Citation:
Ting Ai, Shao-Yu Shi, Li-Ting Chen, Ling Li, Bo Cao, Ying Gao, Hong Chen, Jing Zhou. Synthesis and anti-tumor activity evaluation of novel podophyllotoxin derivatives[J]. Chinese Chemical Letters,
;2013, 24(01): 37-40.

-
In order to investigate the effects on the cytotoxicity of indole-3-oxalylamino podophyllotoxin analogs, seven novel podophyllotoxin derivatives were synthesized.The compounds were tested against Hela, K562 or K562/A02 cancer cells in vitro,four of which showed significant cytotoxicity.Among them 9a,9b and 9c were superior to the positive control VP-16.
-
Keywords:
- Podophyllotoxin,
- C4-N-substitution,
- Indole,
- Cytotoxicity
-
1. Introduction
Monofluoro- and difluoro-containing organic molecules have become tremendously important in pharmaceuticals [1, 2], agrochemicals [3], and materials [4], owing to the unique properties of fluorine atom and its incorporation enhances the chemical and biological properties of the target compounds [5, 6]. In the last decades, numerous mono- and difluoroalkylating precursors and C–F bond formation strategies have been established for the introduction of fluorine atoms into organic compounds [7-11]. Aside from this, the defluorinative functionalization of inert C-F bonds of CF3 compounds is also an important pathway to the synthesis of useful partially fluorinated organic molecules [12-14]. Such strategy has gained an ever-increasing interest, given the low cost of many CF3 sources and numerous routes available to install CF3 motifs [15, 16]. In general, the cleavage of C–F bond of CF3 groups proceeds through heterolytic pathway, affording difluoro-substituted carbon cations or anions as intermediates, and a number of comprehensive reviews have summarized the progress of this research topic [6].
Defluorination reactions via radical intermediates represent a class of powerful transformations, in which the radical species can undergo different reaction pathways as compared to ionic intermediates, and thus providing versatile routes for chemical bond formation. However, such reactions are still insufficiently studied because of the high bond dissociated energy (BDE) of C–F bond that makes homolytic cleavage extremely difficult [17]. Moreover, since C–F bond strength continuously decreases as defluorination proceeds, selectively formation of di- and monofluoroalkyl radical intermediates in a controlled manner becomes exceedingly difficult and exhaustive defluorination is often resulted [18, 19]. So far, there have been some reports on deflurinative generation of radical interemediates and the CF3 group is required to attached to a π-system, such as arenes, alkenes, and carbonyls, so that the substrates can accept a single electron or a radical species and then induces fluoride anion elimination. This review will summarize recent progress of this topic and focus more on the mechanistic discussion. Meanwhile, the synthetic applications of the resulting radical intermediates will also be introduced.
2. C–F bond functionalization of trifluoromethylarenes
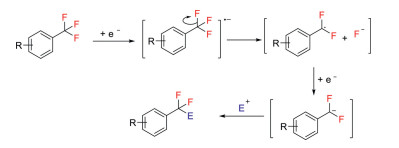
Selective functionalization of C(sp3)−F bond in trifluoromethylarenes has found an important place in modern organic synthesis, which provides direct access to the synthesis of a diverse range of aryldifluoromethyl and arylmonofluoromethyl molecules [14, 20, 21]. Generally, defluorination occurs with the aid of UV irradiation [22, 23] or under reductive reaction conditions. In the later cases, electrochemical reduction [24-26] and the use of Mg metal as the reducing agents are required [27-29]. The reduction mechanism via radical intermediates is shown in Scheme 1. The reaction begins with single-electron reduction of trifluoromethylarenes to generate radical anion intermediates, and subsequent elimination of a fluoride anion gives radical intermediates. These radical species can be easily reduced under strong reductive reaction conditions, affording carbanion intermediates, which can be trapped by electrophiles to deliver difluoro products.
Scheme 1
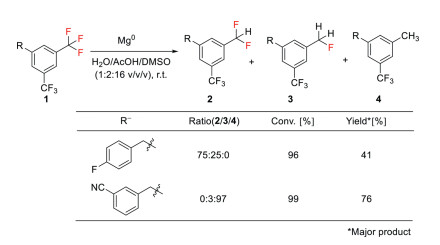
For example, in 2017, Prakash and co-workers demonstrated a magnesium metal-promoted defluorination of bis (trifluoromethyl) arenes in the presence of Brønsted acid for the synthesis of difluoromethyl-containing arenes (Scheme 2) [30]. In this protocol, functional groups like free amine, alcohol are well tolerated. However, reduction of all three C–F bonds was observed in the case of nitrile substituents.
Scheme 2
The strategy shown above is difficult to capture the radical intermediate by various radical traps, as it prefers to undergo further reduction under those strong reductive reaction conditions. To address this challenge, developing new reductive protocols that can prevent the second reduction is desirable. Photoredox catalysis has recently emerged as a powerful method in organic synthesis. Notably, a large number of photoredox catalysts with a broad range of redox potentials are readily available, thus offering ample opportunities to precisely control the redox process. By taking this advantage, some defluorination reactions that can selectively generate difluoromethyl radical without further reduction have been reported. These radicals further participate in various transformations to access valuable ArCF2R derivatives.
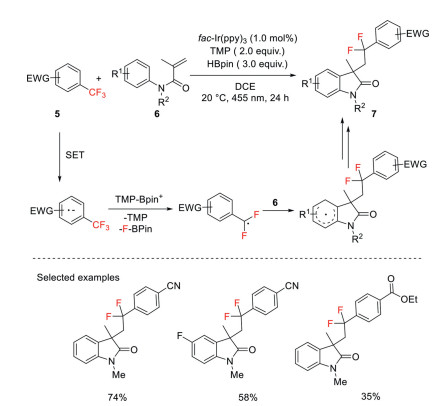
Gschwind and König reported a protocol that emerges photoredox catalysis and Lewis acid activation, by which a single C−F bond of trifluoromethylarenes was selectively cleaved, giving aryldifluoromethyl radical intermediates. Those radicals were capable of performing radical addition to methacrylamides followed by cyclization to afford aryldifluoromethyl-tethered indolinone derivatives. Mechanistic studies suggested that the in situ generated acidic borenium cation serves as an efficient fluoride scavenger that can accelerate the radical generation (Scheme 3) [31].
Scheme 3
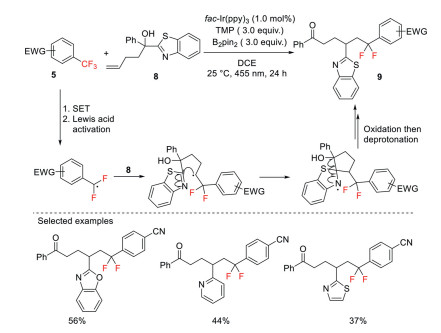
After that, Qiu and Guo have extended this strategy to enable a tandem C(sp3)–F and C–C bond functionalization through defluoroalkylation–distal migration of the heteroaryl group (Scheme 4) [32]. The reaction begins with the reductive generation of α, α-difluorobenzylic radical intermediates, which are then trapped by simple olefins. The resulting alkyl radical attacks an intramolecular heteroaryl ring to trigger a distal aryl migration, and the ensuing oxidation and deprotonation affords ketone products.
Scheme 4
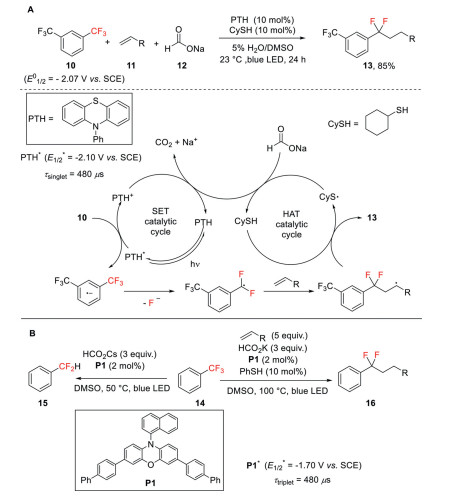
Jui and co-workers developed a new catalytic system for the single C–F bond cleavage of trifluoromethylarenes under visible light irradiation [33]. In this protocol, N-phenylphenothiazine (PTH) was employed as photocatalyst and cyclohexanethiol (CySH) was used to promote hydrogen atom transfer (HAT). Under irradiation by blue LED, the highly reducing excited state PTH* (E1/2* = −2.10 V vs. SCE) can deliver an electron to 1, 3-bistrifluoromethylbenzene (E01/2 = −2.07 V vs. SCE) to generate difluorobenzylic radicals with the elimination of a fluoride anion. These radical intermediates then undergo efficient intermolecular coupling with simple alkenes to forge the desired difluoro products (Scheme 5A). This protocol is highly sensitive to the electronic properties of the trifluoromethylaromatic ring. Only the aryl ring bearing an additional strong electron-withdrawing group, such as CF3, phosphonate, and sulfonamide, is amenable in the reaction.
Scheme 5
To address this limitation, the same group then developed a method employing Miyake's phenoxazine (E1/2* = −1.70 V vs. SCE) as the photocatalyst, which has a long-lived triplet excited state. This method allows monodefluoroalkylation and monohydrodefluorination of unactivated trifluorotoluene derivatives (Scheme 5B) [34]. As reported by the recent work of this group, a powerful single electron reductant (CO2•−) (E1/2* = −2.20 V vs. SCE) can be generated via hydrogen atom transfer from a formate salt to a thiyl radical under the same reaction condition [35] and this reductant is likely also involved in this transformation.
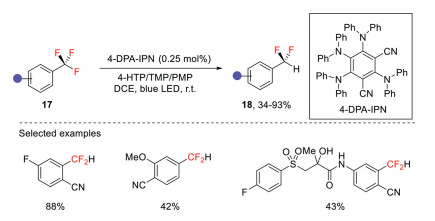
Gouverneur and co-workers quite recently disclosed a phtoredox protocol for the reductive defluorination of electron-poor trifluoromethylarenes under basic conditions (Scheme 6) [36]. In this method, 2, 4, 5, 6-tetrakis(diphenylamino)isophthalonitrile (4-DPA-IPN) was used as the organophotocatalyst, 4-hydroxythiophenol was used as the hydrogen atom donor, under blue light irradiation, a series of complex trifluoromethylated drugs could be transformed into the corresponding difluoromethyl derivatives.
Scheme 6
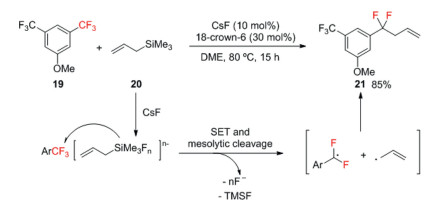
Bander and co-workers developed a fluoride-initiated coupling reaction between trifluoromethylarenes and allylsilanes to access allylated α, α-difluorobenzylic compounds (Scheme 7) [37]. First, fluoride ion act as Lewis base and coordinate to allyltrimethylsilane to give pentacoordinate and hexacoordinate silicate intermediates, which then undergo single electron transfer (SET) to the trifluoromethylarene. The following cleavage of both a C–F and a C–Si bond induces concurrent generation of an α, α-difluorobenzylic radical and an allyl radical, and the quick recombination affords a defluoroallylation product. The expelled fluoride anion then activates another molecule of allyltrimethylsilane.
Scheme 7
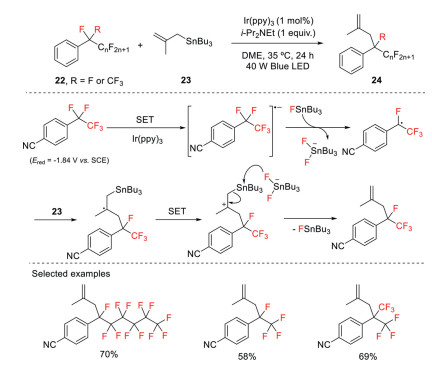
Quite recently, Yasuda and co-workers reported a defluoroallylation reaction of perfluoroalkylarenes using Ir(Ⅲ) photocatalyst and organotin reagent in cooperative mode of catalysis under visible light irradiation [38]. In this transformation, the C–F bond functionalization takes place selectively at the benzylic position through perfluoroalkyl radicals generated from perfluoroalkylarenes by excited Ir(ppy)3 in a single electron transfer pathway (Scheme 8). It should be noted that the destabilization and steric hindrance effects of the resulting perfluoroalkyl radicals are unfavorable for the sequential bond-forming reaction, thereby resulting in a retroprocess including back electron transfer and F− addition. Further DFT calculations suggest that the in situ generated Bu3SnF is capable of trapping F−, which can suppress this retroreaction step. The generated perfluoroalkyl radicals then undergo addition to allylic stannanes followed by single electron oxidation and elimination of the stannyl cation, affording the corresponding defluoroallylation products.
Scheme 8
3. C–F bond functionalizations of trifluoromethyl alkenes
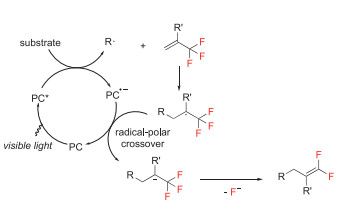
Trifluoromethyl alkenes are privileged structural motifs for synthesizing a diverse range of partially fluorinated or nonfluorinated compounds. Over the past decades, various research groups have used visible-light-mediated reactions of carbon and heteroatom nucleophiles with α-trifluoromethyl alkenes to synthesize gem-difluoroalkenes [14, 21]. The general mechanism is shown in Scheme 9. Initially, the excited photocatalyst (PC*) is reductively quenched by a radical precursor, affording the radical R• and PC•−. Radical addition to trifluoromethyl alkene forms α-CF3 alkyl radical, which would be further reduced by PC•− to give sp3-hybridized carbanion and regenerate PC. Finally, β-fluoride elimination shifts the double bond to give gem-difluoroalkene products
Scheme 9
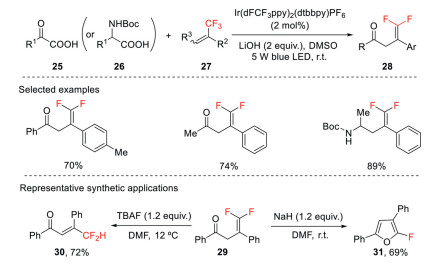
This strategy was applied to the decarboxylative/defluorinative cross coupling of α-keto acids and trifluoromethyl alkenes for the synthesis of γ, γ-difluoroallylic ketones using an Ir-based photocatalyst excited under blue light (Scheme 10) [39]. This reaction features mild reaction conditions, simple operation, and good functional group tolerance. The process could also be extended to N-Boc protected α-amino acids for the synthesis of 1, 1-difluorohomoallylic amines. The resulting functionalized gem-difluoroalkenes can be transformed to various difluoromethylated compounds and monofluorinated heterocycles.
Scheme 10
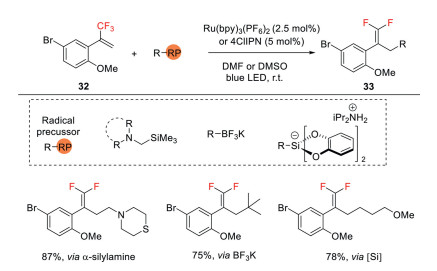
In 2017, Molander also demonstrated a visible light-mediated process for the synthesis of 1, 1-difluoroalkenes by using an array of CF3-substituted alkenes with different carbon-radical precursors via radical defluorinative alkylation process (Scheme 11) [40]. When α-silylamines were employed, various amine-tethered gem-difluoroalkenes were produced. Potassium organotrifluoroborates and alkylbis(catecholato)silicates are also used as competent precursors of carbon centered radicals, providing a route to install a variety of alkyl-substituted gem-difluoroalkenes.
Scheme 11
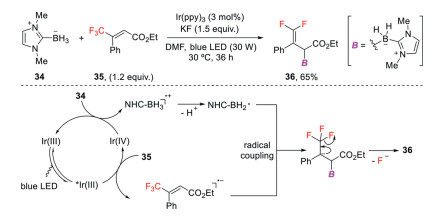
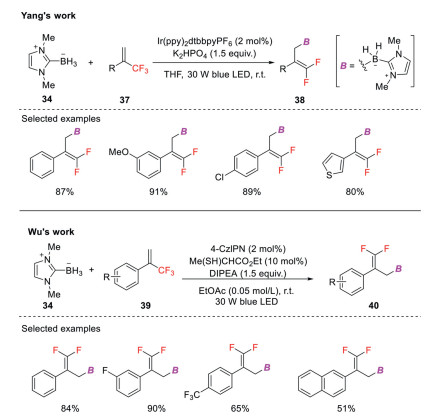
Organoboron compounds have broad applications in chemical synthesis, material sciences, and medicinal chemistry. Recently, the groups of Wang [41], Yang [42] and Wu [43] independently reported photoredox catalysis-enabled radical defluorinative borylations of trifluoromethyl alkenes to afford a wide range of gem-difluoroallylboranes. In Wang's work (Scheme 12), the key step is the generation of N-heterocyclic carbene (NHC)-BH2• via a single-electron oxidation of NHC-BH3 (Ep/2 = + 0.76 V vs. SCE) by IrⅣ(ppy)3 (E1/2red [IrⅣ/IrⅢ] = + 0.77 V vs. SCE), which subsequently undergoes cross-coupling with the in situ generated radical anions to yield the defluoroborylation products [44].
Scheme 12
While in Yang's protocol (Scheme 13), NHC-BH3 is directly oxidized by excited Ir(Ⅲ) species followed by deprotonation to generate NHC-boryl radical. The following addition to CF3-substituted styrene gives an α-trifluoromethyl radical that then undergoes single electron reduction by Ir(Ⅱ) to form a carbanion intermediate. Finally, β-fluoride elimination affords the corresponding gem-difluoroallylboranes. In contrast, NHC-boryl radical is generated by HAT process in Wu's procedure. The excited photocatalyst oxidizes a thiol catalyst to produce a thiyl radical, which then abstracts a hydrogen atom from NHC-BH3 to generate NHC-boryl radical [45]. The ensuring transformation including radical addition, SET reduction by the reduced state of the photocatalyst, and fluoride elimination, provides gem-difluoroallylborane products.
Scheme 13
4. C–F bond functionalization of trifluoromethyl carbonyl compounds
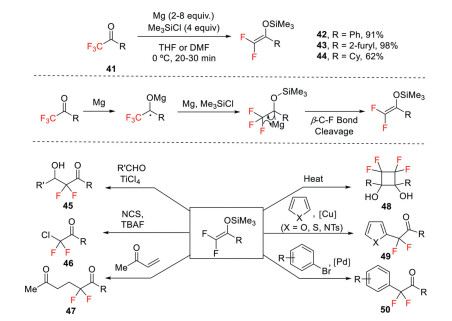
The carbonyl group is an important unit in organic molecules due to its rich chemistry in further transformations. α, α, α-Trifluorocarbonyl compounds are a class of versatile precursors for the synthesis of di- and monofluorocarbonyl products. Previously, the defluorination of these moieties are exploited using reduction methods with low valent metals as the reducing agents [46] or by electrolysis [47]. For example, using Mg as a reducing agent, α, α, α-trifluoroketones are converted to 2, 2-difluoroenol silyl ethers, wherein chlorotrimethyl silane (TMSCl) is used to trap the resulting enolates (Scheme 14) [28]. The possible mechanism involves a two-electron transfer process, As shown in Scheme 14, the first electron transfer from Mg to ketone gives a ketyl species, which is further reduced to an anionic species by Mg. After β-fluoride elimination, 2-fluoroenol silyl ether is formed as final product. Di-fluoroenol silyl ethers are potentially useful building blocks for various difluoro compounds such as β-hydroxy ketones 45, α-halodifluoromethyl ketones 46, 1, 5-dicarbonyl compounds 47. The intramolecular [2 + 2] cycloaddition affords tetrafluorocyclobutanediols 48. It can also undergo transition metal catalyzed cross coupling reactions to give arylated products 49 and 50.
Scheme 14
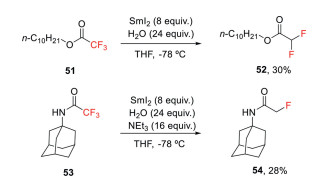
Samarium(Ⅱ) iodide in conjunction with trimethylamine and water is another single-electron reduction approach used for α-defluorination of esters or amides (Scheme 15) [18]. In this defluorination reaction, low temperature (-78 ℃) and the addition of Et3N is crucial for controlling the degree of the defluorination process. However, only moderate yield and selectivity could be obtained by this strategy.
Scheme 15
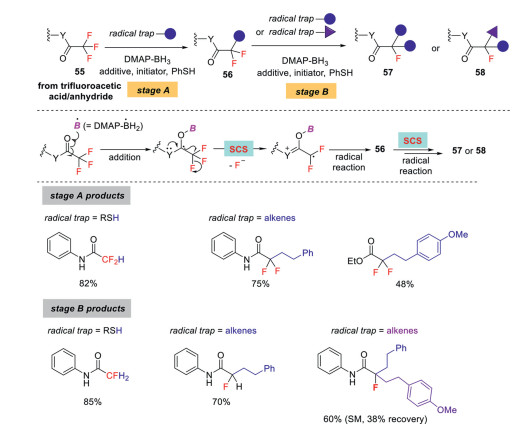
Very recently, Wang's group reported a 4-dimethylamino pyridine-boryl radical promoted sequential C–F bond functionalizations of trifluoromethyl group (Scheme 16) [48]. The strategy comprises a controllable two-stage process, each involving a spin-center shift (SCS) pathway for defluorination. In stage A, the reaction starts by the attack of dimethylaminopyridine-BH2• (DMAP-BH2•) to the carbonyl oxygen atom of CF3-carbonyl molecules, and the following defluorination occurs via an SCS mechanism, giving α, α-difluorocarbonyl radical intermediates. These intermediates are then reduced by a thiol catalyst or captured by alkenes to afford a wide range of difluorocarbonyl products. In stage A, these difluoro compounds repeat the same process, furnishing diverse monofluoro products. As outlined in Scheme 16, this two-stage process shows broad substrate scope and good chemoselectivity. For example, reduction of stages A and B intermediates, wherein RSH was used as the polarity reversal catalyst, can afford di- and monofluoromethyl products selectively, and only minor over-reduction products were observed in the formation of difluoro products. Notably, no trihydrodefluorination product is detected in both cases. Alkenes could be used as the radical trap in both stages A and B, leading to diverse defluorinative coupling products. Interestingly, when two different alkenes were employed in stages A and B, products containing a monofluorinated-tertiary stereogenic center were constructed.
Scheme 16
Further DFT calculations revealed that the chemoselectivity is controlled by the declining reactivity of DMAP-BH2• towards the addition to the defluorinated products, which is attributed to the increasing singly occupied molecular orbital (SOMO)/lowest unoccupied molecular orbital (LUMO) gaps between DMAP-BH2• and the substrates. Therefore, once the first fluoride is removed, the resulting carbonyl group becomes less reactive, thus ensuring excellent chemoselectivity during defluorination [49].
5. Conclusion
C–F bond functionalization of CF3-compounds has recently appeared as an efficient method for the synthesis of partially fluorinated molecules. Recent advances on electrochemistry, photoredox catalysis, and radical chemistry of main group elements have rendered more strategies for C–F bond functionalization reactions. In this review, we summarized recent progress on C–F bond functionalization of CF3 groups involving radical intermediates as the main transformation pathways. Although the C–F bond in the trifluoromethyl group is extremely inert and the defluorination chemoselectivity is difficult to control, important advancement has been made in selective functionalizations of one or two C–F bonds in CF3 groups by different strategies. However, the limitations and challenges are still remained. For example, the sequential functionalization of C–F bonds of trifluoromethylarenes and α, α, α-trifluoroketones with high chemoselectivity has still remained an unsolved challenge. Moreover, the combination of radical chemistry and transition metal catalysis, which is expected to be a powerful tool to construct diversified mono- and difluoro products, has not been well studied. Furthermore, enantioselective transformations of difluoro compounds that are accessed from simple CF3 sources has not been achieved yet. The realization of such strategy would of great value to make monofluorinated tertiary stereogenic centers [50] with high enantioselectiity from simple fluorine sources. We can expect that defluorinative functionalization strategies will continue to make important contributions in organic synthesis, medicinal chemistry, and material science.
Declaration of competing interest
The authors report no declarations of interest.
Acknowledgments
We thank the National Natural Science Foundation of China (No. 21971226) and the Fundamental Research Funds for the Central Universities (No. WK2060000017) for financial support.
References
-
[1]
[1] W.J.Gensler,C.D.Gatsonis,The podophyllotoxin-picropodophyllin equilibrium,J. Org.Chem.31(1966)3224-3227.
-
[2]
[2] Z.Q.Wang,H.Hu,H.X.Chen,Antitumor agents.124.New 4β-substituted aniline derivatives of 6,7-O,O-demethylene-4'-O-demethylopodophyllotoxin and related compounds as potent inhibitors of human DNA topoisomerase Ⅱ,J.Med.Chem.35 (1992)871-877.
-
[3]
[3] L.S.Thurston,H.Irie,S.Tani,Antitumor agents.78.Inhibition of human DNA topoisomerase Ⅱ by podophyllotoxin and α-peltatin analogues,J.Med.Chem.29 (1986)1547-1550.
-
[4]
[4] L.S.Thurston,Y.Imakura,M.Haruna,Antitumor agents.100.Inhibition of human DNA topoisomerase Ⅱ by cytotoxic ether and ester derivatives of podophyllotoxin and α-peltatin,J.Med.Chem.32(1989)604-608.
-
[5]
[5] S.A.Beers,Y.Imakura,H.J.Dai,Antitumor agents.99.Synthetic ring C armatized podophyllotoxin analogues as potential inhibitors of human DNA topoisomerase Ⅱ,J.Nat.Prod.51(1988)901-905.
-
[6]
[6] Y.Hitotsuyanagi,Y.Ichihara,K.Takeya,Synthesis of 4-oxa-2-azapodophyllotoxin, a novel analog of the antitumor lignan podophyllotoxin,Tetrahedron Lett.35 (1994)9401-9402.
-
[7]
[7] W.J.Gensler,C.D.Murthy,M.H.Tranmmell,Nonenolizable podophyllotoxin deri-vatives,J.Med.Chem.20(1977)635-644.
-
[8]
[8] X.M.Zhou,K.J.H.Lee,J.Cheng,Antitumor agents.144.New g-lactone ring-modified arylamino etoposide analogs as inhibitors of human DNA topoisomerase Ⅱ,J.Med.Chem.37(1994)287-292.
-
[9]
[9] H.Hu,S.Y.Liu,Y.C.Cheng,Antitumor agents.123.Synthesis and human DNA topoisomerase Ⅱ inhibitory activity of 20-chloro derivatives of etoposide and 4b-(arylamino)-4'-O-demethylopodophyllotoxins,J.Med.Chem.35(1992) 866-871.
-
[10]
[10] G.Cragg,M.Suffness,Metabolism of plant-derived anticancer agents,Pharmacol. Ther.37(1988)425-461.
-
[11]
[11] A.Wienecke,G.Bacher,Indibulin,a novel microtubule inhibitor,discriminates between mature neuronal and nonneuronal tubulin,Cancer Res.69(2009)171-177.
-
[12]
[12] K.H.Lee,Current developments in the discovery and design of new drug candi-dates from plant natural product leads,J.Nat.Prod.67(2004)273-283.
-
[13]
[13] H.Chen,J.Wang,J.Z.Zhang,L1EPO,a novel podophyllotoxin derivative overcomes P-glycoprotein-mediated multidrug resistance in K562/A02 cell line,Biol.Pharm. Bull.32(2009)609-613.
-
[14]
[14] H.Xu,X.Zhang,X.Tian,Synthesis and insecticidal activity of novel 4b-haloge-nated benzoylamino podophyllotoxins against Pieris rapae LINNAEUS,Chem. Pharm.Bull.50(2002)399-402.
-
[15]
[15] Y.J.Cui,X.Tian,Synthesis and anticancer activity of new derivatives of podo-phyllotoxin,Curr.Sci.75(1998)1383-1386.
-
[16]
[16] S.W.Chen,X.Tian,Y.Q.Tu,Synthesis and cytotoxic activity of novel derivatives of 40-demethylepipodophyllotoxin,Bioorg.Med.Chem.Lett.14(2004)5063-5066.
-
[17]
[17] H.Xu,L.Zhang,X.Tian,A highly improved synthesis of 4b-aminopodophyllo-toxin,Chin.J.Org.Chem.28(2008)1243-1246.
-
[18]
[18] W.L.Xi,Q.Cai,Y.B.Tang,Design and synthesis of novel cytotoxic podophyllotoxin, Chin.Chem.Lett.21(2010)1153-1156.
-
[19]
[19] H.Shang,H.Chen,D.M.Zhao,Synthesis and biological evaluation of 4a/4b-imidazolyl podophyllotoxin analogues as antitumor agents,Arch.Pharm.Chem. Life Sci.345(2012)43-48.
-
[20]
[20] K.H.Lee,S.A.Beers,M.Mori,Antitumor agents.111.New 4-halogenated aniline derivatives of 40-demethylepipodophyllotoxin as potent inhibitors of human DNA topoisomerase Ⅱ,J.Med.Chem.33(1990)1364-1368.
-
[1]
-
-
[1]
[1] W.J.Gensler,C.D.Gatsonis,The podophyllotoxin-picropodophyllin equilibrium,J. Org.Chem.31(1966)3224-3227.
-
[2]
[2] Z.Q.Wang,H.Hu,H.X.Chen,Antitumor agents.124.New 4β-substituted aniline derivatives of 6,7-O,O-demethylene-4'-O-demethylopodophyllotoxin and related compounds as potent inhibitors of human DNA topoisomerase Ⅱ,J.Med.Chem.35 (1992)871-877.
-
[3]
[3] L.S.Thurston,H.Irie,S.Tani,Antitumor agents.78.Inhibition of human DNA topoisomerase Ⅱ by podophyllotoxin and α-peltatin analogues,J.Med.Chem.29 (1986)1547-1550.
-
[4]
[4] L.S.Thurston,Y.Imakura,M.Haruna,Antitumor agents.100.Inhibition of human DNA topoisomerase Ⅱ by cytotoxic ether and ester derivatives of podophyllotoxin and α-peltatin,J.Med.Chem.32(1989)604-608.
-
[5]
[5] S.A.Beers,Y.Imakura,H.J.Dai,Antitumor agents.99.Synthetic ring C armatized podophyllotoxin analogues as potential inhibitors of human DNA topoisomerase Ⅱ,J.Nat.Prod.51(1988)901-905.
-
[6]
[6] Y.Hitotsuyanagi,Y.Ichihara,K.Takeya,Synthesis of 4-oxa-2-azapodophyllotoxin, a novel analog of the antitumor lignan podophyllotoxin,Tetrahedron Lett.35 (1994)9401-9402.
-
[7]
[7] W.J.Gensler,C.D.Murthy,M.H.Tranmmell,Nonenolizable podophyllotoxin deri-vatives,J.Med.Chem.20(1977)635-644.
-
[8]
[8] X.M.Zhou,K.J.H.Lee,J.Cheng,Antitumor agents.144.New g-lactone ring-modified arylamino etoposide analogs as inhibitors of human DNA topoisomerase Ⅱ,J.Med.Chem.37(1994)287-292.
-
[9]
[9] H.Hu,S.Y.Liu,Y.C.Cheng,Antitumor agents.123.Synthesis and human DNA topoisomerase Ⅱ inhibitory activity of 20-chloro derivatives of etoposide and 4b-(arylamino)-4'-O-demethylopodophyllotoxins,J.Med.Chem.35(1992) 866-871.
-
[10]
[10] G.Cragg,M.Suffness,Metabolism of plant-derived anticancer agents,Pharmacol. Ther.37(1988)425-461.
-
[11]
[11] A.Wienecke,G.Bacher,Indibulin,a novel microtubule inhibitor,discriminates between mature neuronal and nonneuronal tubulin,Cancer Res.69(2009)171-177.
-
[12]
[12] K.H.Lee,Current developments in the discovery and design of new drug candi-dates from plant natural product leads,J.Nat.Prod.67(2004)273-283.
-
[13]
[13] H.Chen,J.Wang,J.Z.Zhang,L1EPO,a novel podophyllotoxin derivative overcomes P-glycoprotein-mediated multidrug resistance in K562/A02 cell line,Biol.Pharm. Bull.32(2009)609-613.
-
[14]
[14] H.Xu,X.Zhang,X.Tian,Synthesis and insecticidal activity of novel 4b-haloge-nated benzoylamino podophyllotoxins against Pieris rapae LINNAEUS,Chem. Pharm.Bull.50(2002)399-402.
-
[15]
[15] Y.J.Cui,X.Tian,Synthesis and anticancer activity of new derivatives of podo-phyllotoxin,Curr.Sci.75(1998)1383-1386.
-
[16]
[16] S.W.Chen,X.Tian,Y.Q.Tu,Synthesis and cytotoxic activity of novel derivatives of 40-demethylepipodophyllotoxin,Bioorg.Med.Chem.Lett.14(2004)5063-5066.
-
[17]
[17] H.Xu,L.Zhang,X.Tian,A highly improved synthesis of 4b-aminopodophyllo-toxin,Chin.J.Org.Chem.28(2008)1243-1246.
-
[18]
[18] W.L.Xi,Q.Cai,Y.B.Tang,Design and synthesis of novel cytotoxic podophyllotoxin, Chin.Chem.Lett.21(2010)1153-1156.
-
[19]
[19] H.Shang,H.Chen,D.M.Zhao,Synthesis and biological evaluation of 4a/4b-imidazolyl podophyllotoxin analogues as antitumor agents,Arch.Pharm.Chem. Life Sci.345(2012)43-48.
-
[20]
[20] K.H.Lee,S.A.Beers,M.Mori,Antitumor agents.111.New 4-halogenated aniline derivatives of 40-demethylepipodophyllotoxin as potent inhibitors of human DNA topoisomerase Ⅱ,J.Med.Chem.33(1990)1364-1368.
-
[1]
-

-
-
[1]
Jin Wang , Xiaoyan Pan , Junyu Zhang , Qingqing Zhang , Yanchen Li , Weiwei Guo , Jie Zhang . Active molecule-based theranostic agents for tumor vasculature normalization and antitumor efficacy. Chinese Chemical Letters, 2024, 35(8): 109187-. doi: 10.1016/j.cclet.2023.109187
-
[2]
Chuan Li , Yangyang Han , Yanan Zhai , Ke Li , Xingzhong Liu , Zhuan Zhang , Cai Jia , Yongsheng Che . Phomaketals A and B, pentacyclic meroterpenoids from a eupC overexpressed mutant strain of Phoma sp.. Chinese Chemical Letters, 2024, 35(7): 109019-. doi: 10.1016/j.cclet.2023.109019
-
[3]
Yunhao Zhang , Yinuo Wang , Siran Wang , Dazhen Xu . Progress in Selective Construction of Functional Aromatics from Nitrogenous Cycloalkanes. University Chemistry, 2024, 39(11): 136-145. doi: 10.3866/PKU.DXHX202401083
-
[4]
Ping Sun , Yuanqin Huang , Shunhong Chen , Xining Ma , Zhaokai Yang , Jian Wu . Indole derivatives as agrochemicals: An overview. Chinese Chemical Letters, 2024, 35(7): 109005-. doi: 10.1016/j.cclet.2023.109005
-
[5]
Jing JIN , Zhuming GUO , Zhiyin XIAO , Xiujuan JIANG , Yi HE , Xiaoming LIU . Tuning the stability and cytotoxicity of fac-[Fe(CO)3I3]- anion by its counter ions: From aminiums to inorganic cations. Chinese Journal of Inorganic Chemistry, 2024, 40(5): 991-1004. doi: 10.11862/CJIC.20230458
-
[6]
Yan-Li Li , Zhi-Ming Li , Kai-Kai Wang , Xiao-Long He . Beyond 1,4-addition of in-situ generated (aza-)quinone methides and indole imine methides. Chinese Chemical Letters, 2024, 35(7): 109322-. doi: 10.1016/j.cclet.2023.109322
-
[7]
Wei Zhou , Xi Chen , Lin Lu , Xian-Rong Song , Mu-Jia Luo , Qiang Xiao . Recent advances in electrocatalytic generation of indole-derived radical cations and their applications in organic synthesis. Chinese Chemical Letters, 2024, 35(4): 108902-. doi: 10.1016/j.cclet.2023.108902
-
[8]
Jiajun Wang , Guolin Yi , Shengling Guo , Jianing Wang , Shujuan Li , Ke Xu , Weiyi Wang , Shulai Lei . Computational design of bimetallic TM2@g-C9N4 electrocatalysts for enhanced CO reduction toward C2 products. Chinese Chemical Letters, 2024, 35(7): 109050-. doi: 10.1016/j.cclet.2023.109050
-
[9]
Kai Han , Guohui Dong , Ishaaq Saeed , Tingting Dong , Chenyang Xiao . Morphology and photocatalytic tetracycline degradation of g-C3N4 optimized by the coal gangue. Chinese Journal of Structural Chemistry, 2024, 43(2): 100208-100208. doi: 10.1016/j.cjsc.2023.100208
-
[10]
Xuejiao Wang , Suiying Dong , Kezhen Qi , Vadim Popkov , Xianglin Xiang . Photocatalytic CO2 Reduction by Modified g-C3N4. Acta Physico-Chimica Sinica, 2024, 40(12): 2408005-. doi: 10.3866/PKU.WHXB202408005
-
[11]
Liang Ma , Zhou Li , Zhiqiang Jiang , Xiaofeng Wu , Shixin Chang , Sónia A. C. Carabineiro , Kangle Lv . Effect of precursors on the structure and photocatalytic performance of g-C3N4 for NO oxidation and CO2 reduction. Chinese Journal of Structural Chemistry, 2024, 43(11): 100416-100416. doi: 10.1016/j.cjsc.2024.100416
-
[12]
Yanghanbin Zhang , Dongxiao Wen , Wei Sun , Jiahe Peng , Dezhong Yu , Xin Li , Yang Qu , Jizhou Jiang . State-of-the-art evolution of g-C3N4-based photocatalytic applications: A critical review. Chinese Journal of Structural Chemistry, 2024, 43(12): 100469-100469. doi: 10.1016/j.cjsc.2024.100469
-
[13]
Hong-Tao Ji , Yu-Han Lu , Yan-Ting Liu , Yu-Lin Huang , Jiang-Feng Tian , Feng Liu , Yan-Yan Zeng , Hai-Yan Yang , Yong-Hong Zhang , Wei-Min He . Nd@C3N4-photoredox/chlorine dual catalyzed synthesis and evaluation of antitumor activities of 4-alkylated sulfonyl ketimines. Chinese Chemical Letters, 2025, 36(2): 110568-. doi: 10.1016/j.cclet.2024.110568
-
[14]
Entian Cui , Yulian Lu , Zhaoxia Li , Zhilei Chen , Chengyan Ge , Jizhou Jiang . Interfacial B-O bonding modulated S-scheme B-doped N-deficient C3N4/O-doped-C3N5 for efficient photocatalytic overall water splitting. Chinese Chemical Letters, 2025, 36(1): 110288-. doi: 10.1016/j.cclet.2024.110288
-
[15]
Zhi Zhu , Xiaohan Xing , Qi Qi , Wenjing Shen , Hongyue Wu , Dongyi Li , Binrong Li , Jialin Liang , Xu Tang , Jun Zhao , Hongping Li , Pengwei Huo . Fabrication of graphene modified CeO2/g-C3N4 heterostructures for photocatalytic degradation of organic pollutants. Chinese Journal of Structural Chemistry, 2023, 42(12): 100194-100194. doi: 10.1016/j.cjsc.2023.100194
-
[16]
Xiaoming Fu , Haibo Huang , Guogang Tang , Jingmin Zhang , Junyue Sheng , Hua Tang . Recent advances in g-C3N4-based direct Z-scheme photocatalysts for environmental and energy applications. Chinese Journal of Structural Chemistry, 2024, 43(2): 100214-100214. doi: 10.1016/j.cjsc.2024.100214
-
[17]
Guangming YIN , Huaiyao WANG , Jianhua ZHENG , Xinyue DONG , Jian LI , Yi'nan SUN , Yiming GAO , Bingbing WANG . Preparation and photocatalytic degradation performance of Ag/protonated g-C3N4 nanorod materials. Chinese Journal of Inorganic Chemistry, 2024, 40(8): 1491-1500. doi: 10.11862/CJIC.20240086
-
[18]
Qingwang LIU . MoS2/Ag/g-C3N4 Z-scheme heterojunction: Preparation and photocatalytic performance. Chinese Journal of Inorganic Chemistry, 2025, 41(4): 821-832. doi: 10.11862/CJIC.20240148
-
[19]
Min WANG , Dehua XIN , Yaning SHI , Wenyao ZHU , Yuanqun ZHANG , Wei ZHANG . Construction and full-spectrum catalytic performance of multilevel Ag/Bi/nitrogen vacancy g-C3N4/Ti3C2Tx Schottky junction. Chinese Journal of Inorganic Chemistry, 2024, 40(6): 1123-1134. doi: 10.11862/CJIC.20230477
-
[20]
Jianyu Qin , Yuejiao An , Yanfeng Zhang . In Situ Assembled ZnWO4/g-C3N4 S-Scheme Heterojunction with Nitrogen Defect for CO2 Photoreduction. Acta Physico-Chimica Sinica, 2024, 40(12): 2408002-. doi: 10.3866/PKU.WHXB202408002
-
[1]
Metrics
- PDF Downloads(0)
- Abstract views(813)
- HTML views(2)

 Login In
Login In




 DownLoad:
DownLoad:
 DownLoad:
DownLoad: