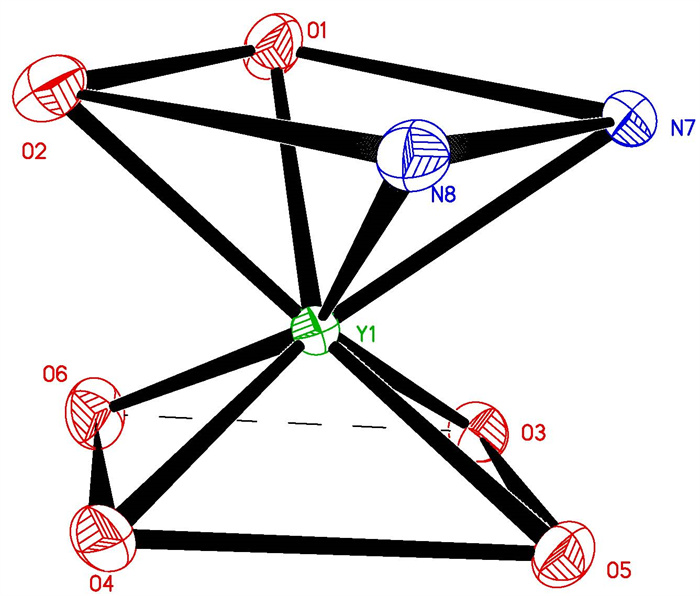
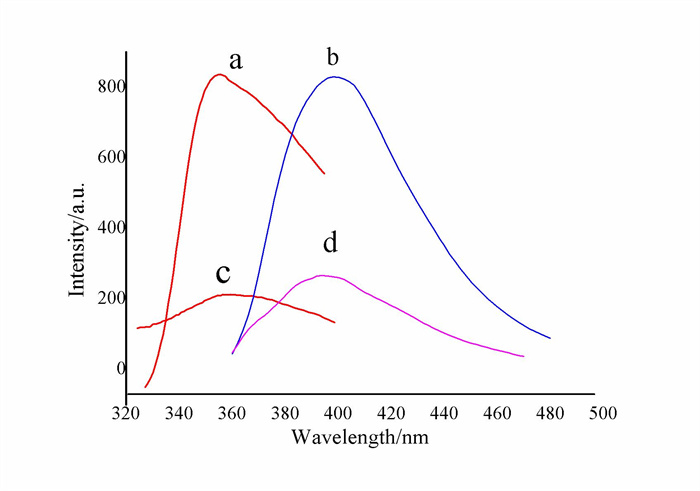
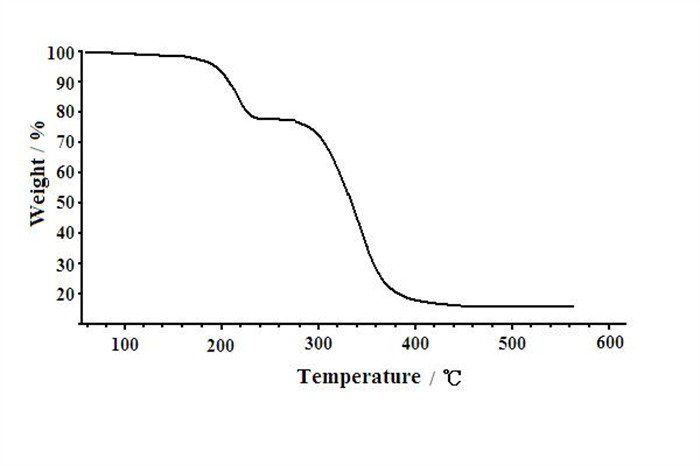
-
[1]
Jung, J.; Puget, M.; Cador, O.; Bernot, K.; Calzado, C.; Guennic, B. L. Analysis of the magnetic exchange interactions in yttrium(III) complexes containing nitronyl nitroxide radicals. Inorg. Chem. 2017, 56, 6788–6801.
doi: 10.1021/acs.inorgchem.6b02952
-
[2]
Sodpiban, O.; Del Gobbo, S.; Barman, S.; Aomchad, V.; Kidkhunthod, P.; Ould-Chikh, S.; Poater, A.; D Elia, V.; Basset, J. M. Synthesis of well-defined yttrium-based Lewis acids by capturing a reaction intermediate and catalytic application for cycloaddition of CO2 to epoxides under atmospheric pressure. Catal. Sci. Technol. 2019, 9, 6152–6165.
doi: 10.1039/C9CY01642B
-
[3]
Zhao, P.; Zhu, Q. H.; Fettinger, J. C.; Power, P. Characterization of a monomeric, homoleptic, solvent free samarium bis(aryloxide). Inorg. Chem. 2018, 57, 14044–14046.
doi: 10.1021/acs.inorgchem.8b02677
-
[4]
Silva, I. G. N.; Morals, A. F.; Mustafa, D. Synthesis, characterization and Judd-Ofelt analysis of Sm3+-doped anhydrous yttrium trimesate MOFs and their Y2O3: Sm3+ low temperature calcination products. J. Lumin. 2019, 210, 335–341.
doi: 10.1016/j.jlumin.2019.02.048
-
[5]
Pousaneh, E.; Preuss, A.; Assim, K.; Ruffer, T.; Korb, M.; Tittmann, J.; Hermann, S.; Schulz, S. Z.; Lang, H. Tetranuclear yttrium and gadolinium 2-acetylcyclopentanoate clusters: synthesis and their use as spin-coating precursors for metal oxide film formation for field-effect transistor fabrication. J. Rare Earths 2018, 36, 1098–1105.
doi: 10.1016/j.jre.2018.02.010
-
[6]
Veauthler, J. M.; Schelter, E. J.; Carlson, C. N.; Scott, B. L.; Re, R. E.; Thopson, J. D.; Klpllnger, J. L.; Morrls, D. E.; John, K. D. Direct comparison of the magnetic and electronic properties of samarocene and ytterbocene terpyridine complexes. Inorg. Chem. 2008, 47, 5841–5849.
doi: 10.1021/ic8001465
-
[7]
Ligny, R.; Guillaume, S. M.; Carpentier, J. F. Yttrium-mediated ring-opening copolymerization of oppositely configurated 4-alkoxymethylene-beta-propiolactones: effective access to highly alternated isotactic functional PHAs. Chem. Eur. J. 2019, 25, 6412–6424.
doi: 10.1002/chem.201900413
-
[8]
Su, Y. C.; Chiu, T. Y.; Liu, Y. T.; Lin, P. H.; Ko, B. T. Significant enhancement of catalytic properties in mononuclear yttrium complexes by nitrophenolate-type ligands: synthesis, structure, and catalysis for lactide polymerization. J. Poly. SCi. Part. A-Poly. Chem. 2019, 57, 2038–2047.
doi: 10.1002/pola.29468
-
[9]
Peewasan, K.; Merkel, M. P.; Fuhr, O.; Powell, A. K. A designed and potentially decadentate ligand for use in lanthanide(III) catalysed biomass transformations: targeting diastereoselective trans-4, 5-diaminocyclopentenone derivatives. Dalton Trans. 2020, 49, 2331–2336.
doi: 10.1039/D0DT00183J
-
[10]
Wu, H. L.; Pan, G. L.; Bai, Y. C.; Wang, H.; Kong, J.; Shi, F. R.; Zhang, Y. R.; Wang, X. L. A. Schiff base bis(N-salicylidene)-3-oxapentane-1, 5-diamine and its yttrium(III) complex: synthesis, crystal structure, DNA-binding properties, and antioxidant activities. J. Coord. Chem. 2013, 64, 2634–2646.
-
[11]
Lu, G.; Wang, K.; Kong, X.; Pan, H.; Zhang, J. H.; Chen, Y. L.; Zhuang, J. Binuclear phthalocyanine dimer-containing yttrium double-decker ambipolar semiconductor with sensitive response toward oxidizing NO2 and reducing NH3. Chem. Electro. Chem. 2018, 5, 605–609.
-
[12]
Gao, J. Q.; Li, D.; Wang, J.; Jin, X. D.; Kang, P. L.; Wu, T.; Li, K.; Zhang, X. D. Synthesis and crystal structure of a 2-D yttrium coordination network with propylenediamine-N, N, N΄, N΄-tetraacetic acid. J. Coord. Chem. 2011, 64, 2284–2291.
doi: 10.1080/00958972.2011.594163
-
[13]
Lu, J. T.; Wang, H. L.; Zhang, X. M. Synthesis and crystal structure of a mixed (phthalocyaninato)(porphyrinato)yttrium double-decher complex. Chin. J. Struct. Chem. 2011, 30, 872–976.
-
[14]
Bhattacharjee, C. R.; Das, G.; Goswami, P.; Mondal, P.; Prasad, K.; Rao, D. S. S. Norel photoluminescent lanthaidomesogens forming bilayer Yectic phase derived from blue light emitting liquid crystalline, one ring o-donor Schiff-base ligands. Polyhedraon 2011, 30, 1040–1047.
doi: 10.1016/j.poly.2011.01.015
-
[15]
Wu, A. Q.; Zheng, F. K.; Liu, X.; Guo, G. C.; Cai, L. Z.; Dong, Z. C.; Takano, Y.; Huang, J. S. A novel bi-layered samarium complex with an unprecedented coordination mode of orotic acid [Y2(HL)2(OX)(H2O)2]n·2.5nH2O (H3L = orotic acid, ox2– = oxalate2–): synthesis, crystal structure and physical properties. Inorg. Chem. Commun. 2006, 9, 347–350.
doi: 10.1016/j.inoche.2005.12.014
-
[16]
Li, W.; Li, C. H.; Yang, Y. Q.; Kuang, Y. F. Synthesis, crystal structure and electrochemical properties of one-dimensional chain coordination polymer [Cu(phen)(2, 4, 6-TMBA)2(H2O)]n. Chin. J. Struct. Chem. 2008, 27, 210–214.
-
[17]
Li, W.; Li, C. H.; Yang, Y. Q.; Li, D. P.; Peng, Y. L.; Kuang, Y. F. Hydrothermal synthesis, crystal structure and thermal stability properties of one dimensional chain coordination polymer [Cd(phen)(2, 4, 6-TMBA)2(H2O)]n. Chin. J. Inorg. Chem. 2007, 23, 2013–2017.
-
[18]
Li, D. P.; Feng, Y. L.; Chen, Z. M.; Kuang, D. Z.; Zhang, C. H.; Yang, Y. Q.; Li, W. Synthesis, crystal structure of a binuclear terbium complex with 2, 4, 6-trimethylbenzoic acid and 1, 10-phenanthroline. Chin. J. Inorg. Chem. 2006, 22, 2097–2100.
-
[19]
(a) Sheldrick, G. M. Program for Bruker Area Detector Absorption Correction. University of Gottingen: Gottingen, Germany 2008. (b) Sheldrick, G. M. SHELXS-97, Program for Crystal Structure Solution. University of Gottingen: Gottingen, Germany 2013.
-
[20]
Gao, J. Q.; Li, D.; Wang, J.; Jin, X. D.; Kang, P. L.; Wu, T.; Li, K.; Zhang, X. D. Synthesis and crystal structure of a 2-D yttrium coordination network with propylenediamine-N, N, N΄, N ΄-tetraacetic acid. J. Coord. Chem. 2011, 64, 2284–2291.
doi: 10.1080/00958972.2011.594163
-
[21]
Wu, H. L.; Pan, G. L.; Bai, Y. C.; Wang, H.; Kong, J.; Shi, F. R.; Zhang, Y. H.; Wang, X. L. A Schiff base bis(N-salicylidene)-3-oxapentane-1, 5-diamine and its yttrium(III) complex: synthesis, crystal structure, DNA-binding properties, and antioxidant activities. J. Coord. Chem. 2013, 66, 2634–2646.
doi: 10.1080/00958972.2013.812725
-
[22]
Chen, X. M.; Cai, J. W. Principle and Practice of Single Crystal Structural Analysis. The first edition, Beijing, Science press 2004.
-
[23]
Nakamota, K. Translated by Huang, D. R.; Wang, R. Q. Infrared and Raman Spectra of Inorganic and Coordination Compounds, 3rd edn. Beijing: Chemical Industry Press 1986.
-
[24]
(a) El, A.; Mohamed, S. Spectroscopic, thermal analysis, and nematicidal evaluation of new mixed ligand complexes of bidentate Schiff base and 1, 10 phenanthroline with some transition metals. J. Chin. Chem. Soci. 2018, 65, 1188–1198. (b) Li, C. H.; Li, W.; Hu, H. X.; Jiang, M. L. Synthesis, crystal structure and fluorescent and thermal stability properties of the zinc(II) complex [Zn(C15H11O3)2(C12H8N2)2]·5H2O. Chin. J. Struct. Chem. 2014, 33, 947–951. (c) Li, J. Rare earth luminescent materials and their applications. Chemical Industry Press. China 2003, 170–172.

 Login In
Login In






 DownLoad:
DownLoad: