triplet ground state (Fig. 1d). The strongest support for the triplet ground state came from temperature- and field-dependent superconducting quantum interference device (SQUID) magnetometry. The χT value of 2-Cu in 2 K was 0.77 cm3 K/mol, and it reached approximately 1 cm3 K/mol at 300 K. The singlet-triplet energy gap was estimated to be 1.66 kcal/mol by fitting the χT–T plot (Fig. 1e). The field-dependent magnetization plot of 2-Cu at 2 K was fitted to a Brillouin function with S = 0.89, which was close to the value (S = 1) corresponding to the triplet ground state (Fig. 1f). The magnetic hysteresis of 2-Cu was observed at 2 K. Moreover, 2-Cu exhibits remarkable stability in air despite its radical character. The calculated density plots of spin and SOMO both demonstrate that the density is concentrated mostly in the inner corrole ring, which is nicely protected by fused benzenes with low reactivity.
The research conducted by Shen's group introduces a new approach to the fine-tuning of interactions between metal center and corrole ligand and provides a promising strategy for the creation of stable corrole radical complexes with distinctive high-spin systems. The strategy will further trigger the development of novel functional materials based on corroles and their work will encourage an increasing amount of spintronics research for the use of innovative magnetic and electrical devices.
References
-
[1]
[1] J. Yamada, T. Sugimoto, TTF Chemistry: Fundamentals and Applications of Tetrathiafulvalene, Kodansha-Springer, Tokyo, 2004.
-
[2]
[2] D. Canevet, M. Sallé, G.X. Zhang, D.Q. Zhang, D.B. Zhu, Tetrathiafulvalene (TTF) derivatives: key buildinγ-blocks for switchable processes, Chem. Commun. (2009) 2245-2269.
-
[3]
[3] C.D. Gutsche, Calixarenes Revisited, The Royal Society of Chemistry, Cambridge, 1998.
-
[4]
[4] C.D. Gutsche, Calixarenes: An Introduction, The Royal Society of Chemistry, Cambridge, 2008.
-
[5]
[5] J. Vicens, J.M. Harrowfield, L. Baklouti, Calixarenes in the Nanoworld, Springer Publisher, Dordrecht, Netherlands, 2007.
-
[6]
[6] J.B. de Vains Regnouf, M. Sallé, R. Lamartine, Conjugated p-(tetrathiafulvalenylmethylideneamino) calix[4]arene, J. Chem. Soc. Perkin Trans. 2 (1997) 2461-2463.
-
[7]
[7] B.T. Zhao, M.J. Blesa, N. Mercier, F. Le Derf, M. Sallé, A tetrathiafulvalene-appended calix[4]arene: synthesis and electrochemical characterization, Supramol. Chem. 17 (2005) 465-468.
-
[8]
[8] B.T. Zhao, M.J. Blesa, N. Mercier, F. Le Derf, M. Sallé, A calixarene-amide-tetrathiafulvalene assembly for the electrochemical detection of anions, New J. Chem. 29 (2005) 1164-1167.
-
[9]
[9] B.T. Zhao, M.J. Blesa, N. Mercier, F. Le Derf, M. Sallé, Biscalix[4]arenes bridged by an electroactive tetrathiafulvalene unit, J. Org. Chem. 70 (2005) 6254-6257.
-
[10]
[10] M.J. Blesa, B.T. Zhao, M. Allain, F. Le Derf, M. Sallé, Bis(calixcrown)tetrathiafulvalene receptors, Chem. Eur. J. 12 (2006) 1906-1914.
-
[11]
[11] J. Lyskawa, M. Sallé, J.Y. Balandier, et al., Monitoring the formation of TTF dimers by Na+ complexation, Chem. Commun. (2006) 2233-2235.
-
[12]
[12] B.T. Zhao, M.J. Blesa, F. Le Derf, et al., Carboxylic acid derivatives of tetrathiafulvalene: key intermediates for the synthesis of redox-active calixarene-based anion receptors, Tetrahedron 63 (2007) 10768-10777.
-
[13]
[13] J. Lyskawa, D. Canevet, M. Allain, M. Sallé, An electron-rich three dimensional receptor based on a calixarene-tetrathiafulvalene assembly, Tetrahedron Lett. 51 (2010) 5868-5872.
-
[14]
[14] B.T. Zhao, L.W. Liu, J.J. Ding, G.R. Qu, Synthesis of tetrathiafulvalene-calixarene derivatives and their intermolecular electron transfer towards tetrachloro-1,4-benzoquinone, Chem. J. Chin. Univ. 32 (2011) 2103-2108.
-
[15]
[15] M.H. Düker, R. Gómez, C.M.L. Vande Velde, V.A. Azov, Upper rim tetrathiafulvalene-bridged calix[4]arenes, Tetrahedron Lett. 52 (2011) 2881-2884.
-
[16]
[16] M.H. Lee, Q.Y. Cao, S.K. Kim, J.L. Sessler, J.S. Kim, Anion responsive TTF-appended calix[4]arenes: synthesis and study of two different conformers, J. Org. Chem. 76 (2011) 870-874.
-
[17]
[17] F. Sun, F. Hu, G.X. Zhang, Q.Y. Zheng, D.Q. Zhang, Calix[4]arenes with electroactive tetrathiafulvalene and quinone units: metal-ion-promoted electron transfer, J. Org. Chem. 76 (2011) 6883-6888.
-
[18]
[18] F. Sun, F. Hu, G.X. Zhang, D.Q. Zhang, Metal-ion-promoted electron transfer between tetrathiafulvalene and quinone units within a calix[4]arene framework and tuning through coordination of the neighboring crown ether with a sodium cation, Chem. Asian J. 7 (2012) 183-189.
-
[19]
[19] K. Flídrová, M. Tkadlecová, K. Lang, P. Lhoták, Anion complexation by calix[4]-areneeTTF conjugates, Dyes Pigments 92 (2011) 668-673.
-
[20]
[20] B.T. Zhao, W.B. Guo, P.Z. Hu, Synthesis, structure and electrochemical behavior of a novel redox-active thiacalix[4]arene-tetrathiafulvalene assembly, Heterocycles 81 (2010) 1661-1667.
-
[21]
[21] B.T. Zhao, Z. Zhou, Z.N. Yan, et al., Synthesis and electrochemical behavior of a model redox-active thiacalix[4]arene-tetrathiafulvalene assembly, Tetrahedron Lett. 51 (2010) 5815-818.
-
[22]
[22] B.T. Zhao, X.M. Zhu, Q.M. Peng, et al., A novel redox-active calix[4]arene-tetrathiafulvalene dyad, Cent. Eur. J. Chem. 9 (2011) 1102-108.
-
[23]
[23] B.T. Zhao, J.J. Li, Z. Zhou, Z.N. Yan, W.M. Zhu, Synthesis and electrochemical behavior of electroactive bistetrathiafulvalene-attached thiacalix[4]arene assemblies, Chem. Res. Chin. Univ. 28 (2012) 828-32.
-
[24]
[24] H.C. Kolb, M.G. Finn, K.B. Sharpless, Click chemistry: diverse chemical function from a few good reactions, Angew. Chem. Int. Ed. 40 (2001) 2004-021.
-
[25]
[25] M. Meldal, C.W. Torn鴈, Cu-catalyzed azide-alkyne cycloaddition, Chem. Rev. 108 (2008) 2952-015.
-
[26]
[26] Y.L. Zhao, W.R. Dichtel, A. Trabolsi, et al., A redox-switchable a-cyclodextrinbased[2]rotaxane, J. Am. Chem. Soc. 130 (2008) 11294-1296.
-
[27]
[27] B.T. Zhao, L.W. Liu, X.C. Li, G.R. Qu, A clicked tetrathiafulvalene-oxyquinoline dyad as an optical and electrochemical Zn2+ probe, Chin. J. Chem. 30 (2012) 254-58.
-
[28]
[28] L.W. Liu, W.B. Guo, X.C. Li, G.R. Qu, B.T. Zhao, Progress on synthesis of calixarene derivatives via click chemistry, Chin. J. Org. Chem. 30 (2010) 1960-974.
-
[29]
[29] E.M. Collins, M.A. McKervey, E. Madigan, et al., Chemically modified calix[4]arenes: regioselective synthesis of 1, 3-(distal) derivatives and related compounds. X-ray crystal structure of a diphenol-initrile, J. Chem. Soc., Perkin Trans. 1 (1991) 3137-142.
-
[1]
-
-
[1]
[1] J. Yamada, T. Sugimoto, TTF Chemistry: Fundamentals and Applications of Tetrathiafulvalene, Kodansha-Springer, Tokyo, 2004.
-
[2]
[2] D. Canevet, M. Sallé, G.X. Zhang, D.Q. Zhang, D.B. Zhu, Tetrathiafulvalene (TTF) derivatives: key buildinγ-blocks for switchable processes, Chem. Commun. (2009) 2245-2269.
-
[3]
[3] C.D. Gutsche, Calixarenes Revisited, The Royal Society of Chemistry, Cambridge, 1998.
-
[4]
[4] C.D. Gutsche, Calixarenes: An Introduction, The Royal Society of Chemistry, Cambridge, 2008.
-
[5]
[5] J. Vicens, J.M. Harrowfield, L. Baklouti, Calixarenes in the Nanoworld, Springer Publisher, Dordrecht, Netherlands, 2007.
-
[6]
[6] J.B. de Vains Regnouf, M. Sallé, R. Lamartine, Conjugated p-(tetrathiafulvalenylmethylideneamino) calix[4]arene, J. Chem. Soc. Perkin Trans. 2 (1997) 2461-2463.
-
[7]
[7] B.T. Zhao, M.J. Blesa, N. Mercier, F. Le Derf, M. Sallé, A tetrathiafulvalene-appended calix[4]arene: synthesis and electrochemical characterization, Supramol. Chem. 17 (2005) 465-468.
-
[8]
[8] B.T. Zhao, M.J. Blesa, N. Mercier, F. Le Derf, M. Sallé, A calixarene-amide-tetrathiafulvalene assembly for the electrochemical detection of anions, New J. Chem. 29 (2005) 1164-1167.
-
[9]
[9] B.T. Zhao, M.J. Blesa, N. Mercier, F. Le Derf, M. Sallé, Biscalix[4]arenes bridged by an electroactive tetrathiafulvalene unit, J. Org. Chem. 70 (2005) 6254-6257.
-
[10]
[10] M.J. Blesa, B.T. Zhao, M. Allain, F. Le Derf, M. Sallé, Bis(calixcrown)tetrathiafulvalene receptors, Chem. Eur. J. 12 (2006) 1906-1914.
-
[11]
[11] J. Lyskawa, M. Sallé, J.Y. Balandier, et al., Monitoring the formation of TTF dimers by Na+ complexation, Chem. Commun. (2006) 2233-2235.
-
[12]
[12] B.T. Zhao, M.J. Blesa, F. Le Derf, et al., Carboxylic acid derivatives of tetrathiafulvalene: key intermediates for the synthesis of redox-active calixarene-based anion receptors, Tetrahedron 63 (2007) 10768-10777.
-
[13]
[13] J. Lyskawa, D. Canevet, M. Allain, M. Sallé, An electron-rich three dimensional receptor based on a calixarene-tetrathiafulvalene assembly, Tetrahedron Lett. 51 (2010) 5868-5872.
-
[14]
[14] B.T. Zhao, L.W. Liu, J.J. Ding, G.R. Qu, Synthesis of tetrathiafulvalene-calixarene derivatives and their intermolecular electron transfer towards tetrachloro-1,4-benzoquinone, Chem. J. Chin. Univ. 32 (2011) 2103-2108.
-
[15]
[15] M.H. Düker, R. Gómez, C.M.L. Vande Velde, V.A. Azov, Upper rim tetrathiafulvalene-bridged calix[4]arenes, Tetrahedron Lett. 52 (2011) 2881-2884.
-
[16]
[16] M.H. Lee, Q.Y. Cao, S.K. Kim, J.L. Sessler, J.S. Kim, Anion responsive TTF-appended calix[4]arenes: synthesis and study of two different conformers, J. Org. Chem. 76 (2011) 870-874.
-
[17]
[17] F. Sun, F. Hu, G.X. Zhang, Q.Y. Zheng, D.Q. Zhang, Calix[4]arenes with electroactive tetrathiafulvalene and quinone units: metal-ion-promoted electron transfer, J. Org. Chem. 76 (2011) 6883-6888.
-
[18]
[18] F. Sun, F. Hu, G.X. Zhang, D.Q. Zhang, Metal-ion-promoted electron transfer between tetrathiafulvalene and quinone units within a calix[4]arene framework and tuning through coordination of the neighboring crown ether with a sodium cation, Chem. Asian J. 7 (2012) 183-189.
-
[19]
[19] K. Flídrová, M. Tkadlecová, K. Lang, P. Lhoták, Anion complexation by calix[4]-areneeTTF conjugates, Dyes Pigments 92 (2011) 668-673.
-
[20]
[20] B.T. Zhao, W.B. Guo, P.Z. Hu, Synthesis, structure and electrochemical behavior of a novel redox-active thiacalix[4]arene-tetrathiafulvalene assembly, Heterocycles 81 (2010) 1661-1667.
-
[21]
[21] B.T. Zhao, Z. Zhou, Z.N. Yan, et al., Synthesis and electrochemical behavior of a model redox-active thiacalix[4]arene-tetrathiafulvalene assembly, Tetrahedron Lett. 51 (2010) 5815-818.
-
[22]
[22] B.T. Zhao, X.M. Zhu, Q.M. Peng, et al., A novel redox-active calix[4]arene-tetrathiafulvalene dyad, Cent. Eur. J. Chem. 9 (2011) 1102-108.
-
[23]
[23] B.T. Zhao, J.J. Li, Z. Zhou, Z.N. Yan, W.M. Zhu, Synthesis and electrochemical behavior of electroactive bistetrathiafulvalene-attached thiacalix[4]arene assemblies, Chem. Res. Chin. Univ. 28 (2012) 828-32.
-
[24]
[24] H.C. Kolb, M.G. Finn, K.B. Sharpless, Click chemistry: diverse chemical function from a few good reactions, Angew. Chem. Int. Ed. 40 (2001) 2004-021.
-
[25]
[25] M. Meldal, C.W. Torn鴈, Cu-catalyzed azide-alkyne cycloaddition, Chem. Rev. 108 (2008) 2952-015.
-
[26]
[26] Y.L. Zhao, W.R. Dichtel, A. Trabolsi, et al., A redox-switchable a-cyclodextrinbased[2]rotaxane, J. Am. Chem. Soc. 130 (2008) 11294-1296.
-
[27]
[27] B.T. Zhao, L.W. Liu, X.C. Li, G.R. Qu, A clicked tetrathiafulvalene-oxyquinoline dyad as an optical and electrochemical Zn2+ probe, Chin. J. Chem. 30 (2012) 254-58.
-
[28]
[28] L.W. Liu, W.B. Guo, X.C. Li, G.R. Qu, B.T. Zhao, Progress on synthesis of calixarene derivatives via click chemistry, Chin. J. Org. Chem. 30 (2010) 1960-974.
-
[29]
[29] E.M. Collins, M.A. McKervey, E. Madigan, et al., Chemically modified calix[4]arenes: regioselective synthesis of 1, 3-(distal) derivatives and related compounds. X-ray crystal structure of a diphenol-initrile, J. Chem. Soc., Perkin Trans. 1 (1991) 3137-142.
-
[1]
-

-
-
[1]
Yiqian Jiang , Zihan Yang , Xiuru Bi , Nan Yao , Peiqing Zhao , Xu Meng . Mediated electron transfer process in α-MnO2 catalyzed Fenton-like reaction for oxytetracycline degradation. Chinese Chemical Letters, 2024, 35(8): 109331-. doi: 10.1016/j.cclet.2023.109331
-
[2]
Quanyou Guo , Yue Yang , Tingting Hu , Hongqi Chu , Lijun Liao , Xuepeng Wang , Zhenzi Li , Liping Guo , Wei Zhou . Regulating local electron transfer environment of covalent triazine frameworks through F, N co-modification towards optimized oxygen reduction reaction. Chinese Chemical Letters, 2025, 36(1): 110235-. doi: 10.1016/j.cclet.2024.110235
-
[3]
Yuwei Liu , Yihui Zhu , Weijian Duan , Yizhuo Yang , Haorui Tuo , Chunhua Feng . Electrocatalytic nitrate reduction on Fe, Fe3O4, and Fe@Fe3O4 cathodes: Elucidating structure-sensitive mechanisms of direct electron versus hydrogen atom transfer. Chinese Chemical Letters, 2025, 36(6): 110347-. doi: 10.1016/j.cclet.2024.110347
-
[4]
Xiuzheng Deng , Yi Ke , Jiawen Ding , Yingtang Zhou , Hui Huang , Qian Liang , Zhenhui Kang . Construction of ZnO@CDs@Co3O4 sandwich heterostructure with multi-interfacial electron-transfer toward enhanced photocatalytic CO2 reduction. Chinese Chemical Letters, 2024, 35(4): 109064-. doi: 10.1016/j.cclet.2023.109064
-
[5]
Hui Li , Yanxing Qi , Jia Chen , Juanjuan Wang , Min Yang , Hongdeng Qiu . Synthesis of amine-pillar[5]arene porous adsorbent for adsorption of CO2 and selectivity over N2 and CH4. Chinese Chemical Letters, 2024, 35(11): 109659-. doi: 10.1016/j.cclet.2024.109659
-
[6]
Chunxiu Yu , Zelin Wu , Hongle Shi , Lingyun Gu , Kexin Chen , Chuan-Shu He , Yang Liu , Heng Zhang , Peng Zhou , Zhaokun Xiong , Bo Lai . Insights into the electron transfer mechanisms of peroxydisulfate activation by modified metal-free acetylene black for degradation of sulfisoxazole. Chinese Chemical Letters, 2024, 35(8): 109334-. doi: 10.1016/j.cclet.2023.109334
-
[7]
Xingyu Ma , Yi-Xin Chen , Zi Ye , Chong-Jing Zhang . Isotope-labeled click-free probes to identify protein targets of lysine-targeting covalent reversible molecules. Chinese Chemical Letters, 2025, 36(5): 110203-. doi: 10.1016/j.cclet.2024.110203
-
[8]
Yi Liu , Zhe-Hao Wang , Guan-Hua Xue , Lin Chen , Li-Hua Yuan , Yi-Wen Li , Da-Gang Yu , Jian-Heng Ye . Photocatalytic dicarboxylation of strained C–C bonds with CO2 via consecutive visible-light-induced electron transfer. Chinese Chemical Letters, 2024, 35(6): 109138-. doi: 10.1016/j.cclet.2023.109138
-
[9]
Yun-Xin Huang , Lin-Qian Yu , Ke-Yu Chen , Hao Wang , Shou-Yan Zhao , Bao-Cheng Huang , Ren-Cun Jin . Biochar with self-doped N to activate peroxymonosulfate for bisphenol-A degradation via electron transfer mechanism: The active edge graphitic N site. Chinese Chemical Letters, 2024, 35(9): 109437-. doi: 10.1016/j.cclet.2023.109437
-
[10]
Yan Fan , Jiao Tan , Cuijuan Zou , Xuliang Hu , Xing Feng , Xin-Long Ni . Unprecedented stepwise electron transfer and photocatalysis in supramolecular assembly derived hybrid single-layer two-dimensional nanosheets in water. Chinese Chemical Letters, 2025, 36(4): 110101-. doi: 10.1016/j.cclet.2024.110101
-
[11]
Manlin Lu , Sheng Liao , Jiayu Li , Zidong Yu , Ningjiu Zhao , Zuoti Xie , Shunli Chen , Li Dang , Ming-De Li . Face-to-face π-π interactions and electron communication boosting efficient reverse intersystem crossing in through-space charge transfer molecules. Chinese Chemical Letters, 2025, 36(6): 110066-. doi: 10.1016/j.cclet.2024.110066
-
[12]
Lumin Zheng , Ying Bai , Chuan Wu . Multi-electron reaction and fast Al ion diffusion of δ-MnO2 cathode materials in rechargeable aluminum batteries via first-principle calculations. Chinese Chemical Letters, 2024, 35(4): 108589-. doi: 10.1016/j.cclet.2023.108589
-
[13]
Saisai Yuan , Yiming Chen , Xijuan Wang , Degui Zhao , Tengyang Gao , Caiyun Wei , Chuanxiang Chen , Yang Yang , Wenjing Hong . Decouple the intermolecular interaction by encapsulating an insulating sheath. Chinese Chemical Letters, 2025, 36(6): 110816-. doi: 10.1016/j.cclet.2025.110816
-
[14]
Qinghong Zhang , Qiao Zhao , Xiaodi Wu , Li Wang , Kairui Shen , Yuchen Hua , Cheng Gao , Yu Zhang , Mei Peng , Kai Zhao . Visible-light-induced ring-opening cross-coupling of cycloalcohols with vinylazaarenes and enones via β-C-C scission enabled by proton-coupled electron transfer. Chinese Chemical Letters, 2025, 36(2): 110167-. doi: 10.1016/j.cclet.2024.110167
-
[15]
Jin Long , Xingqun Zheng , Bin Wang , Chenzhong Wu , Qingmei Wang , Lishan Peng . Improving the electrocatalytic performances of Pt-based catalysts for oxygen reduction reaction via strong interactions with single-CoN4-rich carbon support. Chinese Chemical Letters, 2024, 35(5): 109354-. doi: 10.1016/j.cclet.2023.109354
-
[16]
Yihu Ke , Shuai Wang , Fei Jin , Guangbo Liu , Zhiliang Jin , Noritatsu Tsubaki . Charge transfer optimization: Role of Cu-graphdiyne/NiCoMoO4 S-scheme heterojunction and Ohmic junction. Chinese Journal of Structural Chemistry, 2024, 43(12): 100458-100458. doi: 10.1016/j.cjsc.2024.100458
-
[17]
Kongchuan Wu , Dandan Lu , Jianbin Lin , Ting-Bin Wen , Wei Hao , Kai Tan , Hui-Jun Zhang . Elucidating ligand effects in rhodium(Ⅲ)-catalyzed arene–alkene coupling reactions. Chinese Chemical Letters, 2024, 35(5): 108906-. doi: 10.1016/j.cclet.2023.108906
-
[18]
Jingyu Chen , Sha Wu , Yuhao Wang , Jiong Zhou . Near-perfect separation of alicyclic ketones and alicyclic alcohols by nonporous adaptive crystals of perethylated pillar[5]arene and pillar[6]arene. Chinese Chemical Letters, 2025, 36(4): 110102-. doi: 10.1016/j.cclet.2024.110102
-
[19]
Xin Jiang , Han Jiang , Yimin Tang , Huizhu Zhang , Libin Yang , Xiuwen Wang , Bing Zhao . g-C3N4/TiO2-X heterojunction with high-efficiency carrier separation and multiple charge transfer paths for ultrasensitive SERS sensing. Chinese Chemical Letters, 2024, 35(10): 109415-. doi: 10.1016/j.cclet.2023.109415
-
[20]
Xing Xiao , Yunling Jia , Wanyu Hong , Yuqing He , Yanjun Wang , Lizhi Zhao , Huiqin An , Zhen Yin . Sulfur-defective ZnIn2S4 nanosheets decorated by TiO2 nanosheets with exposed {001} facets to accelerate charge transfer for efficient photocatalytic hydrogen evolution. Chinese Journal of Structural Chemistry, 2024, 43(12): 100474-100474. doi: 10.1016/j.cjsc.2024.100474
-
[1]
Metrics
- PDF Downloads(0)
- Abstract views(846)
- HTML views(1)

 Login In
Login In




 DownLoad:
DownLoad:
 DownLoad:
DownLoad: