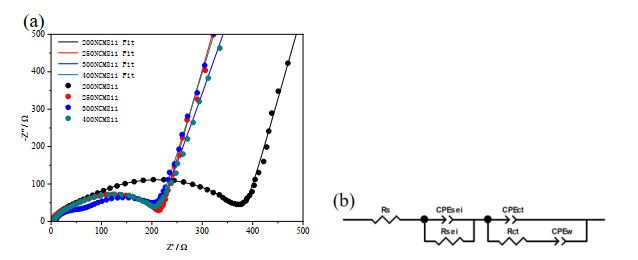
-
[1]
Taracson, J.; Armand, M. Issues and challenges facing lithium ion batteries. Nature 2001, 414, 359–367.
doi: 10.1038/35104644
-
[2]
Whittingham, M. S. Lithium batteries and cathode materials. Chem. Rev. 2004, 104, 4271–4302.
doi: 10.1021/cr020731c
-
[3]
Pelosato, R.; Cordaro, G.; Stucchi, D.; Cristiani, C.; Dotelli, G. Cobalt based layered perovskites as cathode material for intermediate temperature solid oxide fuel cells: a brief review. J. Power. Sources 2015, 298, 46–67.
doi: 10.1016/j.jpowsour.2015.08.034
-
[4]
Yi, T. F.; Zhu, Y. R.; Zhu, X. D.; Shu, J.; Yue, C. B.; Zhou, A. N. A review of recent developments in the surface modification of LiMn2O4 as cathode material of power lithium-ion battery. Ionics. 2009, 15, 779–784.
doi: 10.1007/s11581-009-0373-x
-
[5]
Etacheri, V.; Marom, R.; Elazari, R.; Salitra, G.; Aurbach, D. Challenges in the development of advanced Li-ion batteries: a review. Energ. Environ. Sci. 2011, 4, 3243–3262.
doi: 10.1039/c1ee01598b
-
[6]
Chen, Z.; Dahn, J. Methods to obtain excellent capacity retention in LiCoO2 cycled to 4.5 V. Electrochim. Acta. 2004, 49, 1079–1090.
doi: 10.1016/j.electacta.2003.10.019
-
[7]
Myung, S. T.; Maglia, F.; Park, K. J.; Yoon, C. S.; Lamp, P.; Kim, S. J.; Sun, Y. K. Nickel-rich layered cathode materials for automotive lithium-ion batteries: achievements and perspectives. ACS. Energy. Lett. 2016, 2, 196-223.
-
[8]
Liu, W.; Oh, P.; Liu, X.; Lee, M. J.; Cho, W.; Chae, S.; Kim, Y.; Cho, J. Nickel-rich layered lithium transition-metal oxide for high-energy lithium-ion batteries. Angew. Chem. -Int. Edit. 2015, 54, 4440–4457.
doi: 10.1002/anie.201409262
-
[9]
Manthiram, A.; Knight, J. C.; Myung, S. T.; Oh, S. M.; Sun, Y. K. Nickel-rich and lithium-rich layered oxide cathodes: progress and perspectives. Adv. Energy Mater. 2016, 6, 1501010.
doi: 10.1002/aenm.201501010
-
[10]
Kim, H. S.; Jin, B. S.; Jung, Y. S.; Lee, S.; Jeon, M.; Lim, H. T. In Enhanced electrochemical performance of surface-treated Li [Ni0. 8Co0. 1Mn0. 1]O2 cathode material for lithium-ion batteries, 2016 IEEE Transportation Electrification Conference and Expo, Asia-Pacific (ITEC Asia-Pacific), IEEE: 2016, 641–646.
-
[11]
Li, J.; Li, Y.; Guo, Y.; Lv, J.; Yi, W.; Ma, P. A facile method to enhance electrochemical performance of high-nickel cathode material Li(Ni0.8Co0.1 Mn0.1)O2 via Ti doping. J. Mater. Sci-Mater. El. 2018, 29, 10702–10708.
doi: 10.1007/s10854-018-9093-1
-
[12]
Zhang, J.; Zhong, H.; Zheng, C.; Xia, Y.; Liang, C.; Huang, H.; Gan, Y.; Tao, X.; Zhang, W. All-solid-state batteries with slurry coated LiNi0.8 Co0.1Mn0.1O2 composite cathode and Li6PS5Cl electrolyte: effect of binder content. J. Power Sources 2018, 391, 73–79.
doi: 10.1016/j.jpowsour.2018.04.069
-
[13]
Perednis, D.; Gauckler, L. Solid oxide fuel cells with electrolytes prepared via spray pyrolysis. Solid State Ion 2004, 166, 229–239.
doi: 10.1016/j.ssi.2003.11.011
-
[14]
Li, D.; Haneda, H.; Hishita, S.; Ohashi, N.; Labhsetwar, N. K. Fluorine-doped TiO2 powders prepared by spray pyrolysis and their improved photocatalytic activity for decomposition of gas-phase acetaldehyde. J. Fluorine. Chem. 2005, 126, 69–77.
doi: 10.1016/j.jfluchem.2004.10.044
-
[15]
Hao, J.; Studenikin, S.; Cocivera, M. Blue, green and red cathodoluminescence of Y2O3 phosphor films prepared by spray pyrolysis. J. Lumin. 2001, 93, 313–319.
doi: 10.1016/S0022-2313(01)00207-1
-
[16]
Shinde, V.; Gujar, T.; Lokhande, C. LPG sensing properties of ZnO films prepared by spray pyrolysis method: effect of molarity of precursor solution. Sensor Actuat. B-Chem. 2007, 120, 551–559.
doi: 10.1016/j.snb.2006.03.007
-
[17]
Jung, D. S.; Ko, Y. N.; Kang, Y. C.; Park, S. B. Recent progress in electrode materials produced by spray pyrolysis for next-generation lithium ion batteries. Adv. Powder. Technol. 2014, 25, 18–31.
doi: 10.1016/j.apt.2014.01.012
-
[18]
Liu, W. M.; Hu, G. R.; Peng, Z. D.; Du, K.; Cao, Y. B.; Liu, Q. Synthesis of spherical LiNi0.8Co0.15Al0.05O2 cathode materials for lithium-ion batteries by a co-oxidation-controlled crystallization method. Chin. Chem. Lett. 2011, 22, 1099–1102.
doi: 10.1016/j.cclet.2011.01.041
-
[19]
Hu, G.; Liu, W.; Peng, Z.; Du, K.; Cao, Y. Synthesis and electrochemical properties of LiNi0.8Co0.15Al0.05O2 prepared from the precursor Ni0.8Co0.15Al0.05OOH. J. Power Sources 2012, 198, 258–263.
doi: 10.1016/j.jpowsour.2011.09.101
-
[20]
Wang, M.; Zhang, R.; Gong, Y.; Su, Y.; Xiang, D.; Chen, L.; Chen, Y.; Luo, M.; Chu, M. Improved electrochemical performance of the LiNi0.8Co0.1Mn0.1O2 material with lithium-ion conductor coating for lithium-ion batteries. Solid State Ion 2017, 312, 53–60.
doi: 10.1016/j.ssi.2017.10.017
-
[21]
Zheng, J.; Yan, P.; Estevez, L.; Wang, C.; Zhang, J. G. Effect of calcination temperature on the electrochemical properties of nickel-rich LiNi0.76Mn0.14Co0.10O2 cathodes for lithium-ion batteries. Nano. Energy 2018, 49, 538–548.
doi: 10.1016/j.nanoen.2018.04.077
-
[22]
Nam, K. W.; Bak, S. M.; Hu, E.; Yu, X.; Zhou, Y.; Wang, X.; Wu, L.; Zhu, Y.; Chung, K. Y.; Yang, X. Q. Combining in situ synchrotron X-ray diffraction and absorption techniques with transmission electron microscopy to study the origin of thermal instability in overcharged cathode materials for lithium-ion batteries. Adv. Funct. Mater. 2013, 23, 1047–1063.
doi: 10.1002/adfm.201200693
-
[23]
Hsieh, C. T.; Hsu, H. H.; Hsu, J. P.; Chen, Y. F.; Chang, J. K. Infrared-assisted synthesis of lithium nickel cobalt alumina oxide powders as electrode material for lithium-ion batteries. Electrochim. Acta 2016, 206, 207–216.
doi: 10.1016/j.electacta.2016.04.146
-
[24]
Zheng, X.; Li, X.; Zhang, B.; Wang, Z.; Guo, H.; Huang, Z.; Yan, G.; Wang, D.; Xu, Y. Enhanced electrochemical performance of LiNi0.8Co0.1Mn0.1O2 cathode materials obtained by atomization co-precipitation method. Ceram. Int. 2016, 42, 644–649.
doi: 10.1016/j.ceramint.2015.08.159
-
[25]
Li, H. H.; Yabuuchi, N.; Meng, Y. S.; Kumar, S.; Breger, J.; Grey, C. P.; Shao-Horn, Y. Changes in the cation ordering of layered O3LixNi0.5Mn0.5O2 during electrochemical cycling to high voltages: an electron diffraction study. Chem. Mater. 2007, 19, 2551–2565.
doi: 10.1021/cm070139+
-
[26]
Li, J.; Xiong, S.; Liu, Y.; Ju, Z.; Qian, Y. Uniform LiNi1/3Co1/3Mn1/3O2 hollow microspheres: designed synthesis, topotactical structural transformation and their enhanced electrochemical performance. Nano. Energy 2013, 2, 1249–1260.
doi: 10.1016/j.nanoen.2013.06.003
-
[27]
Liang, C.; Kong, F.; Longo, R. C.; Kc, S.; Kim, J. S.; Jeon, S.; Choi, S.; Cho, K. Unraveling the origin of instability in Ni-rich LiNi1–2xCoxMnxO2 (NCM) cathode materials. J. Phys. Chem. C 2016, 120, 6383–6393.
-
[28]
Yao, Y.; Liu, H.; Li, G.; Peng, H.; Chen, K. Synthesis and electrochemical performance of phosphate-coated porous LiNi1/3Co1/3Mn1/3O2 cathode material for lithium ion batteries. Electrochim. Acta 2013, 113, 340–345.
doi: 10.1016/j.electacta.2013.09.071
-
[29]
Yan, J.; Liu, H.; Wang, Y.; Zhao, X.; Mi, Y.; Xia, B. Enhanced high-temperature cycling stability of LiMn2O4 by LiCoO2 coating as cathode material for lithium ion batteries. J. Mater. Sci. Chem. Eng. 2014, 2, 12–18.
-
[30]
Barsoukov, E.; Kim, D.; Lee, H. S.; Lee, H.; Yakovleva, M.; Gao, Y.; Engel, J. F. Comparison of kinetic properties of LiCoO2 and LiTi0.05Mg0.05Ni0.7Co0.2O2 by impedance spectroscopy. Solid State Ion. 2003, 161, 19–29.
doi: 10.1016/S0167-2738(03)00150-4
-
[31]
Abraham, D.; Twesten, R.; Balasubramanian, M.; Petrov, I.; McBreen, J.; Amine, K. Surface changes on LiNi0.8Co0.2O2 particles during testing of high-power lithium-ion cells. Electrochem. Commun. 2002, 4, 620–625.
doi: 10.1016/S1388-2481(02)00388-0
-
[32]
Jo, M.; Hong, Y. S.; Choo, J.; Cho, J. Effect of LiCoO2 cathode nanoparticle size on high rate performance for Li-ion batteries. J. Electrochem. Soc. 2009, 156, A430–A434.
doi: 10.1149/1.3111031

 Login In
Login In

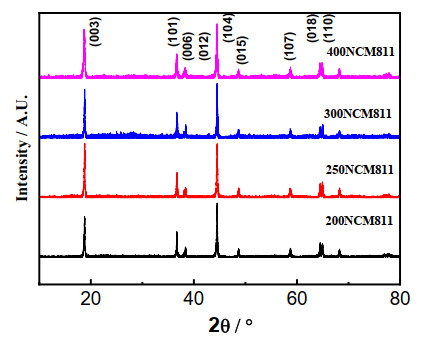





 DownLoad:
DownLoad: