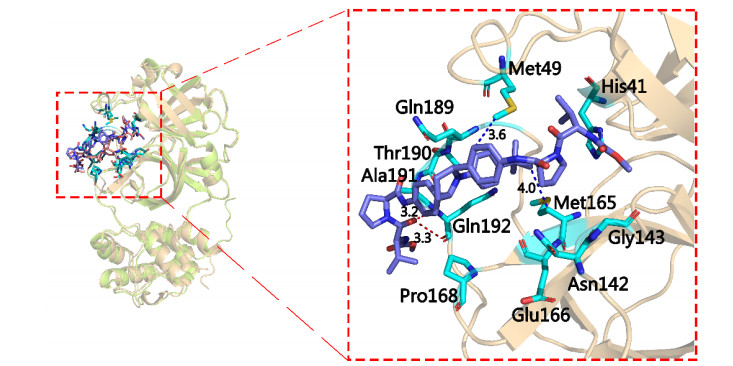
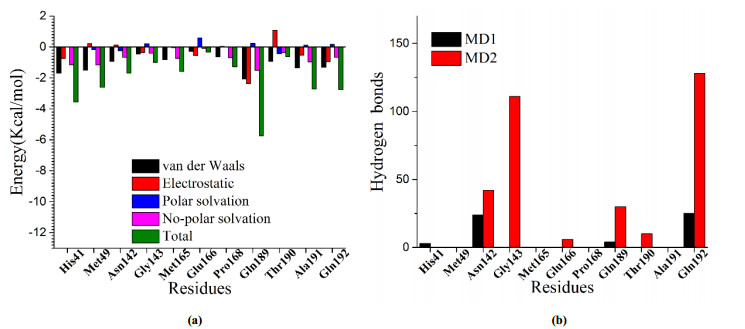
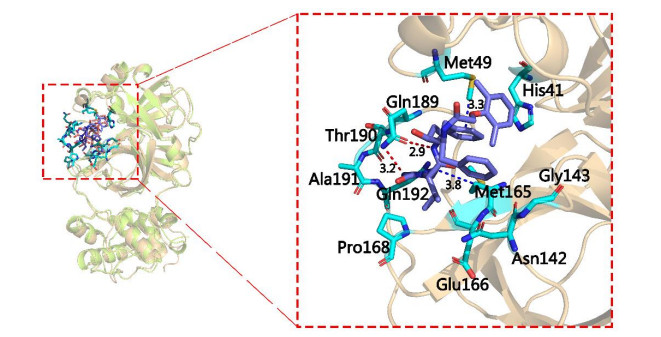
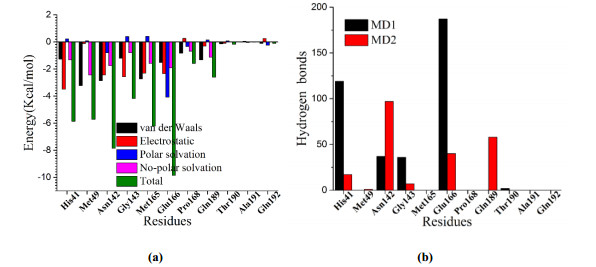
-
[1]
Bassetti, M.; Vena, A.; Roberto Giacobbe Giacobbe, D. R. The novel Chinese coronavirus (2019-nCoV) infections: challenges for fighting the storm. Eur. J. Clin. Invest. 2020, e13209−4.
-
[2]
Chen, N.; Zhou, M.; Dong, X.; Qu, J.; Gong, F.; Han, Y.; Qiu, Y.; Liu, Y.; Wei, Y.; Xia, J. A.; Yu, T.; Zhang, X. X.; Zhang, L. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet 2020, 395, 507−513.
doi: 10.1016/S0140-6736(20)30211-7
-
[3]
Huang, C. L.; Wang, Y. M.; Li, X. W.; Ren, L. L.; Zhao, J. P.; Hu, Y.; Zhang, L.; Fan, G. H.; Xu, J. Y.; Gu, X. Y.; Cheng, Z. S.; Yu, T.; Xia, J. A.; Wei, Y.; Wu, W. J.; Xie, X. L.; Yin, W.; Li, H.; Cao, B. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020, 395, 497−506.
doi: 10.1016/S0140-6736(20)30183-5
-
[4]
Hui, D. S.; Azhar, E. I.; Madani, T. A.; Ntoumi, F.; Kock, R.; Dar, O.; Ippolito, G.; Mchugh, T. D.; Memish, Z. A. Arabia, H. R. S.; Drosten, C.; Germany, B.; Zumla, A.; Petersen, E.; Zumla, A. The continuing 2019-nCoV epidemic threat of novel coronaviruses to global health – The latest 2019 novel coronavirus outbreak in Wuhan, China. Int. J. Infect. Dis. 2020, 91, 264−266.
doi: 10.1016/j.ijid.2020.01.009
-
[5]
Xu, X.; Chen, P.; Wang, J.; Feng, J.; Zhou, H.; Li, X.; Zhong, W.; Hao, P. Evolution of the novel coronavirus from the ongoing Wuhan outbreak and modeling of its spike protein for risk of human transmission. Sci. China Life Sci. 2020, 63, 457−460.
doi: 10.1007/s11427-020-1637-5
-
[6]
Gallagher, T. M.; Buchmeier, M. J. Coronavirus spike proteins in viral entry and pathogenesis. Virology 2001, 279, 371−374.
doi: 10.1006/viro.2000.0757
-
[7]
Liu, X.; Wang, X. J. Potential inhibitors against 2019-nCoV coronavirus M protease from clinically approved medicines. J. Genet. Genom. 2020, 119–121.
-
[8]
Seah, I.; Su, X.; Lingam, G. Revisiting the dangers of the coronavirus in the ophthalmology practice. Nature 2020, 1−3.
-
[9]
Su, S.; Wong, G.; Shi, W.; Liu, J.; Lai, A. C.; Zhou, J.; Liu, W. J.; Bi, Y. H.; Gao, G. F. Epidemiology, genetic recombination, and pathogenesis of coronaviruses. Trends Microbiol. 2016, 24, 490−502.
doi: 10.1016/j.tim.2016.03.003
-
[10]
Wang, W.; Tang, J.; Wei, F. Updated understanding of the outbreak of 2019 novel coronavirus (2019-nCoV) in Wuhan, China. J. Med. Virol. 2020, 92, 441−447.
doi: 10.1002/jmv.25689
-
[11]
Pang, J.; Wang, M. X.; Ang, I. Y. H.; Tan, S. H. X.; Lewis, R. F.; Chen, J. I. P.; Gutierrez, R. A.; Gwee, S.; X.; W.; Chua, P. E. Y.; Yang, Q.; Ng, X. Y.; Yap, R. K. S.; Tan, H. Y.; Teo, Y. Y.; Tan, C. C.; Cook, A. R.; Yap, J. C. H.; Hsu, L. Y. Potential rapid diagnostics, vaccine and therapeutics for 2019 novel coronavirus (2019-nCoV): a systematic review. J. Clin. Med. 2020, 9, 623−623.
doi: 10.3390/jcm9030623
-
[12]
Andersen, K. G.; Rambaut, A.; Lipkin, W. I.; Holmes, E. C. The proximal origin of SARS-CoV-2. Nat. Med. 2020, 26, 450−452.
doi: 10.1038/s41591-020-0820-9
-
[13]
Jain, A. N. Scoring noncovalent protein-ligand interactions: a continuous differentiable function tuned to compute binding affinities. J. Comput. Aid. Mol. Des. 1996, 10, 427−440.
doi: 10.1007/BF00124474
-
[14]
Feng, Z.; Pearce, L. V.; Xu, X.; Yang, X.; Yang, P.; Blumberg, P. M.; Xie, X. Q. Structural insight into tetrameric hTRPV1 from homology modeling, molecular docking, molecular dynamics simulation, virtual screening, and bioassay validations. J. Chem. Inf. Model. 2015, 55, 572−588.
doi: 10.1021/ci5007189
-
[15]
Chen, J. Z.; Wang, J.; Xie, X. Q. GPCR structure-based virtual screening approach for CB2 antagonist search. J. Chem. Inf. Model. 2007, 47, 1626−1637.
doi: 10.1021/ci7000814
-
[16]
Sheng, S.; Wang, J.; Wang, L.; Liu, H.; Li, P.; Liu, M.; Long, C. F.; Xie, C. S.; Xie, X. Q.; Su, W. Network pharmacology analyses of the antithrombotic pharmacological mechanism of Fufang Xueshuantong Capsule with experimental support using disseminated intravascular coagulation rats. J. Ethnopharmacol. 2014, 154, 735−744.
doi: 10.1016/j.jep.2014.04.048
-
[17]
Feng, Z. W.; Kochanek, S.; Close, D.; Wang, L. R.; Srinivasan, A.; Almehizia, A. A.; Iyer, P.; Xie, X. Q.; Johnston, P. A.; Gold, B. Design and activity of AP endonuclease-1 inhibitors. J. Chem. Biol. 2015, 8, 79−93.
doi: 10.1007/s12154-015-0131-7
-
[18]
Feng, Z. W.; Pearce, L. V.; Zhang, Y.; Xing, C.; Herold, B. K.; Ma, S.; Hu, Z.; Turcios, N. A.; Yang, P.; Tong, Q.; Blumberg, P. M.; McCall, A. K. Multi-functional diarylurea small molecule inhibitors of TRPV1 with therapeutic potential for neuroinflammation. AAPS J. 2016, 18, 898−913.
doi: 10.1208/s12248-016-9888-z
-
[19]
Wang, Y. Q.; Guo, H. Q.; Feng, Z. W.; Wang, S. Y.; Wang, Y. X.; He, Q. X.; Li, G. P.; Lin, W. W.; Xie, X. Q.; Lin, Z. H. PD-1-targeted discovery of peptide inhibitors by virtual screening, molecular dynamics simulation, and surface plasmon resonance. Molecules 2019, 24, 3784−15.
doi: 10.3390/molecules24203784
-
[20]
Salomon-Ferrer, R.; Götz, A. W.; Poole, D.; Le Grand, S.; Walker, R. C. Routine microsecond molecular dynamics simulations with AMBER on GPUs. 2. Explicit solvent particle mesh Ewald. J. Chem. Theory Comput. 2013, 9, 3878−3888.
doi: 10.1021/ct400314y
-
[21]
Gotz, A. W.; Williamson, M. J.; Xu, D.; Poole, D.; Le Grand, S.; Walker, R. C. Routine microsecond molecular dynamics simulations with AMBER on GPUs. 1. Generalized born. J. Chem. Theory Comput. 2012, 8, 1542−1555.
doi: 10.1021/ct200909j
-
[22]
Loncharich, R. J.; Brooks, B. R.; Pastor, R. W. Langevin dynamics of peptides: the frictional dependence of isomerization rates of N-acetylalanyl-N΄-methylamide. Biopolymers 1992, 32, 523−535.
doi: 10.1002/bip.360320508
-
[23]
Izaguirre, J. A.; Catarello, D. P.; Wozniak, J. M.; Skeel, R. D. Langevin stabilization of molecular dynamics. J. Phys. Chem. 2001, 114, 2090−2098.
doi: 10.1063/1.1332996
-
[24]
Darden, T.; York, D.; Pedersen, L. Particle mesh Ewald: an N⋅log(N) method for Ewald sums in large systems. J. Phys. Chem. 1993, 98, 10089−10092.
doi: 10.1063/1.464397
-
[25]
Essmann, U.; Perera, L.; Berkowitz, M. L.; Darden, T.; Lee, H.; Pedersen, L. G. A smooth particle mesh Ewald method. J. Phys. Chem. 1995, 103, 8577−8593.
doi: 10.1063/1.470117
-
[26]
Ryckaert, J. P.; Ciccotti, G.; Berendsen, H. J. Numerical integration of the cartesian equations of motion of a system with constraints: molecular dynamics of n-alkanes. J. Comput. Phys. 1977, 23, 327−341.
doi: 10.1016/0021-9991(77)90098-5
-
[27]
Wang, Y. Q.; Lin, W. W.; Wu, N.; Wang, S. Y.; Chen, M. Z.; Lin, Z. H.; Xie, X. Q.; Feng, Z. W. Structural insight into the serotonin (5-HT) receptor family by molecular docking, molecular dynamics simulation and systems pharmacology analysis. Acta Pharmacol. Sin. 2019, 40, 1138−1156.
doi: 10.1038/s41401-019-0217-9
-
[28]
Wang, J.; Hou, T. Develop and test a solvent accessible surface area-based model in conformational entropy calculations. J. Chem. Inf. Model. 2012, 52, 1199−1212.
doi: 10.1021/ci300064d
-
[29]
Hawkins, G. D.; Cramer, C. J.; Truhlar, D. G. Parametrized models of aqueous free energies of solvation based on pairwise descreening of solute atomic charges from a dielectric medium. J. Phys. Chem. 1996, 100, 19824−19839.
doi: 10.1021/jp961710n
-
[30]
Wang, W.; Kollman, P. A. Free energy calculations on dimer stability of the HIV protease using molecular dynamics and a continuum solvent model. J. Mol. Biol. 2000, 303, 567−582.
doi: 10.1006/jmbi.2000.4057
-
[31]
Sun, H.; Duan, L.; Chen, F.; Liu, H.; Wang, Z.; Pan, P.; Zhu, F.; John Zhang, Z. H.; Hou, T. J. Assessing the performance of MM/PBSA and MM/GBSA methods. 7. Entropy effects on the performance of end-point binding free energy calculation approaches. Phys. Chem. Chem. Phys. 2018, 20, 14450−14460.
doi: 10.1039/C7CP07623A
-
[32]
Chen, F.; Liu, H.; Sun, H.; Pan, P.; Li, Y.; Li, D.; Hou, T. Assessing the performance of the MM/PBSA and MM/GBSA methods. 6. Capability to predict protein-protein binding free energies and re-rank binding poses generated by protein-protein docking. Phys. Chem. Chem. Phys. 2016, 18, 22129−22139.
doi: 10.1039/C6CP03670H
-
[33]
Sun, H.; Li, Y.; Shen, M.; Tian, S.; Xu, L.; Pan, P.; Guan, Y.; Hou, T. Assessing the performance of MM/PBSA and MM/GBSA methods. 5. Improved docking performance using high solute dielectric constant MM/GBSA and MM/PBSA rescoring. Phys. Chem. Chem. Phys. 2014, 16, 22035−22045.
doi: 10.1039/C4CP03179B
-
[34]
Sun, H.; Li, Y.; Tian, S.; Xu, L.; Hou, T. Assessing the performance of MM/PBSA and MM/GBSA methods. 4. Accuracies of MM/PBSA and MM/GBSA methodologies evaluated by various simulation protocols using PDBbind data set. Phys. Chem. Chem. Phys. 2014, 16, 16719−16729.
doi: 10.1039/C4CP01388C
-
[35]
Tsui, V.; Case, D. A. Theory and applications of the generalized Born solvation model in macromolecular simulations. Biopolymers 2000, 56, 275−291.
doi: 10.1002/1097-0282(2000)56:4<275::AID-BIP10024>3.0.CO;2-E
-
[36]
Bashford, D.; Case, D. A. Generalized born models of macromolecular solvation effects. Annu. Rev. Phys. Chem. 2000, 51, 129−152.
doi: 10.1146/annurev.physchem.51.1.129
-
[37]
Sitkoff, D.; Sharp, K. A.; Honig, B. Accurate calculation of hydration free energies using macroscopic solvent models. J. Phys. Chem. 1994, 98, 1978−1988.
doi: 10.1021/j100058a043
-
[38]
Still, W. C.; Tempczyk, A.; Hawley, R. C.; Hendrickson, T. Semianalytical treatment of solvation for molecular mechanics and dynamics. J. Chem. Sci. 1990, 112, 6127−6129.
-
[39]
Weiser, J.; Shenkin, P. S.; Still, W. C. Approximate atomic surfaces from linear combinations of pairwise overlaps (LCPO). J. Comput. Chem. 1999, 20, 217−230.
doi: 10.1002/(SICI)1096-987X(19990130)20:2<217::AID-JCC4>3.0.CO;2-A
-
[40]
Hu, J.; Feng, Z.; Ma, S.; Zhang, Y.; Tong, Q.; Alqarni, M. H.; Gou, X.; Xie, X. Q. Difference and influence of inactive and active states of cannabinoid receptor subtype CB2: from conformation to drug discovery. J. Chem. Inf. Model. 2016, 56, 1152−1163.
doi: 10.1021/acs.jcim.5b00739
-
[41]
Ekins, S.; Mottin, M.; Ramos, P. R.; Sousa, B. K.; Neves, B. J.; Foil, D. H.; Zorn, K. M.; Braga, R. C.; Coffee, M.; Southan, C.; Puhl, A. C. Déjà vu: stimulating open drug discovery for SARS-CoV-2. Drug. Discov. Today 2020, 5, 928−941.
-
[42]
Sk, M. F.; Roy, R.; Jonniya, N. A.; Poddar, S.; Kar, P. Elucidating biophysical basis of binding of inhibitors to SARS-CoV-2 main protease by using molecular dynamics simulations and free energy calculations. J. Biomol. Struct. Dyn. 2020, 0739, 1−21.
-
[43]
Yin, W. C.; Mao, C. Y.; Luan, X. D.; Shen, D. D.; Shen, Q. Y.; Su, H. X.; Wang, X. X.; Zhou, F. L.; Zhao, W. F.; Gao, M. Q.; Chang, S. H.; Xie, Y. C.; Tian, G. H.; Jiang, H. W.; Tao, S. C.; Shen, J. S.; Jiang, Y.; Jiang, H. L.; Xu, Y. C.; Zhang, S. Y.; Zhang, Y.; Xu, H. Y. Structural basis for inhibition of the RNA-dependent RNA polymerase from SARS-CoV-2 by Remdesivir. Science 2020, 6498, 1499−1504.

 Login In
Login In






 DownLoad:
DownLoad: