Citation:
Lou Zhen FAN, Yong Fang LI, Feng Ying LI, Yu Liang LI, Dao Ben ZHU. Positive Shifts of the Reduction Potentials of C60 by Substitution of Cyanogens[J]. Chinese Chemical Letters,
;1998, 9(1): 99-101.

-
The electrochemical properties of a new derivative of C60 were investigated by cyclic voltammetry. It was found that the derivative was reduced at a more positive potential than C60, reflecting its significance to accept electron easily. Its NIR characteristic absorption bands of monoanion and dianion were also obtained.
-
Chemotherapy, as one of the most common modalities to treat various diseases, such as cancers and human immunodeficiency virus (HIV), faces a variety of challenges in clinical applications, including but not limited to poor specificity and associated sideeffects. To address these challenges, a plethora of stimuliresponsive prodrugs including dimeric and polymeric prodrugs have been developed and exhibited therapeutic improvements in preclinical and even clinical settings [1-8]. For instance, camptothecin (CPT) dimer, connected by a reduction-labile maleimide thioether bond was designed and loaded into an acid-active nanoplatform for the targeted killing of tumor cells [9]. Moreover, the CPT dimer possesses Förster resonance energy transfer (FRET) effect between CPT and maleimide thioether bond, and the reversion of the dimer to monomeric CPT resulted in the "turnoff" of the FRET signal, making the drug release process trackable. Furthermore, abacavir dimeric prodrug was designed to facilitate the blood brain barrier penetration via inhibiting the drug's efflux by P-glycoprotein, and the dimeric drug was reverted back into potent monomeric therapeutic agent specifically inside target cells [10]. Although these covalent dimeric prodrugs exhibited promising selectivity towards cancer cells, the preparation of these materials often involves ingenious design and complex synthesis, and each synthetic strategy cannot be translated to dimerization of other drug systems. In addition, many of these dimeric prodrugs often need to be further loaded into nanomaterials for improved therapy [2]. A more facile, general approach to prepare dimeric prodrug that may be used directly as a therapeutic agent is highly sought after.
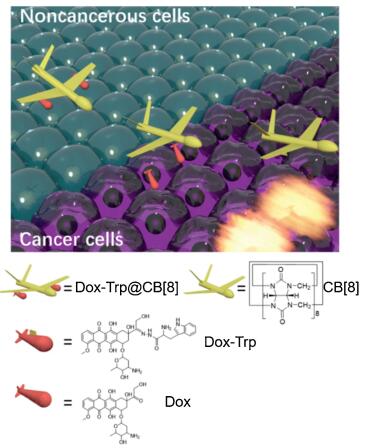
Due to excellent guest-binding behaviours, macrocyclic cucurbit[n]uril (CB[n], n = 5–8 and 10) have attracted increasing attentions in supramolecular chemistry during recent years [11-18]. Recent studies have demonstrated that CB[7] may serve as an effective pharmaceutical excipient that offers a variety of benefits, such as taste-masking and side effect alleviation of the included drugs [19, 20]. On the other hand, CB[8] (Fig. 1) may simultaneously bind two guest drug molecules inside the cavity to form a stable ternary complex, doubling drug loading of that from either CB[7] or CB[8]-based binary complex systems [21-23]. Therefore, CB[8] has been frequently employed as a non-covalent crosslinker in construction of a variety of functional materials [11, 24-28]. One typical example is that peptides or proteins with tryptophan (Trp) or phenylalanine (Phe) residues at N-terminal could be dimerized by CB[8] via homo-ternary complexation, which was firstly discovered by Urbach and coworkers [23]. Such strategy has been applied to dimerize proteins and manipulate their enzymatic activity [29, 30]. Under the same principles, Scherman and co-workers demonstrated the preparation of supramolecular hydrogels by CB[8]-mediated crosslinking of Phe-functionalized polysaccharides, which have exhibited potential applications in controlled drug delivery and tissue engineering [31-33]. Similarly, Wang et al. reported an ecofriendly antibiotic based on CB[8] crosslinked Phe-functionalized polyethylenimine [34]. However, in all of these previous examples, CB[8] was employed to crosslink peptides, proteins and polymers, whereas CB[8]-crosslinked dimeric drug molecules are extremely rare. Herein we designed a doxorubicin (DOX) prodrug DOX-Trp that contains an acid-labile hydrazone bond, and we demonstrate for the first time that a supramolecular dimer of DOX based on CB[8] and Trp modified DOX (DOX-Trp) may act as a stimuli pHresponsive DOX dimer prodrug system that may transport DOX efficiently and selectively to cancer cells. This novel supramolecular drug dimer system resulted in improved therapeutic effect towards cancer cells with minimal cytotoxicity against noncancerous cells.
Figure 1
 Figure 1. Schematic illustration of CB[8] mediated dimerization of DOX-Trp and the selective release of DOX against cancer cells.
Figure 1. Schematic illustration of CB[8] mediated dimerization of DOX-Trp and the selective release of DOX against cancer cells.Firstly, Trp conjugated DOX via a hydrazone bond, DOX-Trp was synthesized. The synthetic procedures are detailed in Scheme S1 (Supporting information), and its chemical structure was confirmed by 1H NMR and 13C NMR and HR-ESI-MS. Supramolecular dimer of DOX, crosslinked by CB[8], was formed when DOX-Trp and CB[8] were mixed at 2:1 molecular ratio in an aqueous solution. 1H NMR, isothermal titration calorimetry (ITC) and ESI-MS were employed to investigate the formation of such a ternary complex. As shown in Fig. S1 (Supporting information), the mixture exhibited significant exchange broadening on 1H NMR spectra during titration with CB[8], consistent with previous observations reported by Urbach and coworkers, which is inconclusive for the interaction and binding ratio between CB[8] and DOX-Trp [23]. The ITC experiment was conducted by injection of DOX-Trp (1.0 mmol/L) into CB[8] solution (0.024mmol/L) in the cell. The integrated thermogram (Fig. 2) performed after the deduction of the blank control was fitted to the "sequentialbinding"model, resultingina2:1binding stoichiometryof DOX-Trp@CB[8], with stepwise bimolecular association constants determined to be Ks1 = 1.99×106 L/mol and Ks2 = 1.14×105 L/mol, respectively, slightly larger than the previously reported values (Ks1 = 1.3×105 L/mol and Ks2 = 2.8×104 L/mol), likely due to the absenceof saltsin theultrapurewater solutionsinour study, instead of PBS used in the previous study [23, 35]. Furthermore, ESI-MS analysis (Fig. S2 in Supporting information) revealed a characteristic triply charged peak at m/z 939.67, corresponding to [(DOXTrp)2@CB[8]+3H]3+ (calcd. value 939.66), further supported the 2:1 binding ratio between DOX-Trp and CB[8].
Figure 2
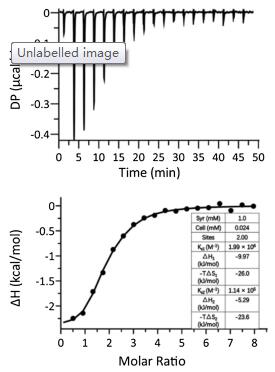 Figure 2. ITC results of the titration of DOX-Trp into CB[8] in aqueous solutions at 25.0 ℃. Top: Thermogram of 19 drops (0.4 μL for the first drop and 2 μL per drop for the rest 18 drops) of DOX-Trp (1.0 mmol/L, 0.04 μL) injected into CB[8] solution (0.024 mmol/L, 0.2 mL). Bottom: The dependence of △H against the molar ratio between DOX-Trp and CB[8] during titration; the solid line represents the best fit plot by using the "sequential binding" binding model.
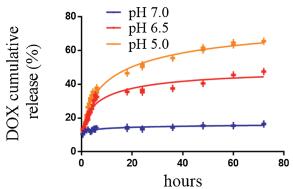
Figure 2. ITC results of the titration of DOX-Trp into CB[8] in aqueous solutions at 25.0 ℃. Top: Thermogram of 19 drops (0.4 μL for the first drop and 2 μL per drop for the rest 18 drops) of DOX-Trp (1.0 mmol/L, 0.04 μL) injected into CB[8] solution (0.024 mmol/L, 0.2 mL). Bottom: The dependence of △H against the molar ratio between DOX-Trp and CB[8] during titration; the solid line represents the best fit plot by using the "sequential binding" binding model.As the prodrug DOX-Trp contained an acid-labile hydrazone bond, it was expected that the dimer system would exhibit pH responsive release of DOX under acidic conditions typically encountered in tumour tissues and cells. As the typical pH in cancer tissues and around cancer cells is approximately 6.5 and even lower in lysosome (pH 5.0), and the pH of extracellular fluids and around normal cells is approximately 7.0 [36-38]. Therefore, we chose pH 5.0, 6.5 and 7.0 to examine the drug release behaviours of DOX-Trp@CB[8]. Thus, DOX release rate of the supramolecular dimer was studied under different pH conditions using HPLC. As shown in Fig. 3, the dimer system showed decent stability when incubated in a PBS solution at pH 7.0, with accumulated DOX release less than 16% after incubation for 72 h. Conversely, the release of free DOX reached up to 62% and 49% when incubated at pH 5.0 and 6.5, respectively, for 72 h, confirming the pH sensitivity of the dimer system.
Figure 3
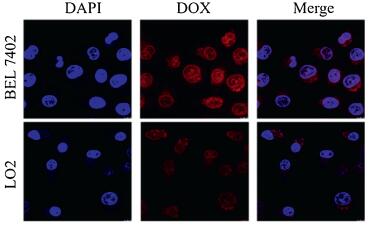
Next, the cellular uptake behaviours of the dimer system by LO2 and BEL 7402 cell lines, as representative noncancerous and cancer cell lines, were investigated via confocal laser scanning microscopy (CLSM) upon incubating the cells with the dimer system for 24 h. As shown in Fig. 4, DAPI was employed to stain the cell nucleus with blue fluorescence, while DOX possesses naturally-occurring red fluorescence. Upon incubation of the cells with DOX-Trp@CB[8] for 24 h, BEL 7402 and LO2 cells exhibited obviously different levels of uptake of DOX, as much higher-intensity of red fluorescence was observed in BEL 7402 cells than that in LO2 cells. In addition, most of red fluorescence in LO2 cells was concentrated in the cytoplasm, while in BEL 7402 cells red fluorescence was mainly situated in the nucleus, suggesting that DOX was effectively released by the dimer system and more efficiently taken up by the cancer cells. With regards to free DOX-Trp (Fig. S3 in Supporting information), both LO2 cells and BEL 7402 cells were infiltrated by red fluorescence produced by DOX, indicating that DOX-Trp does not have the ability to selectively release DOX. In contrast, the dimer system likely remained its integrity in the relatively neutral microenvironment of LO2 cell lines, which has limited its uptake by the cells. Subsequently, the quantitative analysis of cellular uptake by flow cytometry was conducted. As shown in Figs. S4 and S5 (Supporting information), the amount of DOX taken up by BEL 7402 cells was nearly 10-fold higher than that by LO2 cells. This was likely attributed to the different pH micro-environments of cancerous cells and noncancerous cells, where free DOX could not be released at neutral pH conditions encountered near noncancerous cells, resulting in significantly reduced uptake by noncancerous cells.
Figure 4
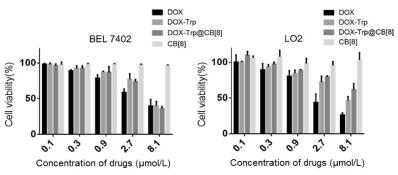
To further investigate the selective drug release profile of the supramolecular dimer system in cancer cells, cytotoxicity experiment was conducted in both cancerous and non-cancerous cell lines. For comparison, each cell line was incubated with free DOX, DOX-Trp, DOX-Trp@CB[8] and CB[8], respectively, and were subsequently investigated via MTT assays. As shown in Fig. 5, after incubation for 48 h, free DOX, DOX-Trp and DOX-Trp@CB[8] exhibited comparable anticancer activities against BEL 7402 cells with the IC50 (inhibitory concentration to produce 50% cell death) values determined to be 4.85±1.28 μmol/L, 7.168±1.23 μmol/L and 4.61±1.01 μmol/L, respectively. In contrast, the DOX-Trp@CB[8] group exhibited remarkably reduced cytotoxicity against LO2 cell line after 48 h of incubation, when compared to DOX-Trp group and free DOX group. The IC50 value was determined to be 13.66± 1.21 μmol/LforDOX-Trp@CB[8], significantly higher than thatoffree DOX (2.11±1.08 μmol/L) and DOX-Trp (8.01±0.88 μmol/L) against LO2 cells, suggesting that the dimer system may set the ammunition (DOX) free at the target site due to the inherent pH sensitivity. The dramatically improved safety profile of the dimer system against noncancerous cells was consistent with the reduced cellular uptake by noncancerous cells, likely attributed to the reduced transmembrane transport of the supramolecular dimer. Our previous studies have exhibited that supramolecular complexation of a guest species by cucurbituril may inhibit its cellular uptake [39, 40]. In fact, DOX has proven anticancer activity, however, its clinical application has been mainly hampered by its systemic toxicity. Thus, the supramolecular dimer system may provide a novel approach to alleviate the cytotoxicity of DOX while maintaining its cytotoxicity against cancer cells in a specific manner.
Figure 5
In summary, we have designed and developed a novel pHsensitive supramolecular DOX dimer system, via CB[8] mediated DOX-Trp homo-dimerization, which has exhibited selective ammunition (DOX) release in cancer cells, thus exerting a significantly improved safety profile against normal cells while maintaining effective cytotoxicity against cancer cells. Under this strategy, many other anticancer drugs could be chemically modified and loaded as a dimeric "ammunition" into this supramolecular dimer system for improved cancer therapy.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This work was supported by the Science and Technology Development Fund, Macau SAR (No. 030/2017/A1), University of Macau (No. MYRG2016-00008-ICMS-QRCM) and the National Natural Science Foundation of China (No. 21871301).
Appendix A. Supplementary data
Supplementary material related to this article canbefound, in the online version, at doi:https://doi.org/10.1016/j.cclet.2019.10.020.
-

-
-
[1]
Zhengzheng LIU , Pengyun ZHANG , Chengri WANG , Shengli HUANG , Guoyu YANG . Synthesis, structure, and electrochemical properties of a sandwich-type {Co6}-cluster-added germanotungstate. Chinese Journal of Inorganic Chemistry, 2024, 40(6): 1173-1179. doi: 10.11862/CJIC.20240039
-
[2]
Hongyu Tang , Dongming Liu , Jinfu Huang , Liang Zhang , Yang Tang , Bin Huang , Yanwei Li , Shunhua Xiao , Yiling Sun , Renheng Wang . Excellent structural stability and electrochemical properties of LiNi0.9Co0.05Mn0.05O2 material by surface Ni2+ anchoring and Cs+ doping. Chinese Chemical Letters, 2025, 36(6): 109987-. doi: 10.1016/j.cclet.2024.109987
-
[3]
Wenjiang LI , Pingli GUAN , Rui YU , Yuansheng CHENG , Xianwen WEI . C60-MoP-C nanoflowers van der Waals heterojunctions and its electrocatalytic hydrogen evolution performance. Chinese Journal of Inorganic Chemistry, 2024, 40(4): 771-781. doi: 10.11862/CJIC.20230289
-
[4]
Tong Li , Leping Pan , Yan Zhang , Jihu Su , Kai Li , Kuiliang Li , Hu Chen , Qi Sun , Zhiyong Wang . Electrochemical construction of 2,5-diaryloxazoles via N–H and C(sp3)-H functionalization. Chinese Chemical Letters, 2024, 35(4): 108897-. doi: 10.1016/j.cclet.2023.108897
-
[5]
Ruike Hu , Kangmin Wang , Junxiang Liu , Jingxian Zhang , Guoliang Yang , Liqiu Wan , Bijin Li . Extended π-conjugated systems by external ligand-assisted C−H olefination of heterocycles: Facile access to single-molecular white-light-emitting and NIR fluorescence materials. Chinese Chemical Letters, 2025, 36(4): 110113-. doi: 10.1016/j.cclet.2024.110113
-
[6]
Jiqing Liu , Qi Dang , Liting Wang , Dejin Wang , Liang Tang . Applications of flexible electrochemical electrodes in wastewater treatment: A review. Chinese Chemical Letters, 2024, 35(8): 109277-. doi: 10.1016/j.cclet.2023.109277
-
[7]
Yi ZHANG , Guang LI , Wenxuan FAN , Qingfeng YI . Influence of bismuth trisulfide on the electrochemical performance of iron electrode. Chinese Journal of Inorganic Chemistry, 2025, 41(6): 1196-1206. doi: 10.11862/CJIC.20240445
-
[8]
Linghui Zou , Meng Cheng , Kaili Hu , Jianfang Feng , Liangxing Tu . Vesicular drug delivery systems for oral absorption enhancement. Chinese Chemical Letters, 2024, 35(7): 109129-. doi: 10.1016/j.cclet.2023.109129
-
[9]
Xiaoyu Chen , Jiahao Hu , Jingyi Lin , Haiyang Huang , Changqing Ye , Hongli Bao . Biisoindolylidene solvatochromic fluorophores: Synthesis and photophysical properties. Chinese Chemical Letters, 2025, 36(2): 109923-. doi: 10.1016/j.cclet.2024.109923
-
[10]
Bowen Song , Chenxu Shi , Yinghao Qu , Hongjun Liu , Hui Yang , Xiaoming Wu , Xijun Liu . The electrical properties and charge transport mechanism of MXenes. Chinese Chemical Letters, 2025, 36(6): 110823-. doi: 10.1016/j.cclet.2025.110823
-
[11]
Lei Wang , Jun-Jie Wu , Chang-Cun Yan , Wan-Ying Yang , Zong-Lu Che , Xin-Yu Xia , Xue-Dong Wang , Liang-Sheng Liao . Near-infrared organic lasers with ultra-broad emission bands by simultaneously harnessing four-level and six-level systems. Chinese Chemical Letters, 2024, 35(8): 109365-. doi: 10.1016/j.cclet.2023.109365
-
[12]
Yang Yang , Jing-Li Luo , Xian-Zhu Fu . Water-oxidation intermediates enabling electrochemical propylene epoxidation. Chinese Journal of Structural Chemistry, 2024, 43(5): 100269-100269. doi: 10.1016/j.cjsc.2024.100269
-
[13]
Kailong Zhang , Chao Zhang , Luanhui Wu , Qidong Yang , Jiadong Zhang , Guang Hu , Liang Song , Gaoran Li , Wenlong Cai . Chloride molten salt derived attapulgite with ground-breaking electrochemical performance. Chinese Chemical Letters, 2024, 35(10): 109618-. doi: 10.1016/j.cclet.2024.109618
-
[14]
Chuang LIU , Lichao SUN , Qingfeng ZHANG . Chiral inorganic nanocatalysts for electrochemical and enzyme-mimicked biosensing. Chinese Journal of Inorganic Chemistry, 2025, 41(1): 59-78. doi: 10.11862/CJIC.20240406
-
[15]
Muhammad Humayun , Mohamed Bououdina , Abbas Khan , Sajjad Ali , Chundong Wang . Designing single atom catalysts for exceptional electrochemical CO2 reduction. Chinese Journal of Structural Chemistry, 2024, 43(1): 100193-100193. doi: 10.1016/j.cjsc.2023.100193
-
[16]
Tianli Hui , Tao Zheng , Xiaoluo Cheng , Tonghui Li , Rui Zhang , Xianghai Meng , Haiyan Liu , Zhichang Liu , Chunming Xu . A review of plasma treatment on nano-microstructure of electrochemical water splitting catalysts. Chinese Journal of Structural Chemistry, 2025, 44(3): 100520-100520. doi: 10.1016/j.cjsc.2025.100520
-
[17]
Liwei Hou , Xianyun Peng , Siliu Lyu , Zhongjian Li , Bin Yang , Qinghua Zhang , Qinggang He , Lecheng Lei , Yang Hou . Advancements in MXene-based nanohybrids for electrochemical water splitting. Chinese Chemical Letters, 2025, 36(6): 110392-. doi: 10.1016/j.cclet.2024.110392
-
[18]
Xiaonan LI , Hui HAN , Yihan ZHANG , Jing XIONG , Tingting GUO , Juanzhi YAN . A viologen‐based Cd(Ⅱ) coordination polymer: Self‐assembly, thermochromism, and electrochemical property. Chinese Journal of Inorganic Chemistry, 2025, 41(7): 1439-1444. doi: 10.11862/CJIC.20240376
-
[19]
Kang Wang , Qinglin Zhou , Weijin Li . Conductive metal-organic frameworks for electromagnetic wave absorption. Chinese Journal of Structural Chemistry, 2024, 43(10): 100325-100325. doi: 10.1016/j.cjsc.2024.100325
-
[20]
Ju Wang , Yongbing Sun , Lingbang Meng , Jianfang Feng , Meng Cheng , Liangxing Tu . Intestinal transporters and oral absorption enhancing strategies based on these transporters. Chinese Chemical Letters, 2025, 36(5): 110529-. doi: 10.1016/j.cclet.2024.110529
-
[1]
Metrics
- PDF Downloads(0)
- Abstract views(715)
- HTML views(6)

 Login In
Login In




 DownLoad:
DownLoad:
 DownLoad:
DownLoad: