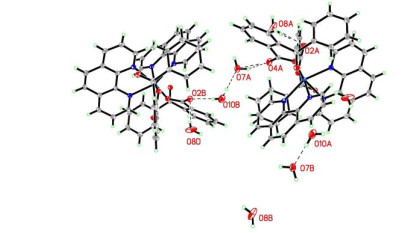
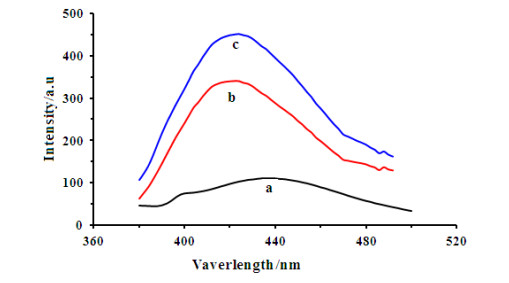
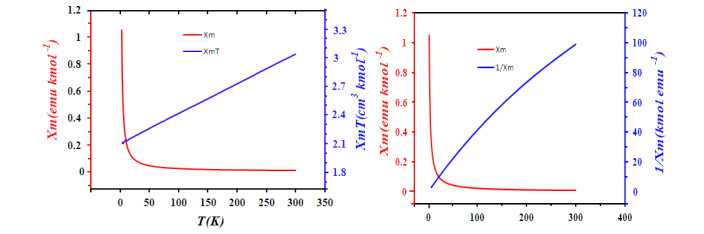
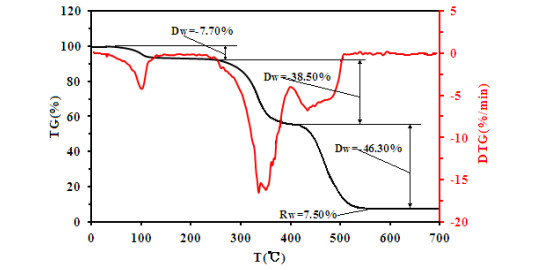
-
[1]
(a) Khullar, S.; Mandal, S. K. Supramolecular assemblies of dimanganese subunits and water clusters organized by strong hydrogen bonding interactions: single crystal to single crystal transformation by thermal de-rehydration processes. Cryst. Growth Des. 2012, 12, 5329–5337. (b) Luo, M.; Frechette, J. Electrochemical stability of low-density carboxylic acid terminated monolayers. J. Phys. Chem. C 2010, 114, 20167–20172. (c) Mundle, S. O. C.; Kluger, R. Decarboxylation via addition of water to a carboxyl group: acid catalysis of pyrrole-2-carboxylic acid. J. Am. Chem. Soc. 2009, 131, 11674–11675. (d) He, H. Y.; Yuan, D. Q.; Ma, H. Q.; Sun, D. F.; Zhang, G. Q.; Zhou, H. C. Control over interpenetration in lanthanide-organic frameworks: synthetic strategy and gas-adsorption properties. Inorg. Chem. 2010, 49, 7605–7607.
-
[2]
(a) Alexandru, M. G.; Visinescu, D.; Shova, S.; Marius, A.; Francesc, L.; Miguel, J. Synthesis, crystal structures, and magnetic properties of two novel cyanido-bridged heterotrimetallic {(CuMnCrIII)-Mn-II-Cr-II} complexes. Inorg. Chem. 2017, 56, 2258–2269. (b) Li, Y.; Wen, B. S.; Zou, X. Z.; Huang, B.; Qiu, W. D.; Zhang, Z. M.; You, A.; Cheng, X. L. Syntheses, crystal structures, and magnetic properties of 0D tetranuclear nickel(II) coordination compound and 1D manganese(II) coordination polymer constructed from biphenyl tricarboxylic acid. Chin. J. Inorg. Chem. 2018, 34, 981–988. (c) Crowston, B. J.; Shipp, J. D.; Chekulaev, D.; Heteronuclear d-d and d-f Ru(II)/M complexes (M = Gd(III), Yb(III), Nd(III), Zn(II) or Mn(II)) of ligands combining phenanthroline and aminocarboxylate binding sites: combined relaxivity, cell imaging and photophysical studies. Dalton Trans. 2019, 48, 6132–6152. (d) Alexandru, M. G.; Visinescu, D.; Braun-Cula, B.; Lloret, F.; Julve, M. Synthesis, crystal structure and magnetic properties of three {(CrMn(II)-Mn(III} heterodimetallic complexes based on heteroleptic cyanido-bearing Cr(III) building blocks. Eur. J. Inorg. Chem. 2018, 3–4, 349–359. (e) Barmpa, A.; Frousiou, O.; Kalogiannis, S.; Perdih, F.; Turel, I.; Psomas, G. Manganese(II) complexes of the quinolone family member flumequine: structure, antimicrobial activity and affinity for albumins and calf-thymus DNA. Polyhedron 2018, 145, 166–175.
-
[3]
(a) Zhang, J. P.; Zhang, Y. B.; Lin, J. B.; Chen, X. M. Metal azolate frameworks: from crystal engineering to functional materials. Chem. Rev. 2012, 112, 1001–1033. (b) Guo, J.; Sun, D.; Zhang, L. L.; Yang, Q.; Zhao, X. L.; Sun, D. F. Cadmium-organic coordination polymers based on N-donor ligands and small anions: syntheses, crystal structures, and photoluminescent properties. Cryst. Growth Des. 2012, 12, 5649–5654. (c) Zeng, M. H.; Tan, Y. X.; He, Y. P.; Yin, Z.; Chen, Q.; Kurmoo, M. A. Porous 4-fold-interpenetrated chiral framework exhibiting vapochromism, single-crystal-to-single-crystal solvent exchange, gas sorption, and a poisoning effect. Inorg. Chem. 2013, 52, 2353–2360. (d) Fei, H. L.; Bresler, M. R.; Oliver, S. R. J. A new paradigm for anion trapping in high capacity and selectivity: crystal-to-crystal transformation of cationic materials. J. Am. Chem. Soc. 2011, 133, 11110–11113.
-
[4]
(a) Guan, L.; Zhou, Y. H.; Zhang, H. Syntheses and crystal structures of two 1D chain coordination polymers based on 2-benzoylbenzoic acid. Chin. J. Struct. Chem. 2010, 29, 690–695. (b) Caglar, S.; Demir, S. K. A crystallographic, spectroscopic, thermal, and DFT investigation of mixed ligand Cu(II) complexes of 2-benzoylbenzoate. Synth. React. Inorg. Met. Org. Chem. 2012, 42, 1449–1458.
-
[5]
(a) Zu, W. X. Inorganic compounds synthesis of the manual. Chemistry Industry Press. 1rd. 2006, 325–326. (b) Li, C. H.; Li, W.; Hu, B. N.; Hu, H. X. Synthesis, crystal structure, thermal stability properties of a new binuclear Cu(II) complex with 2-benzoylbenzoic acid as ligand. Chin. J. Struct. Chem. 2013, 32, 1264–1268. (c) Guan, L.; Zhou, Y. H.; Zhang, H. Syntheses and crystal structures of two 1D chain coordination polymers based on 2-benzoylbenzoic acid. Chin. J. Struct. Chem. 2010, 29, 693–695.
-
[6]
(a) Alexandru, M. G.; Visinescu, D.; Shova, S.; Marius, A.; Francesc, L.; Miguel, J. Synthesis, crystal structures, and magnetic properties of two novel cyanido-bridged heterotrimetallic {(CuMnCrIII)-Mn-II-Cr-II} complexes. Inorg. Chem. 2017, 56, 2258–2269. (b) Li, Y.; Wen, B. S.; Zou, X. Z.; Huang, B.; Qiu, W. D.; Zhang, Z. M.; You, A.; Cheng, X. L. Syntheses, crystal structures, and magnetic properties of 0D tetranuclear nickel(II) coordination compound and 1D manganese(II) coordination polymer constructed from biphenyl tricarboxylic acid. Chin. J. Inorg. Chem. 2018, 34, 981–988. (c) Crowston, B. J.; Shipp, J. D.; Chekulaev, D.; Heteronuclear d-d and d-f Ru(II)/M complexes (M = Gd(III), Yb(III), Nd(III), Zn(II) or Mn(II)) of ligands combining phenanthroline and aminocarboxylate binding sites: combined relaxivity, cell imaging and photophysical studies. Dalton Trans. 2019, 48, 6132–6152. (d) Alexandru, M. G.; Visinescu, D.; Braun-Cula, B.; Lloret, F.; Julve, M. Synthesis, crystal structure and magnetic properties of three {(CrMn(II)-Mn(III} heterodimetallic complexes based on heteroleptic cyanido-bearing Cr(III) building blocks. Eur. J. Inorg. Chem. 2018, 3–4, 349–359. (e) Barmpa, A; Frousiou, O.; Kalogiannis, S.; Perdih, F.; Turel, I.; Psomas, G. Manganese(II) complexes of the quinolone family member flumequine: structure, antimicrobial activity and affinity for albumins and calf-thymus DNA. Polyhedron 2018, 145, 166–175.
-
[7]
(a) Li, S. F.; Shen, Y. M.; Liu, D. B.; Fan, L. B.; Zhao, Y. P. Novel nanomaterials of 2-benzoylbenzoic acid anions intercalated magnesium aluminum layered double hydroxides for UV absorption properties. Sci. Adv. Mater. 2015, 7, 756–761. (b) Zhang, W. L.; Zhu, D. S. Novel diorganotin(IV) carboxylates based on 2-benzoylbenzoic acid: synthesis, characterization, molecular structures and luminescent activities. Mol. Cryst. Liq. Cryst. 2019, 1, 1–12. (c) Yang, Y. Q.; Li, Y. L. Synthesis, fluorescence and magnetic properties of a new europium(III) complex Eu(C14H9O3)2(C12H8N2)2NO3. Chin. J. Struct. Chem. 2018, 37, 1781–1785. (d) Li, W.; Li, C. H.; Yang, Y. Q.; Li, H. F. Crystal structure, electrochemical, fluorescent and magnetic properties of a new complex [Ag(2, 2΄-bipy)(C14H9O3)]·(C14H10O3). Chin. J. Struct. Chem. 2014, 33, 1593–1596.
-
[8]
Sheldrick, G. M. SHELXL-2014, Program for Crystal Structure Refinement. University of Gottingen, Gottingen, Germany 2014.
-
[9]
(a) Li, C. H.; Li, W.; Yang, Y. Q.; Liu, C. X. Synthesis, crystal structure and thermal stability of complex {Mn(phen)2(H2O)[C8H11O2(COO)]}·(ClO4)·H2O. Chin. J. Inorg. Chem. 2007, 23, 1671–1674. (b) Li, W.; Li, C. H.; Li, W.; Yang, Y. Q.; Kuang, Y. F. Hydrothermal synthesis, crystal structure and electrochemical properties of complex Mn2(phen)2(p-CBA)4(H2O). Chin. J. Struct. Chem. 2007, 26, 1057–1060. (c) Li, C. H.; Li, W.; Tan, X. W.; Li, Y. L. Hydrothermal synthesis, structure and thermal stability of binuclear manganese complex with o-acetamidobenzoic acid and 1, 10-phenanthroline. Chin. J. Inorg. Chem. 2010, 26, 1129–1132.
-
[10]
Hu, S. Z.; Zhou, Z. H.; Tsai, K. R. Average vander waals radii of atoms in crystals. Acta. Phys. -Chim. Sin. 2003, 19, 1073–1077.
-
[11]
Ning, Y. C. Structure Identification of Organic Compounds and Organic Spectroscopy. 2rd. Beijing, Science Press 2006, 332.
-
[12]
Chen, X. M.; Cai, J. W. Principle and Practice of Single Crystal Structural Analysis. 1st Ed. Beijing, Science press 2004.
-
[13]
Nakamota, K. Translated by Huang, D. R.; Wang, R. Q. Infrared and Raman Spectra of Inorganic and Coordination Compounds, 3rd edn. Beijing: Chemical Industry Press 1986.
-
[14]
(a) Liu, Y. Z.; Gao, H. Y.; Yi, X. G.; Li, D. P.; Li, Y. X. Crystal structures and DNA binding properties of 2-naphthoxyacetic acid Cu(II) complexes. Chin. J. Struct. Chem. 2019, 38, 1362–16369. (b) Fu, J. D.; Guo, J. K.; Wen, Y. H. Hydrothermal syntheses, crystal structures and luminescent properties of binuclear Mn(II) and Co(II) complexes assembled by 4-carboxymethoxy phenylacetic acid. Chin. J. Inorg. Chem. 2011, 27, 996–1000.
-
[15]
Wang, L. Y.; Liu, Z. L.; Zhang, C. X.; Liu, Z. Q.; Liao, D. Z.; Jiang, Z. H.; Yan, S. P. Syntheses, crystal and magnetic properties of free radical-Mn(II) chain complexes. Sci. in China B 2003, 33, 332–339.
-
[16]
(a) Gou, J. S.; Song, L. M.; Liu, H.; Shen, D. D.; Hu, W. X.; Wang, W. L.; Ren, X. Y.; Chang, J. M. Release profile of nitrogen during thermal treatment of waste wood packaging materials. J. Bioresour. Bioprod. 2019, 4, 51–59. (b) Tang, Q. H.; Fang, L.; Guo, W. J. Effects of bamboo fiber length and loading on mechanical, thermal and pulverization properties of phenolic foam composites. J. Bioresour. Bioprod. 2019, 4, 166–176. (c) Yang, R.; Zhou, X.; Luo, C. Q.; Wang, K. H. Modern Polymeric Testing Technology. 3rd edn. Beijing: Tsinghua University Press 2016, 167.

 Login In
Login In






 DownLoad:
DownLoad: