Citation:
Wen-Sheng Zhang, Wen-Jing Xu, Fei Zhang, Gui-Rong Qu. Synthesis of symmetrical 1, 3-diynes via tandem reaction of (Z)-arylvinyl bromides in the presence of DBU and CuI[J]. Chinese Chemical Letters,
;2013, 24(05): 407-410.

-
Microwave-assisted tandem reaction of (Z)-arylvinyl bromides involving an elimination and homocoupling in the presence of DBU and CuI in DMF affords a variety of symmetrical 1,3-diynes in good to excellent yields. This tandem process, eliminating the need of volatile and savory terminal alkynes, provides an alternative to the conventional homocoupling methods for the synthesis of symmetrical 1,3-diynes.
-
Keywords:
- Catalysis,
- Copper,
- Conjugation,
- Coupling,
- Tandem reaction
-
Light-induced charge transfer and separation are fundamental processes in numerous chemical reactions and are widely used in the design of fluorescent probes. Photoexcitation induced twisted intramolecular charge shuttle (TICS) is a newly discovered photo-induced charge transfer phenomenon, which refers to the role swap of electron donor and electron acceptor after intramolecular rotation when the dye molecule absorbs photons and transitions to the excited state [1]. TICS are ubiquitous in a variety of fluorophores including BODIPY, rhodamine and coumarin. The TICS fluorophore has the following typical structural characteristics. Taking rhodamine as the representative fluorophore which is substituted with a tertiary amine at the meso-position, in the ground state, the carbon atom at the 10-position of xanthene is in a state of lack of electricity, which is prone to nucleophilic substitution reaction. After being excited by light, electrons flow to the 10-carbon, and the consequent twist of the meso C-N bond leads to fluorescence quenching. TICS fluorophores are excellent candidates for designing reactive fluorescent probes based on the nucleophilic substitution reaction at the meso site and the concomitant significant fluorescence enhancement [2-5].
Biothiols are the most widely distributed nucleophilic molecules in living systems, among which cysteine (Cys), homocysteine (Hcy) and glutathione (GSH) mainly exist in cells [6]. The concentration of the first two in cells is about 30–200 µmol/L, respectively, while the highest content of GSH is concentrated in the mitochondria, and the concentration can reach 1–10 mmol/L [7]. GSH plays a key role in mitochondrial respiration and is used to maintain mitochondrial redox balance. GSH also plays an important role in intracellular protein synthesis and metabolism [7, 8]. Detecting and tracking the distribution and content dynamics of intracellular GSH is essential for understanding the redox balance and other physiological states of cells, which has stimulated the research upsurge of fluorescent probes for intracellular imaging of GSH [9-13].
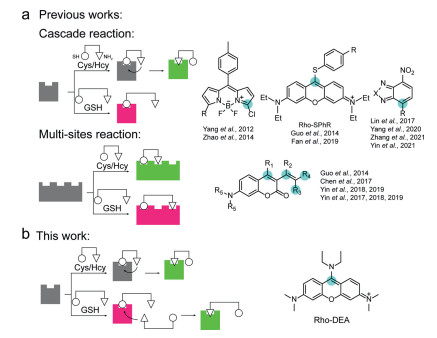
GSH probes are mainly based on the electrophilicity of thiol or amino groups, and are designed by reactions such as nucleophilic addition, nucleophilic cleavage, or ligand exchange [9, 14, 15]. Since Cys and Hcy also contain thiol and amino groups, probes that can distinguish GSH from Cys/Hcy have always been a challenge. Niu et al. found that the thiol group of Cys or Hcy would first undergo nucleophilic substitution reaction with BODIPY derivative, and then the amino group would undergo intramolecular substitution of thiol group. However, due to the long distance between the thiol group and the amino group in GSH, this continuous intramolecular nucleophilic substitution is difficult to occur (Scheme 1a) [16]. Thiol substituted and amino substituted fluorophores show two colors of fluorescence with very different wavelengths, thus effectively distinguishing GSH from Cys/Hcy. The mechanism by which the difference in the distance between the thiol and amino groups in the molecule leads to different final thiol or amino substituted products has been widely used in the design of fluorescent probes to distinguish GSH from Cys/Hcy [17-24]. In addition to this single-site cascade reaction, Liu et al. proposed a strategy to simultaneously react with thiol and amino groups at multiple reactive sites within the probe (Scheme 1a), respectively [25]. Depending on the position difference of the reaction site, the conjugated system after the reaction is different, and different fluorescence channels are formed, so as to achieve the effect of distinguishing GSH and Cys/Hcy. This strategy was subsequently used to design more functionally enhanced fluorescent probes [26-31].
Scheme 1
So far, only Cys and Hcy have been found to be able to undergo a cascade of thiol/amino substitution reactions, sometimes accompanied by the transition from long-wavelength fluorescence to short-wavelength fluorescence [17], while GSH does not have this reaction characteristic, so the reported GSH probes show for single wavelength fluorescence enhancement.
In this paper, we report the first probe capable of cascading nucleophilic substitution reactions with the thiol and amino groups of GSH successively at a single reaction site, and demonstrated dual-color recognition of GSH (Scheme 1b). The probe Rho-DEA is based on the TICS fluorophore, and the continuous intramolecular nucleophilic substitution reaction occurs with Cys/Hcy, while the intermolecular nucleophilic substitution reaction occurs with GSH. Since the intersystem crossing to triplet state of the thiol substitution in the first step leads to fluorescence quenching for Cys/Hcy, GSH can be selectively recognized from the fluorescence signals. Due to the excellent membrane permeability and the accurate mitochondrial localization properties of Rho-DEA itself, also profit from the excellent brightness and photostability of the reaction product with GSH, Rho-DEA can be used for GSH dynamic tracking in live cell mitochondria. Other four compounds were synthesized and their fluorescence response with biothiols was studied for reference (Scheme S1 in Supporting information).
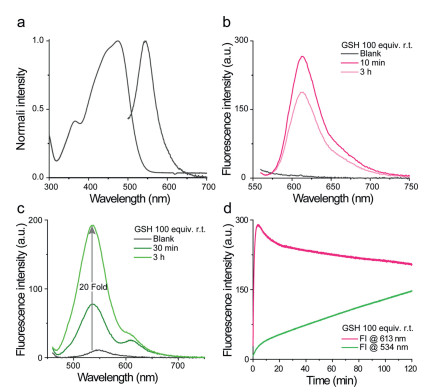
The spectral properties of probe Rho-DEA were first investigated. The maximum absorption in water was about 470 nm while the maximum emission was about 550 nm (Fig. 1a). Other main spectral properties of Rho-DEA and ethylamine substituted non-TICS reference compound Rho-EA in various solvents were summarized in Table S1 (Supporting information). The TICS-based probe Rho-DEA has an anti-solvation effect in both the ground state (UV-vis absorption) and the excited state (emission), which is due to the absence of charge separation. In contrast, for Rho-EA, due to the presence of an obvious intramolecular dipole, with the increase of solvent polarity, the charge transfer is enhanced, and the charge separation is intensified, resulting in a red-shift in both the absorption and the emission. From the perspective of quantum yield, TICS molecule Rho-DEA exhibits a very low quantum yield (< 0.01) in most solvents, that is, it is in a dark state, while Rho-EA exhibited pretty good optical properties in most solvents with high quantum yields.
Figure 1
By testing the spectroscopic properties of Rho-DEA in aqueous solutions of different pH, we found that with the increase of basicity, the maximum absorption of the compound at 470 nm decreased sharply, and a new absorption peak appeared at 400 nm (Fig. S1a in Supporting information). Correspondingly, the maximum emission intensity at 550 nm decreased, a new emission peak appears at 475 nm, and the intensity gradually increased (Fig. S1b in Supporting information). This change was completely irreversible, that was, once absorption at 400 nm and emission peak at 475 nm appeared upon increase of alkalinity of the solution, the original absorption and emission cannot be restored by lowering the pH of the solution. This phenomenon indicated that the compound undergoes irreversible structural changes at this time. Meanwhile, we noted that the spectra profile in this condition was very close to that of the starting material DMBPN (Fig. S1c in Supporting information). Combined with spectral properties and HRMS analysis, we proposed that DMBPN was generated when Rho-DEA was treated with alkaline conditions. As shown in Fig. S1d (Supporting information), the meso-C of Rho-DEA was attacked by hydroxyl groups which was abundant herein. Then the diethylamine group as a large sterically hindered leaving group quickly left, resulting in the stable resonance form DMBPN. This property of the probe Rho-DEA in alkaline solution demonstrated its typical TICS properties: The meso site is easily attacked by nucleophiles. Then we nest tested its reaction with biothiols in detail.
Considering the concentration of GSH in physiological environment, we first tested the reaction of the probe Rho-DEA with 1 mmol/L GSH in 200 mmol/L PB buffer. Referring to previous work, we also initially speculated that the probe Rho-DEA would generate Rho-S (GSH) accompanied by significant long-wavelength fluorescence. Spectral test showed that when the probe Rho-DEA (10 µmol/L) was mixed with 100 equiv. GSH (1 mmol/L) for 10 min, the UV-vis absorption peak was obviously narrowed, the maximum absorption around 470 nm was slightly reduced, and a new absorption peak around 590 nm appeared (Fig. S2 in Supporting information), associating with the appearance of an emission at 613 nm (Fig. 1b). The above spectra profiles were quite similar to that of reference compound Rho-SEA (Fig. S3 in Supporting information). The new emission band at 613 nm and absorption band at 590 nm were ascribed to the thiol-substituted product of Rho-DEA with GSH. After 3 h, the emission at 613 nm decreased, while a short-wavelength emission at 534 nm appeared and increased quickly (Fig. 1c), associated with the increase of a new absorption band at 430 nm (Fig. S4 in Supporting information). The solution color changed from bright purple to slightly green. Based on the optical properties of reference compound Rho-EA, the emission band at 534 nm and absorption band at 430 nm belong to the subsequent substitution reaction of amino group in GSH with the thiol-substituted product (Table S1, Scheme 1b). From the kinetics of the cascade reactions (Fig. 1d), the reaction of thiol substitution was rapid and reached the maximum fluorescence intensity quickly. The subsequent amino substitution reaction was slower and was affected by the concentration of GSH, which indicated that the second step was an intermolecular amino substitution reaction.
However, the previously reported meso-S-substituted pyronine derivative Rho-SPhR did not find a nucleophilic substitution reaction with the amino group of GSH (Scheme 1a) [18, 19]. We speculate that the reactivity of such molecules is affected by the amino substituent on xanthene. For a TICS fluorophore, the greater the electron-donating ability of the amino substituent on xanthene, the lower the charge deficiency of the meso-C, and the lower reactivity. Conversely, reducing the reactivity of the amino substituent enhanced the reactivity of the meso-C (Fig. 2a). Based on such a mechanism, we performed calculations on the Gibbs free energy difference between S-substituted and N-substituted pyronine derivatives with different amino substituents. We found that as the electron-donating ability of the amino substituent on xanthene increases, (represented by the S-substituted Rho-TDEA), the Gibbs free energy difference decreased, associated with the decreased reaction rate. When the concentration of GSH was increased in the solution of Rho-TDEA, the products of GSH amino-substitution were indeed found, but the reaction rate was much slower than that of Rho-DEA (Fig. 2b). Accordingly, when the concentration of GSH was reduced in the solution of Rho-DEA, the green fluorescent specie of the amino substituted product was not found in a short period of time (Fig. 2c and Fig. S5 in Supporting information). The above results were consistent with the calculations of Gibbs free energy. Finally, the process of nucleophilic substitution reaction of Rho-DEA with GSH thiol group and then a cascade reaction with another GSH amino group was confirmed (Fig. 2d).
Figure 2
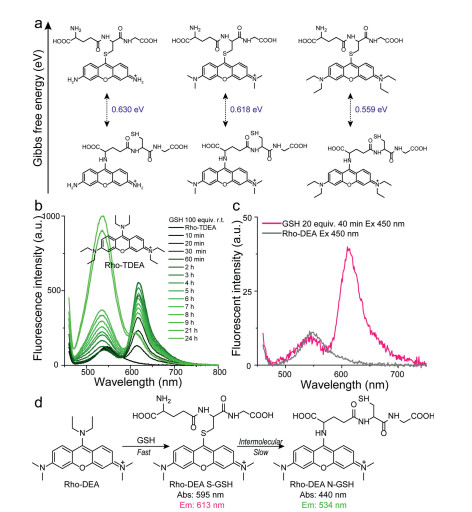 Figure 2. (a) The Gibbs free energy differences between Rho-S(GSH) and Rho-N(GSH) for pyronins with different substituents. (b) The fluorescence changes over time after mixing Rho-TDEA with 100 equiv. GSH and (c) Rho-DEA with 20 equiv. GSH. Probe concentration: 10 µmol/L, PB buffer: 0.2 mol/L, pH 7.4. (d) The process of cascade reaction of Rho-DEA with GSH.
Figure 2. (a) The Gibbs free energy differences between Rho-S(GSH) and Rho-N(GSH) for pyronins with different substituents. (b) The fluorescence changes over time after mixing Rho-TDEA with 100 equiv. GSH and (c) Rho-DEA with 20 equiv. GSH. Probe concentration: 10 µmol/L, PB buffer: 0.2 mol/L, pH 7.4. (d) The process of cascade reaction of Rho-DEA with GSH.Subsequently, we explored the reaction between Rho-DEA and Cys. With the presence of a high concentration of Cys, it was observed that the absorption peak was significantly narrowed with a large blue shift from ~470 nm to ~430 nm, and a strong new absorption peak at ~590 nm appeared in 5 min (Fig. 3a), while the fluorescence intensity at 534 nm was significantly enhanced (48 fold) (Fig. 3b). Based on the above results and the spectra of reference compounds Rho-EA and Rho-SEA, the absorption at 430 nm and the fluorescence emission at 534 nm were ascribed to the product Rho-N(Cys), and the absorption at 590 nm to the product Rho-S(Cys) with none long wavelength emission. After 30 min, the absorption and emission spectra of the solution did not change significantly, and even the maximum emission intensity at 534 nm was slightly reduced (Fig. S6 in Supporting information). During this process, the S-substituted specie of Rho-DEA with Cys was difficult to be observed. This may be due to the extremely fast reaction rate of Rho-DEA with Cys thiol group, which can be completed within tens of minutes (refer to the dynamic study in Fig. 3c). When we reduced the concentration of Cys to avoid the rapid consumption of the stage product Rho-S(Cys), the absorption band of S-substituted specie of Rho-DEA with Cys thiol group could not be observed (Fig. 3d). The UV-vis absorption at 590 nm showed a trend of increasing first and then decreasing (Fig. 3d), while the fluorescence intensity at 534 nm continued to increase (Fig. S7 in Supporting information). It means that Rho-S (Cys) has a process of generation, accumulation and further consumption. Therefore, the reaction of Rho-DEA with Cys also undergoes the cascade reaction process with thiol and then amino groups (Fig. 3e).
Figure 3
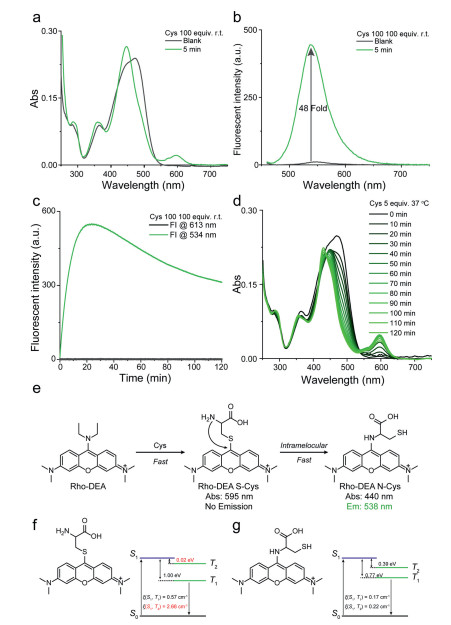 Figure 3. (a–c) The fluorescence changes over time after mixing Rho-DEA with 100 equiv. Cys. (d) The UV-vis absorption changes over time after mixing Rho-DEA with 5 equiv. Cys. Probe concentration: 10 µmol/L, PB buffer: 0.2 mol/L, pH 7.4. (e) The mechanism of TICS probe Rho-DEA reaction with Cys. (f, g) Calculation of single-triplet state energy gap and spin-orbit coupling of (f) Rho-S(Cys) and (g) Rho-N(Cys) at their ground state geometries.
Figure 3. (a–c) The fluorescence changes over time after mixing Rho-DEA with 100 equiv. Cys. (d) The UV-vis absorption changes over time after mixing Rho-DEA with 5 equiv. Cys. Probe concentration: 10 µmol/L, PB buffer: 0.2 mol/L, pH 7.4. (e) The mechanism of TICS probe Rho-DEA reaction with Cys. (f, g) Calculation of single-triplet state energy gap and spin-orbit coupling of (f) Rho-S(Cys) and (g) Rho-N(Cys) at their ground state geometries.Unlike the reaction between GSH and Rho-DEA, the thiol substitution product between Cys and Rho-DEA is fluorescence quenched. Our calculations showed that Rho-S(Cys) exhibited smaller energy gap (ΔEST) and larger spin-orbit coupling (SOC) between the S1 and the second triplet state (T2) at the ground state geometry, which leads to high intersystem crossing probability and generate triplet state species [32] (Fig. 3f). As for Rho-N(Cys), although it also has a small energy gap (ΔEST), the spin-orbit coupling (SOC) between S1 and T1/T2 is too low to generate triplet species (Fig. 3g). For the same reason, Rho-S (GSH) is also unable to produce triplet products and thus has strong long-wavelength fluorescence (Fig. S8 in Supporting information). We conclude that the main reason for the fluorescence quenching of the Rho-S (Cys) is due to intersystem crossing to triplet state, not a photo-induced electron transfer (PET) process from Cys amino to the fluorophore pyronin [18].
To further investigate the selectivity of Rho-DEA, we measured the fluorescent spectra of Rho-DEA with GSH and 20 various amino acids including Cys and Hcy (1 mmol/L). As shown in Figs. 4a and b, dual color fluorescence was only observed at 613 nm and 534 nm simultaneously when GSH was added. The high selectivity of Rho-DEA to GSH is attributed to the unique cascade reaction with thiol and then amino groups, with the formation of reactive intermediates Rho-S and then stable products Rho-N.
Figure 4
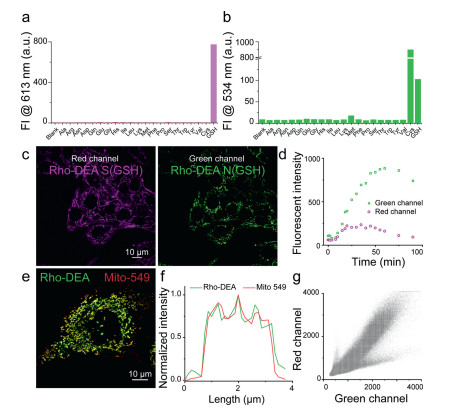 Figure 4. (a) Fluorescence intensity changes at 613 nm and (b) 534 nm after mixing Rho-DEA with 100 equiv. various amino acids in 30 min. Probe concentration 10: µmol/L, PB buffer: 0.2 mol/L, pH 7.4. (c) Dual-color confocal imaging of HeLa cells cultured with 0.5 µmol/L probe Rho-DEA for 4 min and (d) fluorescence intensity changes during this process. (e) Co-localization imaging of HeLa cells cultured with 0.5 µmol/L Rho-DEA for 2 h and then with Mito 549. (f, g) Fluorescence signal distribution and the Pearson's coefficient in (e).
Figure 4. (a) Fluorescence intensity changes at 613 nm and (b) 534 nm after mixing Rho-DEA with 100 equiv. various amino acids in 30 min. Probe concentration 10: µmol/L, PB buffer: 0.2 mol/L, pH 7.4. (c) Dual-color confocal imaging of HeLa cells cultured with 0.5 µmol/L probe Rho-DEA for 4 min and (d) fluorescence intensity changes during this process. (e) Co-localization imaging of HeLa cells cultured with 0.5 µmol/L Rho-DEA for 2 h and then with Mito 549. (f, g) Fluorescence signal distribution and the Pearson's coefficient in (e).The Rho-DEA can target and enrich in mitochondria selectively because of the cationic rhodamine scaffold. It has been shown that the accumulation of cationic compounds in mitochondria can reach the millimole level concentration [33, 34]. In our case, Rho-DEA can fast target to mitochondria and accumulate to a high concentration, triggering the localized reaction with GSH in mitochondria both at millimolar level. At such high concentration, the conversion from Rho-S(GSH) to Rho-N(GSH) by the attack of free GSH would occur rapidly, meanwhile, the effect of other biothiols can be negligible due to their low concentration.
We incubated HeLa cells with Rho-DEA and tracked the fluorescence changes immediately. As shown in Figs. 4c and d, the fluorescent signals in red channel and green channel appeared rapidly after adding Rho-DEA to cell culture medium. The fluorescence intensity in red channel exhibited increasing first and then decreased, finally quenched completely after 100 min, while the fluorescence intensity of green channel continued to increase until achieving a maximum, which was completely consistent with the phenomenon of the probe reacted with GSH in vitro. It can be seen that the product of the reaction between Rho-DEA and GSH was prominent in mitochondria. By colocalization experiments with the commercial mitochondria probe Mito-549, it is observed that Rho-N (GSH) was mainly distributed in mitochondria, the fluorescence signals of two probes were well overlapped with a Pearson's coeff. of 0.93 (Figs. 4e–g). In addition, the changes of fluorescent signal during the incubation of Rho-DEA also reflected the redox states in mitochondria.
In conclusion, we report a TICS probe, Rho-DEA, which selectively recognizes GSH with dual-color fluorogenicity, which can be used in the tracking of glutathione dynamics and redox balance dynamics in cellular mitochondria.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgment
This work is supported by the National Natural Science Foundation of China (Nos. 22078314, 21878286, 21908216).
Supplementary materials
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.cclet.2022.03.121.
References
-
[1]
[1] (a) X.S. Jia, K. Yin, C. Li, J. Li, H.S. Bian, Copper-catalyzed oxidative alkyne homocoupling without palladium, ligands and bases, Green Chem. 13 (2011) 2175-2178;
-
[2]
(b) M.L. Lerch, M.K. Harper, D.J. Faulkner, Brominated polyacetylenes from the philippines sponge diplastrella sp, J. Nat. Prod. 66 (2003) 667-670;
-
[3]
(c) D. Lechner, M. Stavri, M. Oluwatuyi, R. Perda-Miranda, S. Gibbons, The antistaphylococcal activity of angelica dahurica, Phytochemistry 65 (2004) 331-335;
-
[4]
(d) Y.Z. Zhou, H.Y. Ma, H. Chen, et al., New acetylenic glucosides from carthamus tinctorius, Chem. Pharm. Bull. 54 (2006) 1455-1456;
-
[5]
(e) M. Ladika, T.E. Fisk, W.W. Wu, S.D. Jons, High-stability liposomes from macrocyclic diyne phospholipids, J. Am. Chem. Soc. 116 (1994) 12093-12094;
-
[6]
(f) S.F. Mayer, A. Steinreiber, R.V.A. Orru, K. Faber, Chemoenzymatic asymmetric total syntheses of antitumor agents (3R,9R,10R)-and (3S,9R,10R)-panaxytriol and (R)-and (S)-falcarinol from panax ginseng using an enantioconvergent enzymetriggered cascade reaction, J. Org. Chem. 67 (2002) 9115-9121;
-
[7]
(g) G. Zeni, R.B. Panatieri, E. Lissner, et al., Synthesis of polyacetylenic acids isolated from heisteria acuminata, Org. Lett. 3 (2001) 819-821;
-
[8]
(h) A. Stüts, Allylamine derivatives—a new class of active substances in antifungal chemotherapy, Angew. Chem. Int. Ed. Engl. 26 (1987) 320-328;
-
[9]
(i) Q. Yang, L.C. Li, S.H. Li, D.M. Yue, C.H. Xu, Synthesis and photophysical properties of poly(aryleneethnylene)s containing dibenzosilole unit, Chin. Chem. Lett. 23 (2012) 1303-1306.
-
[10]
[2] M. Gholami, R.R. Tykwinski, Oligomeric and polymeric systems with a crossconjugated π-framework, Chem. Rev. 106 (2006) 4997-5027.
-
[11]
[3] (a) P.N.W. Baxter, R. Dali-Youcef, Nitrogen heterocyclic carbon-rich materials: synthesis and spectroscopic properties of dehydropyridoannulene macrocycles, J. Org. Chem. 70 (2005) 4935-4953;
-
[12]
(b) J.A. Marsden, M.M. Haley, Carbon networks based on dehydrobenzoannulenes. 5. Extension of two-dimensional conjugation in graphdiyne nanoarchitectures, J. Org. Chem. 70 (2005) 10213-10226.
-
[13]
[4] J.D. Crowley, S.M. Goldup, A.L. Lee, D.A. Leigh, R.T. McBurney, Active metal template synthesis of rotaxanes, catenanes and molecular shuttles, Chem. Soc. Rev. 38 (2009) 1530-1541.
-
[14]
[5] (a) P. Siemsen, R.C. Livingston, F. Diederich, Acetylenic coupling: a powerful tool in molecular construction, Angew. Chem. Int. Ed. 39 (2000) 2632-2657;
-
[15]
(b) M.M. Haley, Synthesis and properties of annulenic subunits of graphyne and graphdiyne nanoarchitectures, Pure Appl. Chem. 80 (2008) 519-532;
-
[16]
(c) A.S. Hay, Oxidative coupling of acetylenes, J. Org. Chem. 27 (1962) 3320-3321;
-
[17]
(d) E. Valenti, M.A. Pericas, F. Serratosa, 1,4-Dialkoxy-1,3-butadiynes, J. Am. Chem. Soc. 112 (1990) 7405-7746;
-
[18]
(e) Y. Liao, R. Fathi, Z. Yang, Aliphatic acetylenic homocoupling catalyzed by a novel combination of AgOTs-CuCl2-TMEDA and its application for the solid-phase synthesis of bis-benzo[b]furan-linked 1,3-diynes, Org. Lett. 5 (2003) 909-912;
-
[19]
(f) T. Oishi, T. Katayama, K. Yamaguchi, N. Mizuno, Heterogeneously catalyzed efficient alkyne-alkyne homocoupling by supported copper hydroxide on titanium oxide, Chem. Eur. J. 15 (2009) 7539-7542;
-
[20]
(g) S. Adimurthy, C.C. Malakar, U. Beifuss, Influence of bases and ligands on the outcome of the Cu(i)-catalyzed oxidative homocoupling of terminal alkynes to 1,4-disubstituted 1,3-diynes using oxygen as an oxidant, J. Org. Chem. 74 (2009) 5648-5651;
-
[21]
(h) G. Eglington, R. Galbraith, Macrocyclic acetylenic compounds. Part I. Cyclotetradeca-1,3-diyne and related compounds, J. Chem. Soc. (1959) 889-896;
-
[22]
(i) K. Sonogashira, Y. Tohda, N. Hagihara, A convenient synthesis of acetylenes: catalytic substitutions of acetylenic hydrogen with bromoalkenes, iodoarenes and bromopyridines, Tetrahedron Lett. 50 (1975) 4467-4470;
-
[23]
(j) J. Yan, J. Wu, H. Jin, An efficient synthesis of diynes using (diacetoxyiodo)-benzene, J. Organomet. Chem. 692 (2007) 3636-3639;
-
[24]
(k) Q. Zheng, R. Hua, Y. Wan, An alternative CuCl-piperidine-catalyzed oxidative homocoupling of terminal alkynes affording 1,3-diynes in air, Appl. Organomet. Chem. 24 (2010) 314-316;
-
[25]
(l) P. Kuhn, A. Alix, M. Kumarraja, B. Louis, P. Pale, J. Sommer, Copper-zeolites as catalysts for the coupling of terminal alkynes: an efficient synthesis of diynes, Eur. J. Org. Chem. (2009) 423-429;
-
[26]
(m) A.L.K. Shi Shun, R.R. Tykwinski, Synthesis of naturally occurring polyynes, Angew. Chem. Int. Ed. 45 (2006) 1034-1057;
-
[27]
(n) Z. Chen, H. Jiang, A. Wang, S. Yang, Transition-metal-free homocoupling of 1-haloalkynes: a facile synthesis of symmetrical 1,3-diynes, J. Org. Chem. 75 (2010) 6700-6703;
-
[28]
(o) H.A. Stefani, A.S. Guarezemini, R. Cella, Homocoupling reactions of alkynes, alkenes and alkyl compounds, Tetrahedron 66 (2010) 7871-7918;
-
[29]
(p) K. Kamata, S. Yamaguchi, M. Kotani, K. Yamaguchi, N. Mizuno, Efficient oxidative alkyne homocoupling catalyzed by a monomeric dicopper-substituted silicotungstate, Angew. Chem. Int. Ed. 47 (2008) 2407-2410;
-
[30]
(q) J.D. Crowley, S.M. Goldup, N.D. Gowans, et al., An unusual nickel-coppermediated alkyne homocoupling reaction for the active-template synthesis of[2]rotaxanes, J. Am. Chem. Soc. 132 (2010) 6243-6248;
-
[31]
(r) K. Homo-Balaraman, V. Kesavan, Efficient copper(ii) acetate catalyzed homoand heterocoupling of terminal alkynes at ambient conditions, Synthesis 20 (2010) 3461-3466;
-
[32]
(s) A. Coste, F. Couty, G. Evano, Copper-mediated homocoupling of vinyl dibromides to symmetrical diynes, Synthesis 9 (2010) 1500-1504;
-
[33]
(t) S.L. Zhang, X.Y. Liu, T.Q. Wang, An efficient copper-catalyzed homocoupling of terminal alkynes to give symmetrical 1,4-disubstituted 1,3-diynes, Adv. Synth. Catal. 353 (2011) 1463-1466;
-
[34]
(u) S.N. Chen, W.Y. Wu, F.Y. Tsai, Homocoupling reaction of terminal alkynes catalyzed by a reusable cationic 2,2'-bipyridyl palladium(Ⅱ)/CuI system in water, Green Chem. 11 (2009) 269-274;
-
[35]
(v) K. Yin, C.J. Li, J. Li, X.S. Jia, CuCl-catalyzed green oxidative alkyne homocoupling without palladium, ligands and bases, Green Chem. 13 (2011) 591-593.
-
[36]
[6] C. Glaser, Beiträge zur Kenntnis des Acetenylbenzols, Ber. Dtsch. Chem. Ges. 2 (1869) 422-424.
-
[37]
[7] (a) C.X. Kuang, H. Senboku, M. Tokuda, Convenient and stereoselective synthesis of (Z)-1-bromo-1-alkenes by microwave-induced reaction, Tetrahedron Lett. 42 (2001) 3893-3896;
-
[38]
(b) W.S. Zhang, C.X. Kuang, Q. Yang, Stereoselective synthesis of (Z)-4-(2-bromovinyl) benzenesulfonyl azide and its synthetic utility for the transformation to (Z)-N-[4-(2-bromovinyl)benzenesulfonyl]imidates, Chin. J. Chem. 27 (2009) 1727-1732;
-
[39]
(c) W.S. Zhang, C.X. Kuang, Q. Yang, One-pot synthesis of (Z)-4-(2-bromovinyl) arylsulfonamides by microwave-induced simultaneous debrominative decarboxylation and sulfamation of anti-3-(4-chlorosulfonylbenzyl)-2,3-dibromopropanoic acid, Chin. Chem. Lett. 20 (2009) 48-51.
-
[40]
[8] P. Espinet, A.M. Echavarren, The mechanisms of the stille reaction, Angew. Chem. Int. Ed. 43 (2004) 4704-4734, and references cited therein.
-
[41]
[9] N. Miyaura, A. Suzuki, Palladium-catalyzed cross-coupling reactions of organoboron compounds, Chem. Rev. 95 (1995) 2457-2483.
-
[42]
[10] E. Negishi, L. Anastasia, Palladium-catalyzed alkynylation, Chem. Rev. 103 (2003) 1979-2018.
-
[43]
[11] L. Jiang, G.E. Job, A. Klapars, S.L. Buchwald, Copper-catalyzed coupling of amides and carbamates with vinyl halides, Org. Lett. 5 (2003) 3667-3669.
-
[44]
[12] Q. Liao, Y.X. Wang, L.Y. Zhang, C.J. Xi, A general copper-catalyzed coupling of azoles with vinyl bromides, J. Org. Chem. 74 (2009) 6371-6373.
-
[45]
[13] (a) C.X. Kuang, H. Senboku, M. Tokuda, A one-pot synthesis of terminal alkynes from anti-3-aryl-2,3-dibromopropanoic acids under microwave irradiation, Chem. Lett. 34 (2005) 28-29;
-
[46]
(b) W.S. Zhang, C.X. Kuang, Q. Yang, Efficient one-pot synthesis of 4-ethynylbenzenesulfonamides, Z. Naturforsch. B 64b (2009) 292-296;
-
[47]
(c) Y.B. Jiang, C.X. Kuang, Synthesis of 1,2,3-triazole derivatives, Prog. Chem. 24 (2012) 1983-1994.
-
[48]
[14] J. Hui, C.X. Kuang, Ligand-free copper-catalyzed synthesis of symmetrical diynes from 1,1-dibromo-1-alkenes, J. Chin. Chem. 29 (2011) 592-594.
-
[49]
[15] J.C. Yan, L. Wang, First example of transition-metal-free glaser-type coupling reaction, Synth. Commun. 35 (2005) 2333-2338.
-
[1]
-
-
[1]
[1] (a) X.S. Jia, K. Yin, C. Li, J. Li, H.S. Bian, Copper-catalyzed oxidative alkyne homocoupling without palladium, ligands and bases, Green Chem. 13 (2011) 2175-2178;
-
[2]
(b) M.L. Lerch, M.K. Harper, D.J. Faulkner, Brominated polyacetylenes from the philippines sponge diplastrella sp, J. Nat. Prod. 66 (2003) 667-670;
-
[3]
(c) D. Lechner, M. Stavri, M. Oluwatuyi, R. Perda-Miranda, S. Gibbons, The antistaphylococcal activity of angelica dahurica, Phytochemistry 65 (2004) 331-335;
-
[4]
(d) Y.Z. Zhou, H.Y. Ma, H. Chen, et al., New acetylenic glucosides from carthamus tinctorius, Chem. Pharm. Bull. 54 (2006) 1455-1456;
-
[5]
(e) M. Ladika, T.E. Fisk, W.W. Wu, S.D. Jons, High-stability liposomes from macrocyclic diyne phospholipids, J. Am. Chem. Soc. 116 (1994) 12093-12094;
-
[6]
(f) S.F. Mayer, A. Steinreiber, R.V.A. Orru, K. Faber, Chemoenzymatic asymmetric total syntheses of antitumor agents (3R,9R,10R)-and (3S,9R,10R)-panaxytriol and (R)-and (S)-falcarinol from panax ginseng using an enantioconvergent enzymetriggered cascade reaction, J. Org. Chem. 67 (2002) 9115-9121;
-
[7]
(g) G. Zeni, R.B. Panatieri, E. Lissner, et al., Synthesis of polyacetylenic acids isolated from heisteria acuminata, Org. Lett. 3 (2001) 819-821;
-
[8]
(h) A. Stüts, Allylamine derivatives—a new class of active substances in antifungal chemotherapy, Angew. Chem. Int. Ed. Engl. 26 (1987) 320-328;
-
[9]
(i) Q. Yang, L.C. Li, S.H. Li, D.M. Yue, C.H. Xu, Synthesis and photophysical properties of poly(aryleneethnylene)s containing dibenzosilole unit, Chin. Chem. Lett. 23 (2012) 1303-1306.
-
[10]
[2] M. Gholami, R.R. Tykwinski, Oligomeric and polymeric systems with a crossconjugated π-framework, Chem. Rev. 106 (2006) 4997-5027.
-
[11]
[3] (a) P.N.W. Baxter, R. Dali-Youcef, Nitrogen heterocyclic carbon-rich materials: synthesis and spectroscopic properties of dehydropyridoannulene macrocycles, J. Org. Chem. 70 (2005) 4935-4953;
-
[12]
(b) J.A. Marsden, M.M. Haley, Carbon networks based on dehydrobenzoannulenes. 5. Extension of two-dimensional conjugation in graphdiyne nanoarchitectures, J. Org. Chem. 70 (2005) 10213-10226.
-
[13]
[4] J.D. Crowley, S.M. Goldup, A.L. Lee, D.A. Leigh, R.T. McBurney, Active metal template synthesis of rotaxanes, catenanes and molecular shuttles, Chem. Soc. Rev. 38 (2009) 1530-1541.
-
[14]
[5] (a) P. Siemsen, R.C. Livingston, F. Diederich, Acetylenic coupling: a powerful tool in molecular construction, Angew. Chem. Int. Ed. 39 (2000) 2632-2657;
-
[15]
(b) M.M. Haley, Synthesis and properties of annulenic subunits of graphyne and graphdiyne nanoarchitectures, Pure Appl. Chem. 80 (2008) 519-532;
-
[16]
(c) A.S. Hay, Oxidative coupling of acetylenes, J. Org. Chem. 27 (1962) 3320-3321;
-
[17]
(d) E. Valenti, M.A. Pericas, F. Serratosa, 1,4-Dialkoxy-1,3-butadiynes, J. Am. Chem. Soc. 112 (1990) 7405-7746;
-
[18]
(e) Y. Liao, R. Fathi, Z. Yang, Aliphatic acetylenic homocoupling catalyzed by a novel combination of AgOTs-CuCl2-TMEDA and its application for the solid-phase synthesis of bis-benzo[b]furan-linked 1,3-diynes, Org. Lett. 5 (2003) 909-912;
-
[19]
(f) T. Oishi, T. Katayama, K. Yamaguchi, N. Mizuno, Heterogeneously catalyzed efficient alkyne-alkyne homocoupling by supported copper hydroxide on titanium oxide, Chem. Eur. J. 15 (2009) 7539-7542;
-
[20]
(g) S. Adimurthy, C.C. Malakar, U. Beifuss, Influence of bases and ligands on the outcome of the Cu(i)-catalyzed oxidative homocoupling of terminal alkynes to 1,4-disubstituted 1,3-diynes using oxygen as an oxidant, J. Org. Chem. 74 (2009) 5648-5651;
-
[21]
(h) G. Eglington, R. Galbraith, Macrocyclic acetylenic compounds. Part I. Cyclotetradeca-1,3-diyne and related compounds, J. Chem. Soc. (1959) 889-896;
-
[22]
(i) K. Sonogashira, Y. Tohda, N. Hagihara, A convenient synthesis of acetylenes: catalytic substitutions of acetylenic hydrogen with bromoalkenes, iodoarenes and bromopyridines, Tetrahedron Lett. 50 (1975) 4467-4470;
-
[23]
(j) J. Yan, J. Wu, H. Jin, An efficient synthesis of diynes using (diacetoxyiodo)-benzene, J. Organomet. Chem. 692 (2007) 3636-3639;
-
[24]
(k) Q. Zheng, R. Hua, Y. Wan, An alternative CuCl-piperidine-catalyzed oxidative homocoupling of terminal alkynes affording 1,3-diynes in air, Appl. Organomet. Chem. 24 (2010) 314-316;
-
[25]
(l) P. Kuhn, A. Alix, M. Kumarraja, B. Louis, P. Pale, J. Sommer, Copper-zeolites as catalysts for the coupling of terminal alkynes: an efficient synthesis of diynes, Eur. J. Org. Chem. (2009) 423-429;
-
[26]
(m) A.L.K. Shi Shun, R.R. Tykwinski, Synthesis of naturally occurring polyynes, Angew. Chem. Int. Ed. 45 (2006) 1034-1057;
-
[27]
(n) Z. Chen, H. Jiang, A. Wang, S. Yang, Transition-metal-free homocoupling of 1-haloalkynes: a facile synthesis of symmetrical 1,3-diynes, J. Org. Chem. 75 (2010) 6700-6703;
-
[28]
(o) H.A. Stefani, A.S. Guarezemini, R. Cella, Homocoupling reactions of alkynes, alkenes and alkyl compounds, Tetrahedron 66 (2010) 7871-7918;
-
[29]
(p) K. Kamata, S. Yamaguchi, M. Kotani, K. Yamaguchi, N. Mizuno, Efficient oxidative alkyne homocoupling catalyzed by a monomeric dicopper-substituted silicotungstate, Angew. Chem. Int. Ed. 47 (2008) 2407-2410;
-
[30]
(q) J.D. Crowley, S.M. Goldup, N.D. Gowans, et al., An unusual nickel-coppermediated alkyne homocoupling reaction for the active-template synthesis of[2]rotaxanes, J. Am. Chem. Soc. 132 (2010) 6243-6248;
-
[31]
(r) K. Homo-Balaraman, V. Kesavan, Efficient copper(ii) acetate catalyzed homoand heterocoupling of terminal alkynes at ambient conditions, Synthesis 20 (2010) 3461-3466;
-
[32]
(s) A. Coste, F. Couty, G. Evano, Copper-mediated homocoupling of vinyl dibromides to symmetrical diynes, Synthesis 9 (2010) 1500-1504;
-
[33]
(t) S.L. Zhang, X.Y. Liu, T.Q. Wang, An efficient copper-catalyzed homocoupling of terminal alkynes to give symmetrical 1,4-disubstituted 1,3-diynes, Adv. Synth. Catal. 353 (2011) 1463-1466;
-
[34]
(u) S.N. Chen, W.Y. Wu, F.Y. Tsai, Homocoupling reaction of terminal alkynes catalyzed by a reusable cationic 2,2'-bipyridyl palladium(Ⅱ)/CuI system in water, Green Chem. 11 (2009) 269-274;
-
[35]
(v) K. Yin, C.J. Li, J. Li, X.S. Jia, CuCl-catalyzed green oxidative alkyne homocoupling without palladium, ligands and bases, Green Chem. 13 (2011) 591-593.
-
[36]
[6] C. Glaser, Beiträge zur Kenntnis des Acetenylbenzols, Ber. Dtsch. Chem. Ges. 2 (1869) 422-424.
-
[37]
[7] (a) C.X. Kuang, H. Senboku, M. Tokuda, Convenient and stereoselective synthesis of (Z)-1-bromo-1-alkenes by microwave-induced reaction, Tetrahedron Lett. 42 (2001) 3893-3896;
-
[38]
(b) W.S. Zhang, C.X. Kuang, Q. Yang, Stereoselective synthesis of (Z)-4-(2-bromovinyl) benzenesulfonyl azide and its synthetic utility for the transformation to (Z)-N-[4-(2-bromovinyl)benzenesulfonyl]imidates, Chin. J. Chem. 27 (2009) 1727-1732;
-
[39]
(c) W.S. Zhang, C.X. Kuang, Q. Yang, One-pot synthesis of (Z)-4-(2-bromovinyl) arylsulfonamides by microwave-induced simultaneous debrominative decarboxylation and sulfamation of anti-3-(4-chlorosulfonylbenzyl)-2,3-dibromopropanoic acid, Chin. Chem. Lett. 20 (2009) 48-51.
-
[40]
[8] P. Espinet, A.M. Echavarren, The mechanisms of the stille reaction, Angew. Chem. Int. Ed. 43 (2004) 4704-4734, and references cited therein.
-
[41]
[9] N. Miyaura, A. Suzuki, Palladium-catalyzed cross-coupling reactions of organoboron compounds, Chem. Rev. 95 (1995) 2457-2483.
-
[42]
[10] E. Negishi, L. Anastasia, Palladium-catalyzed alkynylation, Chem. Rev. 103 (2003) 1979-2018.
-
[43]
[11] L. Jiang, G.E. Job, A. Klapars, S.L. Buchwald, Copper-catalyzed coupling of amides and carbamates with vinyl halides, Org. Lett. 5 (2003) 3667-3669.
-
[44]
[12] Q. Liao, Y.X. Wang, L.Y. Zhang, C.J. Xi, A general copper-catalyzed coupling of azoles with vinyl bromides, J. Org. Chem. 74 (2009) 6371-6373.
-
[45]
[13] (a) C.X. Kuang, H. Senboku, M. Tokuda, A one-pot synthesis of terminal alkynes from anti-3-aryl-2,3-dibromopropanoic acids under microwave irradiation, Chem. Lett. 34 (2005) 28-29;
-
[46]
(b) W.S. Zhang, C.X. Kuang, Q. Yang, Efficient one-pot synthesis of 4-ethynylbenzenesulfonamides, Z. Naturforsch. B 64b (2009) 292-296;
-
[47]
(c) Y.B. Jiang, C.X. Kuang, Synthesis of 1,2,3-triazole derivatives, Prog. Chem. 24 (2012) 1983-1994.
-
[48]
[14] J. Hui, C.X. Kuang, Ligand-free copper-catalyzed synthesis of symmetrical diynes from 1,1-dibromo-1-alkenes, J. Chin. Chem. 29 (2011) 592-594.
-
[49]
[15] J.C. Yan, L. Wang, First example of transition-metal-free glaser-type coupling reaction, Synth. Commun. 35 (2005) 2333-2338.
-
[1]
-

-
-
[1]
Fangling Cui , Zongjie Hu , Jiayu Huang , Xiaoju Li , Ruihu Wang . MXene-based materials for separator modification of lithium-sulfur batteries. Chinese Journal of Structural Chemistry, 2024, 43(7): 100337-100337. doi: 10.1016/j.cjsc.2024.100337
-
[2]
Liangfeng Yang , Liang Zeng , Yanping Zhu , Qiuan Wang , Jinheng Li . Copper-catalyzed photoredox 1,4-amidocyanation of 1,3-enynes with N-amidopyridin-1-ium salts and TMSCN: Facile access to α-amido allenyl nitriles. Chinese Chemical Letters, 2024, 35(11): 109685-. doi: 10.1016/j.cclet.2024.109685
-
[3]
Fengxing Liang , Yongzheng Zhu , Nannan Wang , Meiping Zhu , Huibing He , Yanqiu Zhu , Peikang Shen , Jinliang Zhu . Recent advances in copper-based materials for robust lithium polysulfides adsorption and catalytic conversion. Chinese Chemical Letters, 2024, 35(11): 109461-. doi: 10.1016/j.cclet.2023.109461
-
[4]
Mengxiang Zhu , Tao Ding , Yunzhang Li , Yuanjie Peng , Ruiping Liu , Quan Zou , Leilei Yang , Shenglei Sun , Pin Zhou , Guosheng Shi , Dongting Yue . Graphene controlled solid-state growth of oxygen vacancies riched V2O5 catalyst to highly activate Fenton-like reaction. Chinese Chemical Letters, 2024, 35(12): 109833-. doi: 10.1016/j.cclet.2024.109833
-
[5]
Shuai Li , Liuting Zhang , Fuying Wu , Yiqun Jiang , Xuebin Yu . Efficient catalysis of FeNiCu-based multi-site alloys on magnesium-hydride for solid-state hydrogen storage. Chinese Chemical Letters, 2025, 36(1): 109566-. doi: 10.1016/j.cclet.2024.109566
-
[6]
Jiajun Lu , Zhehui Liao , Tongxiang Cao , Shifa Zhu . Synergistic Brønsted/Lewis acid catalyzed atroposelective synthesis of aryl-β-naphthol. Chinese Chemical Letters, 2025, 36(1): 109842-. doi: 10.1016/j.cclet.2024.109842
-
[7]
Gangsheng Li , Xiang Yuan , Fu Liu , Zhihua Liu , Xujie Wang , Yuanyuan Liu , Yanmin Chen , Tingting Wang , Yanan Yang , Peicheng Zhang . Three-step synthesis of flavanostilbenes with a 2-cyclohepten-1-one core by Cu-mediated [5 + 2] cycloaddition/decarboxylation cascade. Chinese Chemical Letters, 2025, 36(2): 109880-. doi: 10.1016/j.cclet.2024.109880
-
[8]
Xiao-Hong Yi , Chong-Chen Wang . Metal-organic frameworks on 3D interconnected macroporous sponge foams for large-scale water decontamination: A mini review. Chinese Chemical Letters, 2024, 35(5): 109094-. doi: 10.1016/j.cclet.2023.109094
-
[9]
Haodong Wang , Xiaoxu Lai , Chi Chen , Pei Shi , Houzhao Wan , Hao Wang , Xingguang Chen , Dan Sun . Novel 2D bifunctional layered rare-earth hydroxides@GO catalyst as a functional interlayer for improved liquid-solid conversion of polysulfides in lithium-sulfur batteries. Chinese Chemical Letters, 2024, 35(5): 108473-. doi: 10.1016/j.cclet.2023.108473
-
[10]
Longlong Geng , Huiling Liu , Wenfeng Zhou , Yong-Zheng Zhang , Hongliang Huang , Da-Shuai Zhang , Hui Hu , Chao Lv , Xiuling Zhang , Suijun Liu . Construction of metal-organic frameworks with unsaturated Cu sites for efficient and fast reduction of nitroaromatics: A combined experimental and theoretical study. Chinese Chemical Letters, 2024, 35(8): 109120-. doi: 10.1016/j.cclet.2023.109120
-
[11]
Manoj Kumar Sarangi , L․D Patel , Goutam Rath , Sitansu Sekhar Nanda , Dong Kee Yi . Metal organic framework modulated nanozymes tailored with their biomedical approaches. Chinese Chemical Letters, 2024, 35(11): 109381-. doi: 10.1016/j.cclet.2023.109381
-
[12]
Haijiang Gong , Qingtan Zeng , Shili Gai , Yaqian Du , Jing Zhang , Qingyu Wang , He Ding , Lichun Wu , Anees Ahmad Ansari , Piaoping Yang . Enzyme-based colorimetric signal amplification strategy in lateral flow immunoassay. Chinese Chemical Letters, 2025, 36(5): 110059-. doi: 10.1016/j.cclet.2024.110059
-
[13]
Junxin Li , Chao Chen , Yuzhen Dong , Jian Lv , Jun-Mei Peng , Yuan-Ye Jiang , Daoshan Yang . Ligand-promoted reductive coupling between aryl iodides and cyclic sulfonium salts by nickel catalysis. Chinese Chemical Letters, 2024, 35(11): 109732-. doi: 10.1016/j.cclet.2024.109732
-
[14]
Ruilong Geng , Lingzi Peng , Chang Guo . Dynamic kinetic stereodivergent transformations of propargylic ammonium salts via dual nickel and copper catalysis. Chinese Chemical Letters, 2024, 35(8): 109433-. doi: 10.1016/j.cclet.2023.109433
-
[15]
Rong-Nan Yi , Wei-Min He . Visible light/copper catalysis enabled radial type ring-opening of sulfonium salts. Chinese Chemical Letters, 2025, 36(4): 110787-. doi: 10.1016/j.cclet.2024.110787
-
[16]
Yuhao Guo , Na Li , Tingjiang Yan . Tandem catalysis for photoreduction of CO2 into multi-carbon fuels on atomically thin dual-metal phosphochalcogenides. Chinese Journal of Structural Chemistry, 2024, 43(7): 100320-100320. doi: 10.1016/j.cjsc.2024.100320
-
[17]
Jieshuai Xiao , Yuan Zheng , Yue Zhao , Zhuangzhi Shi , Minyan Wang . Asymmetric Nozaki-Hiyama-Kishi (NHK)-type reaction of isatins with aromatic iodides by cobalt catalysis. Chinese Chemical Letters, 2025, 36(5): 110243-. doi: 10.1016/j.cclet.2024.110243
-
[18]
Baokang Geng , Xiang Chu , Li Liu , Lingling Zhang , Shuaishuai Zhang , Xiao Wang , Shuyan Song , Hongjie Zhang . High-efficiency PdNi single-atom alloy catalyst toward cross-coupling reaction. Chinese Chemical Letters, 2024, 35(7): 108924-. doi: 10.1016/j.cclet.2023.108924
-
[19]
Mengli Xu , Zhenmin Xu , Zhenfeng Bian . Achieving Ullmann coupling reaction via photothermal synergy with ultrafine Pd nanoclusters supported on mesoporous TiO2. Chinese Journal of Structural Chemistry, 2024, 43(7): 100305-100305. doi: 10.1016/j.cjsc.2024.100305
-
[20]
Kai An , Qinglong Qiao , Lovelesh , Syed Ali Abbas Abedi , Xiaogang Liu , Zhaochao Xu . "Superimposed" spectral characteristics of fluorophores arising from cross-conjugation hybridization. Chinese Chemical Letters, 2025, 36(1): 109786-. doi: 10.1016/j.cclet.2024.109786
-
[1]
Metrics
- PDF Downloads(0)
- Abstract views(1035)
- HTML views(32)

 Login In
Login In




 DownLoad:
DownLoad:
 DownLoad:
DownLoad: