Citation:
Chun-Hua Ge, Rui Zhang, Ping Fan, Xiang-Dong Zhang, Li-Juan Wang, Fang-Fang Wang. Supramolecular assembly of 2,4,5-trifluorobenzoate complex based on weak interactions involving fluorine atoms[J]. Chinese Chemical Letters,
;2013, 24(01): 73-75.

-
The complex[Cd(tfbz)2(phen)]2(1)(tfbz=2,4,5-trifluorobenzoate,phen=1,10-phenanthro-line)was synthesized using trifluorobenzoic acid ligand.The single-crystal structure of 1 has been determined by X-ray crystallography.The packing structure is characterized by the formation of an intricate three-dimensional supramolecular network that depends on the C-H…F,F…F,F(lp)…π(lp=lone pair) interactions.
-
Keywords:
- 2,4,5-Trifluorobenzoate,
- Crystal structure,
- Weak interaction
-
Attribute to high power density, and long cycle life, electric double-layer capacitors (EDLCs) or supercapacitors (SCs) are becoming very promising in the fields that need high power output, such as electric vehicles, and military equipment [1-4]. Nowadays, most electrolytes for commercial SCs use organic solvents such as acetonitrile (AN) for the wider electrochemical stability voltage window which offers a higher energy density and better cycle stability [5, 6]. However, the ionic conductivity of the organic electrolyte is not satisfied, which leads to the relatively high equivalent series resistance (ESR) and low power density of the SCs. Also, most organic electrolytes are poisonous, flammable, expensive, and ecologically unfriendly. Moreover, organic electrolytes need specific, severe manufacturing conditions (strict waterfree and oxygen-free conditions). As a contrast, aqueous electrolytes are nonflammable, nontoxic, environmentally friendly, and low-costed. Their high ionic conductivity and small ion size endow aqueous SCs with excellent electrochemical performances (high specific capacitance, low ESR, and high power density), which fulfill the needs of future development of SCs [7, 8].
Nevertheless, aqueous electrolytes have a narrow electrochemical window (most of them ≤ 1.23 V) [9], which limits the energy density and the practical applications of aqueous SCs. Therefore, many research focused on improving the electrochemical stability of aqueous electrolytes. The neutral salt solutions such as Li2SO4 [10, 11], Na2SO4 [12, 13], and KCl [14] were proved to be able to run at higher voltages than aqueous acidic or alkali electrolytes. However, subject to the strong hydration of the ions, the rate performances of SCs using these neutral salts solutions are not satisfying [15]. Most recently, a kind of in situ electrodeposited poly (vinyl alcohol) potassium borate (PVAPB) hydrogel electrolyte (HGE) with excellent electrochemical stability was developed [16, 17]. It showed not only high operation voltage (2 V) but also excellent rate performance in the symmetric activated carbon (AC) SCs. The HGE structure also avoided the weaknesses of liquid aqueous electrolytes (internal corrosion, leaking, and complicate packaging), and made it possible to build flexible SCs.
Besides the electrochemical performance, the temperature stability was another important property of electrolytes, which directly affected the operating temperature range of SCs. The liquid electrolytes would be inactive when their solvents began to freeze or boil. Therefore, the operating temperature range of liquid electrolytes was mainly determined by the solvents they used [18].The freezing point of water is higher than those of most organic solvents, so the low-temperature stability of most aqueous electrolytes are inferior to those of organic electrolytes [8]. As to the HGEs, the states of water in them are not the same as those of water in liquid aqueous electrolytes, and some polymer gel skeletons are not stable in certain temperature ranges, so their temperature failure mechanisms might be different from those of normal liquid aqueous electrolytes [19].
As a new high voltage HGEs for SCs, the PVAPB HGE presents an exceptional ionic transport mechanism, and the water molecules in PVAPB HGE was speculated to ionize and restore during charging and discharging processes of SCs [17]. Thus, the temperature stability of PVAPB HGE might be quite different from those of liquid aqueous electrolytes. In this study, the AC/PVAPB HGE/AC SCs were fabricated and tested at various temperatures (from -5 ℃ to 80 ℃). The results were compared with the SCs using liquid aqueous electrolytes to examine the temperature stability of PVAPB HGEs.
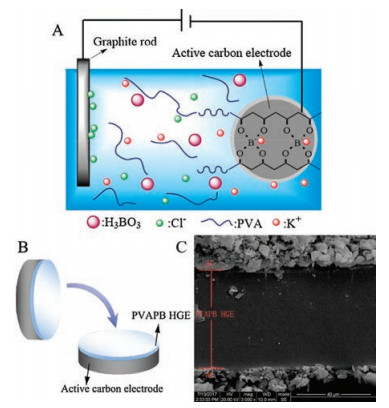
The AC/PVAPB HGE/AC SCs were fabricated according to the following steps. First, PVA (10 g), boric acid (1.75 g), KCl (33.55 g, 0.45 mol) were dissolved in about 450 mL deionized water at 95 ℃. Then the solution was cooling down to 40 ℃, and additional deionized water was added to form 500 mL electrodeposition solutions with 0.9 mol/L of K+ ions. Then the AC electrodes were immersed in this electrodeposition solution, and cathodic electrodeposited with graphite rode anode at 2.75 V for 3 min. As a result, PVAPB HGEs were prepared on the surface of AC electrodes, as shown in Fig. 1A. After electrodeposition, the surfaces of wet PVAPB HGEs were treated with dry filter paper to remove excessive electrodeposition solution. Finally, AC electrodes covered with PVAPB HGEs were assembled into CR 2032 coin-type cell to build symmetric SCs. During the cell assembling, the two working electrodes were placed with the sides covered with PVAPB HGEs facing each other. No additional separator or liquid electrolyte was added during cell assembling, as shown in Fig. 1B. For comparison, the SCs control samples were prepared by using 0.9 mol/L KCl aqueous solution (the same KCl concentration was used in preparing the PVAPB HGEs) and 1 mol/L Na2SO4 aqueous solution (the widely used electrolyte in aqueous high-voltage SCs) as the liquid electrolytes and glass microfiber membrane as the separator.
图 1
 图 1 (A) Schematic of in situ electrodeposition of PVAPB HGE on AC electrode. (B) Schematic of fabrication of symmetric SCs. (C) SEM image of the cross-section of PVAPB HGE layer.Figure 1. (A) Schematic of in situ electrodeposition of PVAPB HGE on AC electrode. (B) Schematic of fabrication of symmetric SCs. (C) SEM image of the cross-section of PVAPB HGE layer.
图 1 (A) Schematic of in situ electrodeposition of PVAPB HGE on AC electrode. (B) Schematic of fabrication of symmetric SCs. (C) SEM image of the cross-section of PVAPB HGE layer.Figure 1. (A) Schematic of in situ electrodeposition of PVAPB HGE on AC electrode. (B) Schematic of fabrication of symmetric SCs. (C) SEM image of the cross-section of PVAPB HGE layer.The cross-section morphology structure of PVAPB HGE between the two AC electrodes was observed with JSM-5900LV scanning electron microscopy (SEM) (JEOL Ltd., Japan). And its SEM image was shown in Fig. 1C. It was found that the PVAPB HGE layer was smooth and intact with the thickness of about 50 μm. The PVAPB HGE closely attached on the AC electrode, and the interface between them was tight and compact, which could provide the SCs with low interface resistance and good electrochemical performance.
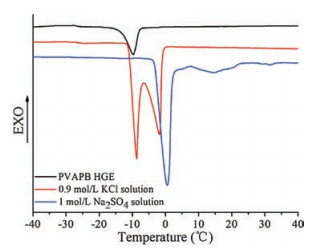
The stability of aqueous SCs at low temperature mainly depends on the freezing point of the electrolyte. Therefore, The PVAPB HGE and the liquid electrolytes (0.9 mol/L KCl and 1 mol/L Na2SO4) were analyzed with differential scanning calorimetry (DSC) (NETZSCH DSC 204 F1, Germany) to observe their temperature stability. DSC samples (5–8 mg) were sealed in aluminum pans and then cooled to -40 ℃ at a rate of 10 ℃/min in a nitrogen atmosphere. After the freezing process, the samples were heated to 40 ℃ at a speed of 2 ℃/min. DSC diagrams of PVAPB HGE, 0.9 mol/L KCl, and 1 mol/L Na2SO4 electrolytes were shown in Fig. 2 to compare their thermal properties at low temperature. There was one endothermic peak at about 0.57 ℃ in DSC diagram of 1 mol/L Na2SO4 solution, which was attributed to the fact that saturated Na2SO4 solution would hardly change the freezing point of water [20]. By contrast, KCl salt had a higher solubility than Na2SO4 salt in water at low temperature, resulting in the phenomenon that the addition of KCl salt would obviously impact on the freezing point of water. There were two endothermic peaks at about -8.7 ℃ and -1.9 ℃ in the DSC diagram of 0.9 mol/L KCl solution. The peak at -8.7 ℃ was attributed to the freezing of water molecules that interacted with K+ and Cl- ions, and that at -1.9 ℃ indicated the free water molecules in 0.9 mol/L KCl solution. As for PVAPB HGE, There was only one endothermic peak at about -9.4 ℃ in its DSC diagram, which was different from that of 0.9 mol/L KCl solution. It indicates that the water molecules in PVAPB HGEs are mainly in bonding state. These bonding water molecules produced by the strong hydrogen bond interaction between water molecules and PVA in PVAPB HGEs may not even be frozen, which can reduce the freezing point of PVAPB HGE. The lowest freezing point of PVAPB HGE suggested that it should be more stable than other liquid aqueous electrolytes at low temperature.
图 2
The electrochemical performances of the SCs using different aqueous electrolytes were all evaluated from -5 ℃ to 80 ℃. The test voltage ranges were set as 0–1.5 V for PVAPB HGE and 1 mol/L Na2SO4, and 0–1.1 V for 0.9 mol/L KCl (because of its relatively narrow electrochemical window). Cyclic voltammetry (CV) and electrochemical impedance spectroscopy (EIS) measurements were carried out by the CHI660E electrochemical workstation. The charge-discharge tests were performed on LANHE CT2001A supercapacitor test system. The SC samples were kept at the experimental temperatures for 2 h before tests to accomplish the temperature equilibrium. It could be seen from Fig. 3A that the CV curves of the PVAPB HGE SCs had a much better rectangular profile than those of SCs with liquid aqueous electrolytes (Fig. S1 in Supporting information), which indicated its better capacitive behavior. With the increase of the operating temperature, the specific capacitance of all the SCs increased, and the specific capacitance of the SC assembled with PVAPB HGE was greater than those of the SCs assembled with liquid aqueous electrolytes. All the SCs would lose function below -5 ℃ or beyond 80 ℃. The SCs using PVAPB HGEs could stably operate from -5 ℃ to 80 ℃, but the SCs with 0.9 mol/L KCl and 1 mol/L Na2SO4 stopped working at -5 ℃, which indicated that the low-temperature stability of PVAPB HGE was better than those liquid aqueous electrolytes because of the unique bonding state of water molecules in PVAPB HGE. Fig. 3B showed the Nyquist plots of the PVAPB HGE SCs measured from 106 Hz to 10-1 Hz at different temperatures, which displayed a typical capacitive behavior. It was found that the bulk resistances (Rb) of PVAPB HGE is higher than that of 0.9 mol/L KCl but smaller than that of 1 mol/L Na2SO4 (Fig. S2 in Supporting information). The Rb and the charge transfer resistances (Rct) of all the SCs decreased with the increase of temperature. It could be seen that the Rb and Rct of SCs using 1 mol/L Na2SO4 were most sensitively affected by the temperature, while those of SCs using 0.9 mol/L KCl seemed insensitive to the temperature, and the influence of the temperatures on the SCs using PVAPB HGE was medium. These phenomena can be explained by the hydration of ions in the electrolytes. The hydrated ions are very sensitive to the temperature, and high temperature will lower the hydration numbers of ions, which is a benefit for the mobility of the ions. Na2SO4 has stronger hydration than KCl, so its ionic conductivity is more temperature-sensitive than that of KCl. The ions in PVAPB HGE cannot be simply described as hydrated ions, but the result still indicated that higher temperature is more suitable for the ion mobility in the PVAPB HGE.
图 3
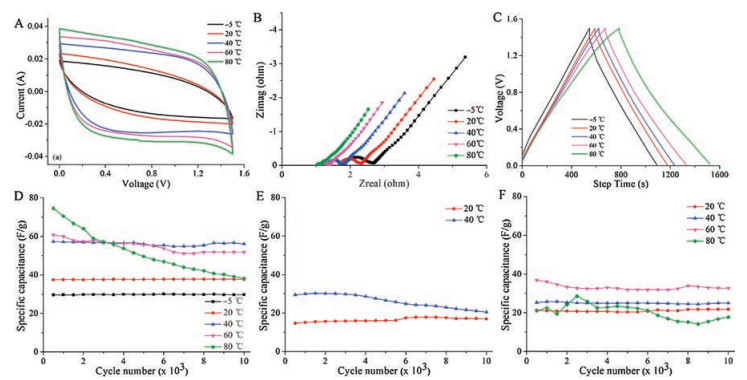 图 3 (A) CV curves o the PVAPB HGE SCs with a scan rate of 10 mV/s at different temperatures. (B) The Nyquist plots of the PVAPB HGE SCs at different temperatures. (C) The galvanostatic charge-discharge curves of the PVAPB HGE SCs with the current density of 0.1 A/g at different temperatures. The cycle life of the PVAPB HGE SCs (D), KCl SCs (E), Na2SO4 SCs (F) at the different operating temperature.Figure 3. (A) CV curves o the PVAPB HGE SCs with a scan rate of 10 mV/s at different temperatures. (B) The Nyquist plots of the PVAPB HGE SCs at different temperatures. (C) The galvanostatic charge-discharge curves of the PVAPB HGE SCs with the current density of 0.1 A/g at different temperatures. The cycle life of the PVAPB HGE SCs (D), KCl SCs (E), Na2SO4 SCs (F) at the different operating temperature.
图 3 (A) CV curves o the PVAPB HGE SCs with a scan rate of 10 mV/s at different temperatures. (B) The Nyquist plots of the PVAPB HGE SCs at different temperatures. (C) The galvanostatic charge-discharge curves of the PVAPB HGE SCs with the current density of 0.1 A/g at different temperatures. The cycle life of the PVAPB HGE SCs (D), KCl SCs (E), Na2SO4 SCs (F) at the different operating temperature.Figure 3. (A) CV curves o the PVAPB HGE SCs with a scan rate of 10 mV/s at different temperatures. (B) The Nyquist plots of the PVAPB HGE SCs at different temperatures. (C) The galvanostatic charge-discharge curves of the PVAPB HGE SCs with the current density of 0.1 A/g at different temperatures. The cycle life of the PVAPB HGE SCs (D), KCl SCs (E), Na2SO4 SCs (F) at the different operating temperature.The ionic conductivity σ of electrolytes at different temperatures can be calculated as follows:
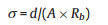
Where A is the surface area of electrodes; d is the distance between the positive and negative electrodes (the thickness of the PVAPB HGE, KCl electrolyte, and the Na2SO4 electrolyte is about 50 μm, 110 μm, and 110 μm, respectively.); Rb is the bulk resistances of the electrolytes. The Rb and σ calculated from Fig. 3B and Fig. S2 were shown in Table 1.
表 1
 表 1 Rb and σ of the electrolytes at different temperatures.Table 1. Rb and σ of the electrolytes at different temperatures.
表 1 Rb and σ of the electrolytes at different temperatures.Table 1. Rb and σ of the electrolytes at different temperatures.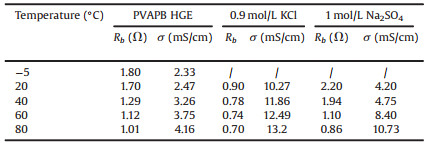
The temperature dependence of the conductivity of electrolyte follow the Arrhenius equation: σ = σ0exp(-Ea/RT), where σ0 is the pre-exponential factor; Ea is the activation energy; T is the absolute temperature, and R is the gas constant. The plot of lnσ versus 1/T for three kinds of electrolytes was shown in Fig. S3 (Supporting information). From the slope of the linear fit line of lnσ versus 1/T plot, the Ea of PVAPB HGE, 0.9 mol/L KCl, and 1 mol/L Na2SO4 were calculated to be 5.81 kJ/mol, 3.52 kJ/mol and 14.44 kJ/mol, respectively. The relatively low Ea of PVAPB HGE indicates its excellent temperature stability. The galvanostatic charge-discharge curves of PVAPB HGE SCs in Fig. 3C behave as a mirror-like shape during the charge-discharge process, which means that the SCs using PVAPB HGEs own a favorable electrochemical capacitance performance in wide temperature region. However, the charge-discharge curves of SCs using liquid aqueous electrolytes (Fig. S4 in Supporting information) show distorted shapes especially at low temperatures, which indicates their poor reversibility at low temperatures. It is noticeable that the SCs using 0.9 mol/L KCl failed in the galvanostatic charge-discharge test at 60 ℃ and 80 ℃, though it could run in the CV test at the same temperatures. The reason comes from that the activity of the water molecules in 0.9 mol/L KCl solution increased at high temperature, which lowers down its electrochemical stability and made 0.9 mol/L KCl SCs stop running at high temperature [21]. The water molecules in 1 mol/L Na2SO4 also has a similar tendency at high temperature, but the strong hydration of Na2SO4 salt could still keep its electrolyte stable at 60 ℃ and 80 ℃.
The long-term cycle performance tests were employed at the current density of 0.5 A/g from -5 ℃ to 80 ℃ to further examine the stability of the SCs at different temperatures. Results were shown in Figs. 3D–F. It was found that 0.9 mol/L KCl SCs had relatively low specific capacitance and poor cycle performance, compared with PVAPB HGE SCs and 1 mol/L Na2SO4 SCs. Although the cyclic stability of 1 mol/L Na2SO4 SCs was as good as that of PVAPB HGE SCs, its specific capacitance was far lower than that of PVAPB HGE SCs. The capacitance of both PVAPB HGE SCs and Na2SO4 SCs dropped a lot during cycle test at 80 ℃, which may be caused by the drastic water evaporation at 80 ℃ in the electrolyte. The capacitance attenuation of the PVAPB HGE SCs was hardly observed at -5 ℃, 20 ℃, and 40 ℃, which indicates its excellent reversibility in wide temperature range, and potential application in the relatively extreme environment.
In summary, the electrochemical performance of the AC/AC SCs assembled with in situ electrodeposited PVAPB HGEs, and liquid aqueous electrolytes (0.9 mol/L KCl and 1 mol/L Na2SO4) were systematically studied in a wide temperature range from -5 ℃ to 80 ℃. In general, as a new aqueous electrolyte, the PVAPB HGE presents better temperature stability than traditional liquid aqueous electrolytes in symmetric AC/AC supercapacitors. Compared with liquid aqueous electrolytes (0.9 mol/L KCl and 1 mol/L Na2SO4) SCs, PVAPB HGE SCs possessed higher specific capacitance in the operating temperature range from -5 ℃ to 80 ℃. The activation energy of ionic conductivity of PVAPB HGE was calculated to be 5.81 kJ/mol, indicating its temperature-insensitivity, and stable electrochemical performance in a wide temperature range. The PVAPB HGE SCs also show good cycle performance at a temperature range from -5 ℃ to 60 ℃, which is better than those of SCs using liquid aqueous electrolytes. The excellent temperature stability of PVAPB HGE made it possible be used in the harsh temperature environment with which traditional liquid aqueous electrolyte can not meet.
Appendix A. Supplementary data
Supplementary data associated with this article can be found, in the online version, at https://doi.org/10.1016/j.cclet.2017.11.035.
References
-
[1]
[1] M.O'keeffe,M.A.Peskov,S.J.Ramsden,O.M.Yaghi,The reticular chemistry structure resource(RCSR)database of,and symbols for,crystal nets,Acc.Chem. Res.41(2008)1782-1789.
-
[2]
[2] D.J.Tranchemontagne,Z.Ni,M.O'Keeffe,O.M.Yaghi,Reticular chemistry of metal-organic polyhedra,Angew.Chem.Int.Ed.47(2008)5136-5147.
-
[3]
[3] K.S.Park,Z.Ni,A.P.Côté,et al.,Exceptional chemical and thermal stability of zeolitic imidazolate frameworks,Proc.Natl.Acad.Sci.U.S.A.103(2006) 10186-10191.
-
[4]
[4] L.Ma,W.Lin,Chirality-controlled and solvent-templated catenation isomerism in metal-organic frameworks,J.Am.Chem.Soc.130(2008)13834-13835.
-
[5]
[5] C.A.Bauer,T.V.Timofeeva,T.B.Settersten,et al.,Influence of connectivity and porosity on ligand-based luminescence in zinc metal-organic frameworks,J.Am. Chem.Soc.129(2007)7136-7144.
-
[6]
[6] I.A.Koval,P.Gamez,O.Roubeau,et al.,Century-known copper salt Cu(OAc)(OMe) proven to be a unique magnetic lattice composed of tetranuclear copper(Ⅱ) species with a rare binding mode of the acetate anion,Inorg.Chem.42(2003) 868-872.
-
[7]
[7] Y.Liu,W.Xuan,H.Zhang,Y.Cui,Chirality- and threefold-symmetry-directed assembly of homochiral octupolar metal-organoboron frameworks,Inorg.Chem. 48(2009)10018-10023.
-
[8]
[8] H.Furukawa,J.Kim,N.W.Ockwig,M.O'Keeffe,O.M.Yaghi,Control of vertex geometry,structure dimensionality,functionality,and pore metrics in the retic-ular synthesis of crystalline metal-organic frameworks and polyhedra,J.Am. Chem.Soc.130(2008)11650-11661.
-
[9]
[9] J.A.K.Howard,V.J.Hoy,D.O'Hagan,G.T.Smith,How good is fluorine as a hydrogen bond acceptor,Tetrahedron 52(1996)12613-12622.
-
[10]
[10] J.D.Dunitz,R.Taylor,Organic fluorine hardly ever accepts hydrogen bonds,Chem. Eur.J.3(1997)89-98.
-
[11]
[11] S.Purser,P.R.Moore,S.Swallow,V.Gouverneur,Fluorine in medicinal chemistry, Chem.Soc.Rev.37(2008)320-330.
-
[12]
[12] X.Wang,C.Qin,E.Wang,et al.,Syntheses,structures,and photoluminescence of a novel class of d10 metal complexes constructed from pyridine-3,4-dicarboxylic acid with different coordination architectures,Inorg.Chem.43(2004) 1850-1852.
-
[13]
[13] G.B.Deacon,S.Hein,P.C.Junk,et al.,Structural variations in rare earth benzoate complexes.Part Ⅱ:Yttrium and terbium,Cryst.Eng.Commun.9(2007) 1110-1123.
-
[14]
[14] Y.Jin,I.I.Yoon,J.Seo,et al.,Cadmium(Ⅱ)and mercury(Ⅱ)complexes of an NO2S2-donor macrocycle and its ditopic xylyl-bridged analogue,Dalton Trans.(2005) 788-796.
-
[15]
[15] G.P.Yong,S.Qiao,Z.Y.Wang,Y.Cui,Five-,seven-,and eight-coordinate Cd(Ⅱ) coordination polymers built by anthranilic acid derivatives:synthesis,structures and photoluminescence,Inorg.Chim.Acta 358(2005)3905-3913.
-
[16]
[16] A.M.Baruah,A.Karmakar,J.B.Baruah,Manganese and cadmium benzoate com-plexes having 8-aminoquinoline ancillary ligand,Open Inorg.Chem.J.2(2008) 62-68.
-
[17]
[17] E.D'Oria,J.J.Novoa,On the hydrogen bond nature of the C-H F interactions in molecular crystals.An exhaustive investigation combining a crystallographic database search and ab initio theoretical calculations,Cryst.Eng.Commun.10 (2008)423-436.
-
[18]
[18] D.Chopra,T.N.G.Row,Role of organic fluorine in crystal engineering,Cryst.Eng. Commun.13(2011)2175-2186.
-
[19]
[19] T.J.Mooibroek,P.Gamez,J.Reedijk,Lone pair-p interactions:a new supramo-lecular bond?Cryst.Eng.Commun.10(2008)1501-1515.
-
[20]
[20] Y.Li,F.K.Zheng,X.Liu,et al.,Crystal structures and magnetic and luminescent properties of a series of homodinuclear lanthanide complexes with 4-cyanoben-zoic ligand,Inorg.Chem.45(2006)6308-6316.
-
[21]
[21] Y.Q.Yang,C.H.Li,W.Li,Z.J.Yi,Synthesis,crystal structure and fluorescence characterization of cadmium(Ⅱ)coordination polymer with 4-acetamidobenzole acid and 4,4'-bipyridine,Chin.J.Inorg.Chem.25(2009)1304-1307.
-
[1]
-
-
[1]
[1] M.O'keeffe,M.A.Peskov,S.J.Ramsden,O.M.Yaghi,The reticular chemistry structure resource(RCSR)database of,and symbols for,crystal nets,Acc.Chem. Res.41(2008)1782-1789.
-
[2]
[2] D.J.Tranchemontagne,Z.Ni,M.O'Keeffe,O.M.Yaghi,Reticular chemistry of metal-organic polyhedra,Angew.Chem.Int.Ed.47(2008)5136-5147.
-
[3]
[3] K.S.Park,Z.Ni,A.P.Côté,et al.,Exceptional chemical and thermal stability of zeolitic imidazolate frameworks,Proc.Natl.Acad.Sci.U.S.A.103(2006) 10186-10191.
-
[4]
[4] L.Ma,W.Lin,Chirality-controlled and solvent-templated catenation isomerism in metal-organic frameworks,J.Am.Chem.Soc.130(2008)13834-13835.
-
[5]
[5] C.A.Bauer,T.V.Timofeeva,T.B.Settersten,et al.,Influence of connectivity and porosity on ligand-based luminescence in zinc metal-organic frameworks,J.Am. Chem.Soc.129(2007)7136-7144.
-
[6]
[6] I.A.Koval,P.Gamez,O.Roubeau,et al.,Century-known copper salt Cu(OAc)(OMe) proven to be a unique magnetic lattice composed of tetranuclear copper(Ⅱ) species with a rare binding mode of the acetate anion,Inorg.Chem.42(2003) 868-872.
-
[7]
[7] Y.Liu,W.Xuan,H.Zhang,Y.Cui,Chirality- and threefold-symmetry-directed assembly of homochiral octupolar metal-organoboron frameworks,Inorg.Chem. 48(2009)10018-10023.
-
[8]
[8] H.Furukawa,J.Kim,N.W.Ockwig,M.O'Keeffe,O.M.Yaghi,Control of vertex geometry,structure dimensionality,functionality,and pore metrics in the retic-ular synthesis of crystalline metal-organic frameworks and polyhedra,J.Am. Chem.Soc.130(2008)11650-11661.
-
[9]
[9] J.A.K.Howard,V.J.Hoy,D.O'Hagan,G.T.Smith,How good is fluorine as a hydrogen bond acceptor,Tetrahedron 52(1996)12613-12622.
-
[10]
[10] J.D.Dunitz,R.Taylor,Organic fluorine hardly ever accepts hydrogen bonds,Chem. Eur.J.3(1997)89-98.
-
[11]
[11] S.Purser,P.R.Moore,S.Swallow,V.Gouverneur,Fluorine in medicinal chemistry, Chem.Soc.Rev.37(2008)320-330.
-
[12]
[12] X.Wang,C.Qin,E.Wang,et al.,Syntheses,structures,and photoluminescence of a novel class of d10 metal complexes constructed from pyridine-3,4-dicarboxylic acid with different coordination architectures,Inorg.Chem.43(2004) 1850-1852.
-
[13]
[13] G.B.Deacon,S.Hein,P.C.Junk,et al.,Structural variations in rare earth benzoate complexes.Part Ⅱ:Yttrium and terbium,Cryst.Eng.Commun.9(2007) 1110-1123.
-
[14]
[14] Y.Jin,I.I.Yoon,J.Seo,et al.,Cadmium(Ⅱ)and mercury(Ⅱ)complexes of an NO2S2-donor macrocycle and its ditopic xylyl-bridged analogue,Dalton Trans.(2005) 788-796.
-
[15]
[15] G.P.Yong,S.Qiao,Z.Y.Wang,Y.Cui,Five-,seven-,and eight-coordinate Cd(Ⅱ) coordination polymers built by anthranilic acid derivatives:synthesis,structures and photoluminescence,Inorg.Chim.Acta 358(2005)3905-3913.
-
[16]
[16] A.M.Baruah,A.Karmakar,J.B.Baruah,Manganese and cadmium benzoate com-plexes having 8-aminoquinoline ancillary ligand,Open Inorg.Chem.J.2(2008) 62-68.
-
[17]
[17] E.D'Oria,J.J.Novoa,On the hydrogen bond nature of the C-H F interactions in molecular crystals.An exhaustive investigation combining a crystallographic database search and ab initio theoretical calculations,Cryst.Eng.Commun.10 (2008)423-436.
-
[18]
[18] D.Chopra,T.N.G.Row,Role of organic fluorine in crystal engineering,Cryst.Eng. Commun.13(2011)2175-2186.
-
[19]
[19] T.J.Mooibroek,P.Gamez,J.Reedijk,Lone pair-p interactions:a new supramo-lecular bond?Cryst.Eng.Commun.10(2008)1501-1515.
-
[20]
[20] Y.Li,F.K.Zheng,X.Liu,et al.,Crystal structures and magnetic and luminescent properties of a series of homodinuclear lanthanide complexes with 4-cyanoben-zoic ligand,Inorg.Chem.45(2006)6308-6316.
-
[21]
[21] Y.Q.Yang,C.H.Li,W.Li,Z.J.Yi,Synthesis,crystal structure and fluorescence characterization of cadmium(Ⅱ)coordination polymer with 4-acetamidobenzole acid and 4,4'-bipyridine,Chin.J.Inorg.Chem.25(2009)1304-1307.
-
[1]
-

-
-
[1]
Ke-Ai Zhou , Lian Huang , Xing-Ping Fu , Li-Ling Zhang , Yu-Ling Wang , Qing-Yan Liu . Fluorinated metal-organic framework for methane purification from a ternary CH4/C2H6/C3H8 mixture. Chinese Journal of Structural Chemistry, 2023, 42(11): 100172-100172. doi: 10.1016/j.cjsc.2023.100172
-
[2]
Muhammad Riaz , Rakesh Kumar Gupta , Di Sun , Mohammad Azam , Ping Cui . Selective adsorption of organic dyes and iodine by a two-dimensional cobalt(II) metal-organic framework. Chinese Journal of Structural Chemistry, 2024, 43(12): 100427-100427. doi: 10.1016/j.cjsc.2024.100427
-
[3]
Zhexin Chen , Yuqing Shi , Fang Zhong , Kai Zhang , Furong Zhang , Shenghong Xie , Zhongbin Cheng , Qian Zhou , Yi-You Huang , Hai-Bin Luo . Discovery of amentoflavone as a natural PDE4 inhibitor with anti-fibrotic effects. Chinese Chemical Letters, 2025, 36(4): 109956-. doi: 10.1016/j.cclet.2024.109956
-
[4]
Tiantian Li , Ruochen Jin , Bin Wu , Dongming Lan , Yunjian Ma , Yonghua Wang . A novel insight of enhancing the hydrogen peroxide tolerance of unspecific peroxygenase from Daldinia caldariorum based on structure. Chinese Chemical Letters, 2024, 35(4): 108701-. doi: 10.1016/j.cclet.2023.108701
-
[5]
Xinyi Cao , Yucheng Jin , Hailong Wang , Xu Ding , Xiaolin Liu , Baoqiu Yu , Xiaoning Zhan , Jianzhuang Jiang . A tetraaldehyde-derived porous organic cage and covalent organic frameworks: Syntheses, structures, and iodine vapor capture. Chinese Chemical Letters, 2024, 35(9): 109201-. doi: 10.1016/j.cclet.2023.109201
-
[6]
Huiying Xu , Minghui Liang , Zhi Zhou , Hui Gao , Wei Yi . Application of Quantum Chemistry Computation and Visual Analysis in Teaching of Weak Interactions. University Chemistry, 2025, 40(3): 199-205. doi: 10.12461/PKU.DXHX202407011
-
[7]
Lu LIU , Huijie WANG , Haitong WANG , Ying LI . Crystal structure of a two-dimensional Cd(Ⅱ) complex and its fluorescence recognition of p-nitrophenol, tetracycline, 2, 6-dichloro-4-nitroaniline. Chinese Journal of Inorganic Chemistry, 2024, 40(6): 1180-1188. doi: 10.11862/CJIC.20230489
-
[8]
Liping GUO . Synthesis and crystal structure characterization of yttrium imido complex: The reactivity of 2-substituted-1-amino-o-carborane with yttrium dialkyl complex. Chinese Journal of Inorganic Chemistry, 2025, 41(7): 1409-1415. doi: 10.11862/CJIC.20250065
-
[9]
Heng Gao , Zhaocong Cheng , Guangshui Tu , Zonglin Qiu , Xieyi Xiao , Haotian Zhou , Handou Zheng , Haiyang Gao . Thermally robust bis(imino)pyridyl iron catalysts for ethylene polymerization: Synergy effects of weak π-π interaction, steric bulk, and electronic tuning. Chinese Chemical Letters, 2025, 36(5): 110762-. doi: 10.1016/j.cclet.2024.110762
-
[10]
Jinfeng Chu , Lan Jin , Yu-Fei Song . Exploration and Practice of Flipped Classroom in Inorganic Chemistry Experiment: a Case Study on the Preparation of Inorganic Crystalline Compounds. University Chemistry, 2024, 39(2): 248-254. doi: 10.3866/PKU.DXHX202308016
-
[11]
Yan Liu , Yuexiang Zhu , Luhua Lai . Introduction to Blended and Small-Class Teaching in Structural Chemistry: Exploring the Structure and Properties of Crystals. University Chemistry, 2024, 39(3): 1-4. doi: 10.3866/PKU.DXHX202306084
-
[12]
Junqiao Zhuo , Xinchen Huang , Qi Wang . Symbol Representation of the Packing-Filling Model of the Crystal Structure and Its Application. University Chemistry, 2024, 39(3): 70-77. doi: 10.3866/PKU.DXHX202311100
-
[13]
Yuyao Wang , Zhitao Cao , Zeyu Du , Xinxin Cao , Shuquan Liang . Research Progress of Iron-based Polyanionic Cathode Materials for Sodium-Ion Batteries. Acta Physico-Chimica Sinica, 2025, 41(4): 100035-. doi: 10.3866/PKU.WHXB202406014
-
[14]
Wenyan Dan , Weijie Li , Xiaogang Wang . The Technical Analysis of Visual Software ShelXle for Refinement of Small Molecular Crystal Structure. University Chemistry, 2024, 39(3): 63-69. doi: 10.3866/PKU.DXHX202302060
-
[15]
Xiaofen GUAN , Yating LIU , Jia LI , Yiwen HU , Haiyuan DING , Yuanjing SHI , Zhiqiang WANG , Wenmin WANG . Synthesis, crystal structure, and DNA-binding of binuclear lanthanide complexes based on a multidentate Schiff base ligand. Chinese Journal of Inorganic Chemistry, 2024, 40(12): 2486-2496. doi: 10.11862/CJIC.20240122
-
[16]
Yao HUANG , Yingshu WU , Zhichun BAO , Yue HUANG , Shangfeng TANG , Ruixue LIU , Yancheng LIU , Hong LIANG . Copper complexes of anthrahydrazone bearing pyridyl side chain: Synthesis, crystal structure, anticancer activity, and DNA binding. Chinese Journal of Inorganic Chemistry, 2025, 41(1): 213-224. doi: 10.11862/CJIC.20240359
-
[17]
Lulu DONG , Jie LIU , Hua YANG , Yupei FU , Hongli LIU , Xiaoli CHEN , Huali CUI , Lin LIU , Jijiang WANG . Synthesis, crystal structure, and fluorescence properties of Cd-based complex with pcu topology. Chinese Journal of Inorganic Chemistry, 2025, 41(4): 809-820. doi: 10.11862/CJIC.20240171
-
[18]
Jia JI , Zhaoyang GUO , Wenni LEI , Jiawei ZHENG , Haorong QIN , Jiahong YAN , Yinling HOU , Xiaoyan XIN , Wenmin WANG . Two dinuclear Gd(Ⅲ)-based complexes constructed by a multidentate diacylhydrazone ligand: Crystal structure, magnetocaloric effect, and biological activity. Chinese Journal of Inorganic Chemistry, 2025, 41(4): 761-772. doi: 10.11862/CJIC.20240344
-
[19]
Zhaodong WANG . In situ synthesis, crystal structure, and magnetic characterization of a trinuclear copper complex based on a multi-substituted imidazo[1,5-a]pyrazine scaffold. Chinese Journal of Inorganic Chemistry, 2025, 41(3): 597-604. doi: 10.11862/CJIC.20240268
-
[20]
Liang Ma , Zhou Li , Zhiqiang Jiang , Xiaofeng Wu , Shixin Chang , Sónia A. C. Carabineiro , Kangle Lv . Effect of precursors on the structure and photocatalytic performance of g-C3N4 for NO oxidation and CO2 reduction. Chinese Journal of Structural Chemistry, 2024, 43(11): 100416-100416. doi: 10.1016/j.cjsc.2024.100416
-
[1]
Metrics
- PDF Downloads(0)
- Abstract views(837)
- HTML views(6)

 Login In
Login In




 下载:
下载:
 DownLoad:
DownLoad: