Bio-electronic bandage: Self-powered performances to accelerate intestinal wound healing
-
* Corresponding authors.
E-mail addresses: xht0071@sina.com (H.-T. Xu),
shenjl@wiucas.ac.cn, sjl1@wmu.edu.cn (J. Shen).
Citation:
Saadullah Khattak, Hong-Tao Xu, Jianliang Shen. Bio-electronic bandage: Self-powered performances to accelerate intestinal wound healing[J]. Chinese Chemical Letters,
;2024, 35(12): 110210.
doi:
10.1016/j.cclet.2024.110210

Intestinal wound healing is complex because of the intestine's intricate structure and the environment, which contains its microbes and digestive modules. Traditional suturing is the utmost standard surgical methodology, which can imply hyperplasia and obstruction, two probable harmful consequences. Thus, patients often face a delayed healing time and constrained healing experiences. Choosing replacement surgical processes containing compression rings or staples takes the threat of more rigorous tissue injury and impending obstruction with the usual movement of the intestines [1]. Improving cell enhancement and reproduction is necessary to cure intestinal wounds appropriately. This group incorporates the cells that contain the blood vessels, the cells that operate in movement, the cells that line the intestines, and the intestinal epithelium [2]. Possibly, accelerated wound healing and resurgence could be achieved by mixing hydrogel with stem cells or regenerative components, such as naturally biodegradable sutures or epidermal growth factors. Another option is an adhesive designed for tissues. However, they cannot affect the genetic activities in cells, such as transforming DNA into RNA and RNA into proteins [2]. Because of this restriction, the effective extracellular distribution of epidermal growth factor (EGF) and its incentive of fast cell proliferation are the foremost wound-healing processes far from their restriction. Postoperative difficulties, for instance, tissue injury and prolonged healing times, prevail even through the extensive utilization of surgical sutures to assure intestinal wounds [1]. To accelerate the curing approach of wounds, some encounters should be defeated before hydrogels or tissue sealants containing therapeutic components and stem cells may be employed commercially [2].
Their ineffectiveness in healing stems partly from the fact that these technologies cannot actively regulate the transcription and translation of genes within cells [3]. As an alternative, electrostimulation can excite cells or tissues by applying electric currents. The current influences the cell membrane directly, which in turn causes biochemical reactions inside the cells. These reactions can activate various signaling pathways, change the intracellular and extracellular environments, and promote cell proliferation, which is crucial for wound healing. Numerous technological obstacles have limited the clinical application of electrostimulation, which has thus far been limited to superficial tissues and organs. An implanted and regulated power source is usually necessary for electrostimulation to deliver on-demand stimulation; however, this source must deteriorate to prevent surgical removal, which is still a challenge. The biocompatible dual-network composite hydrogel (DNCGel) demonstrates excessive probable for smart and personalized medicine through increasing gel strength, conductivity, and bacterial inhibition, although endorsing wound healing and permitting real-time health monitoring. This investigation covers the method for future innovations in smart hydrogels for biomedical applications, specifically in smart wound dressings and flexible wearable sensors. The other research proposes the collective result of electric stimulation and multiple metal ions, showing a new attempt at creating iTENG patches for the therapy of hard-to-heal wounds [4]. An electronic bandage that uses dual electrostimulation to speed up the healing of intestinal wounds was recently described in an article by Wu et al. [5] in Nat. Electron. The bandage is self-powered and made of soft, biodegradable materials. It uses direct current stimulation to increase the release of these healing factors and pulsed electrostimulation for electrotransfection, which boosts the expression of epithelial cell growth factors. The bandage's galvanic cell-driving healing factor exocytosis ensures cell survival and high transfection efficiency. This method speeds healing more effectively than traditional sutures or single-stimulation bandages, as shown in Fig. 1. These results ought to stimulate novel therapeutic approaches to circumvent the healing of intestinal wounds.
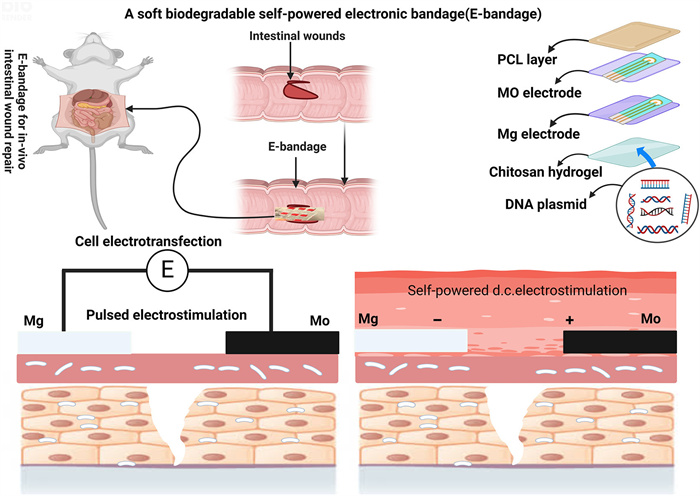
The most common postoperative consequences in individuals after conventional procedures to close intestinal wounds are hyperplasia and obstructions, which are responsible for around 17% of the total cases. Therefore, Wu et al. [5] thoroughly assessed the healing efficacy of intestinal wounds via an E-bandage utilizing dual electrostimulation at 7- and 14-days post-implantation, juxtaposed with wounds closed through standard clinical procedures. With traditional closure using absorbable sutures, significant hyperplasia was evident at the wound site. In stark contrast, the application of the E-bandage yielded minimal hyperplasia within a fortnight, irrespective of how the electrodes were positioned to the wound. Quantitative evaluations revealed that, by the 14th day, the E-bandage had substantially decreased the extent of hyperplasia (about 2%), signifying an enhancement of roughly 30% over electrostimulation (ES) treatment, a 27% improvement over pulsed electrostimulation (ET) treatment, and a pronounced 203% benefit in comparison to the standard surgical closure.
Additionally, Wu et al. reported pronounced intestinal obstruction brought on through hyperplasia in the suture group, with obstruction levels at 110% by day 7, escalating to 151% by day 14. The devices lacking electrostimulation mirrored the hyperplasia and obstruction outcomes associated with conventional surgical methods, underlining that the device did not lead to any physical constriction of the intestine. In contrast, the cohort treated with the E-bandage exhibited negligible intestinal obstruction (3% on day 7, decreasing to 1.47% by day 14). This underscores the potential of the dual-electrostimulation approach as a significantly less invasive option for intestinal wound healing. The levels of hyperplasia and obstruction might also act as valuable indicators for gauging the likelihood of these postoperative complications in more extensive studies.
The negligible levels of hyperplasia and obstruction following E-bandage treatment imply a decreased risk of these postoperative complications when compared to traditional surgical closure methods. Wu et al. also studied the impact of intestinal hyperplasia and obstruction on food digestion by observing body weight changes in mice. Daily weight measurements indicated a significant decrease in weight within a week after conventional surgery, followed by a marginal increase between the third and seventh day, but then a continued decline to 75% of pre-surgical weight by the fourteenth day. In sharp contrast, mice receiving E-bandage treatment showed a highly effective weight recovery, returning to 106% of their initial weight by the seventh day and maintaining it, thus demonstrating reduced post-surgical issues due to the dual-electrostimulation treatment. Clinically, Wu et al. assessed the healing of intestine lesions in mice by looking for signs of hyperplasia, blockage, and changes in body weight. The E-bandage group outperformed the surgery closure group by a factor of three, while the ES and ET groups performed around 15% and 10% better, respectively, due to the synergistic benefits of pulsed and direct current electrostimulation.
Additional evaluation of the E-bandage's healing effectiveness was accomplished by Wu et al. [5] applying immunocytochemistry with hematoxylin and eosin and Masson's trichrome staining studies two weeks post-treatment. The E-bandage group has been proven to promote EGF levels corresponding to immunohistochemistry findings and associated statistical data. Also, the group that stained their cells with alpha-smooth muscle actin had augmented the proliferation of fibroblasts and smooth muscle cells, proposing that the improved extracellular EGF was beneficial to all layers of tissue [2]. According to histological evaluations of granulation tissue, muscle structure, and mucosal continuity, the E-bandage group showed the best wound healing results. The E-bandage group showed faster wound remodeling and greater healing efficacy at 14 days, as shown by Masson's trichrome staining, demonstrating a denser and more distributed collagen fiber region. This provides more evidence that the collagen network in this group was stronger. As Wu et al. acknowledged, the gut microbiota has a significant role in host metabolic control and immune system maturation. Therefore, to evaluate the restoration of normal intestinal functions, sequencing the microbiota of the intestines was a crucial aspect of their study. The intestines treated with the E-bandage were found to have significantly higher relative abundances of the bacterium Akkermansia muciniphila, Lachnospiraceae_NK4A136 group, and the Lactobacillus genus, which are known to maintain gut homeostasis. The study found that the relative abundance of probiotic species increased significantly even when pulsed electrostimulation or direct current (d.c) electrostimulation was used independently. Conventional surgical closure patients in the control group had fewer than 10% of their microbiota using these probiotics. After the therapy, functional cluster analysis of the gut microbiota revealed that the treated intestines were most energetic after receiving dual-electrostimulation from the E-bandage. This liveliness included metabolic and cellular activity. The E-bandage treatment is more successful in establishing intestinal health than pulse electrostimulation or direct current electrostimulation.
A state-of-the-art bioelectronic platform, the E-bandage, was created by Wu et al. to facilitate the healing of intestinal lesions. Electrostimulation, genetic engineering, and biomolecular transport are the cellular-level tools used by this platform. One of the many advantages of the device is its ability to lessen the risk of postoperative problems, which are critical to the health and well-being of the patient. Even with all these benefits, some things could still be improved. There must be more than the present E-bandage design for frequent pulsed electrostimulation situations. Even though it may be required to sustain therapeutic medicines, the E-bandage has not yet accomplished a controlled release mechanism for the integrated hydrogel payload.
Consequently, Wu et al. are looking to refine their technology further. Future development aims to create devices suitable for long-term establishment capable of delivering repeated electrical stimulations and controlling the release of their drugs. This could significantly enhance the therapeutic potential of the device. Although the current testing has been exclusive to intestinal wounds, there is potential for this technology to be adapted to aid the healing of various other organ and tissue injuries, including those in the nervous system and muscles. With the promise shown by the dual-electrostimulation strategy, there is optimism that developments in bioelectronics from Wu et al.'s pioneering work could eventually lead to novel treatments being made available in clinical settings, improving patient care and outcomes. Future research directions could include enhancing the efficacy and stability of self-powered systems, improving biocompatibility, and integrating advanced sensors for real-time monitoring of wound healing. Current challenges consist of attaining stable and uniform power generation within the body's environment, decreasing possible opposing consequences, and developing accessible manufacturing developments. Overwhelming these challenges will be necessary for transitioning these modern bandages from experimental phases to extensive clinical application.
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Saadullah Khattak: Writing – original draft. Hong-Tao Xu: Writing – review & editing, Supervision. Jianliang Shen: Writing – review & editing, Supervision.
J.C. Slieker, F. Daams, I.M. Mulder, et al., JAMA Surg. 148 (2013) 190–201.
doi: 10.1001/2013.jamasurg.33
J. Wu, H. Yuk, T.L. Sarrafian, et al., Sci. Translat. Med. 14 (2022) eabh2857.
M.M. Martino, Science 343 (2014) 885–888.
doi: 10.1126/science.1247663
Y. Zhang, C. Xue, Y. Zhang, et al., Chin. Chem. Lett. 35 (2024) 109196.
H. Wu, Y. Wang, H. Li, et al., Nat. Electron. 7 (2024) 299–312.
doi: 10.1038/s41928-024-01138-8
J.C. Slieker, F. Daams, I.M. Mulder, et al., JAMA Surg. 148 (2013) 190–201.
doi: 10.1001/2013.jamasurg.33
J. Wu, H. Yuk, T.L. Sarrafian, et al., Sci. Translat. Med. 14 (2022) eabh2857.
M.M. Martino, Science 343 (2014) 885–888.
doi: 10.1126/science.1247663
Y. Zhang, C. Xue, Y. Zhang, et al., Chin. Chem. Lett. 35 (2024) 109196.
H. Wu, Y. Wang, H. Li, et al., Nat. Electron. 7 (2024) 299–312.
doi: 10.1038/s41928-024-01138-8

Qinghong Pan , Huafang Zhang , Qiaoling Liu , Donghong Huang , Da-Peng Yang , Tianjia Jiang , Shuyang Sun , Xiangrong Chen . A self-powered cathodic molecular imprinting ultrasensitive photoelectrochemical tetracycline sensor via ZnO/C photoanode signal amplification. Chinese Chemical Letters, 2025, 36(1): 110169-. doi: 10.1016/j.cclet.2024.110169
Xin Dong , Tianqi Chen , Jing Liang , Lei Wang , Huajie Wu , Zhijin Xu , Junhua Luo , Li-Na Li . Structure design of lead-free chiral-polar perovskites for sensitive self-powered X-ray detection. Chinese Journal of Structural Chemistry, 2024, 43(6): 100256-100256. doi: 10.1016/j.cjsc.2024.100256
Yixia Zhang , Caili Xue , Yunpeng Zhang , Qi Zhang , Kai Zhang , Yulin Liu , Zhaohui Shan , Wu Qiu , Gang Chen , Na Li , Hulin Zhang , Jiang Zhao , Da-Peng Yang . Cocktail effect of ionic patch driven by triboelectric nanogenerator for diabetic wound healing. Chinese Chemical Letters, 2024, 35(8): 109196-. doi: 10.1016/j.cclet.2023.109196
Jingwen Zhao , Jianpu Tang , Zhen Cui , Limin Liu , Dayong Yang , Chi Yao . A DNA micro-complex containing polyaptamer for exosome separation and wound healing. Chinese Chemical Letters, 2024, 35(9): 109303-. doi: 10.1016/j.cclet.2023.109303
Jiliang Deng , Guoliang Shi , Zhihang Ye , Quan Xiao , Xiaoting Zhang , Lei Ren , Fangyu Yang , Miao Wang . Unveiling and swift diagnosing chronic wound healing with artificial intelligence assistance. Chinese Chemical Letters, 2025, 36(3): 110496-. doi: 10.1016/j.cclet.2024.110496
Haijun Shen , Yi Qiao , Chun Zhang , Yane Ma , Jialing Chen , Yingying Cao , Wenna Zheng . A matrix metalloproteinase-sensitive hydrogel combined with photothermal therapy for transdermal delivery of deferoxamine to accelerate diabetic pressure ulcer healing. Chinese Chemical Letters, 2024, 35(12): 110283-. doi: 10.1016/j.cclet.2024.110283
Salim Ullah , Jianliang Shen , Hong-Tao Xu . Innovative self-healing conductive organogel: Pioneering the future of electronics. Chinese Chemical Letters, 2025, 36(3): 110553-. doi: 10.1016/j.cclet.2024.110553
Peizhe Li , Qiaoling Liu , Mengyu Pei , Yuci Gan , Yan Gong , Chuchen Gong , Pei Wang , Mingsong Wang , Xiansong Wang , Da-Peng Yang , Bo Liang , Guangyu Ji . Chlorogenic acid supported strontium polyphenol networks ensemble microneedle patch to promote diabetic wound healing. Chinese Chemical Letters, 2024, 35(8): 109457-. doi: 10.1016/j.cclet.2023.109457
Fereshte Hassanzadeh-Afruzi , Mina Azizi , Iman Zare , Ehsan Nazarzadeh Zare , Anwarul Hasan , Siavash Iravani , Pooyan Makvandi , Yi Xu . Advanced metal-organic frameworks-polymer platforms for accelerated dermal wound healing. Chinese Chemical Letters, 2024, 35(11): 109564-. doi: 10.1016/j.cclet.2024.109564
Jiajia Wang , XinXin Ge , Yajing Xiang , Xiaoliang Qi , Ying Li , Hangbin Xu , Erya Cai , Chaofan Zhang , Yulong Lan , Xiaojing Chen , Yizuo Shi , Zhangping Li , Jianliang Shen . An ionic liquid functionalized sericin hydrogel for drug-resistant bacteria-infected diabetic wound healing. Chinese Chemical Letters, 2025, 36(2): 109819-. doi: 10.1016/j.cclet.2024.109819
Yunfen Gao , Liying Wang , Chufan Zhou , Yi Zhao , Hai Huang , Jun Wu . Low-dimensional antimicrobial nanomaterials in anti-infection treatment and wound healing. Chinese Chemical Letters, 2025, 36(3): 110028-. doi: 10.1016/j.cclet.2024.110028
Supphachok Chanmungkalakul , Syed Ali Abbas Abedi , Federico J. Hernández , Jianwei Xu , Xiaogang Liu . The dark side of cyclooctatetraene (COT): Photophysics in the singlet states of “self-healing” dyes. Chinese Chemical Letters, 2024, 35(8): 109227-. doi: 10.1016/j.cclet.2023.109227
Yueying Wang , Jianming Xiong , Linwei Xin , Yuanyuan Li , He Huang , Wenjun Miao . Photosensitizer-synergized g-carbon nitride nanosheets with enhanced photocatalytic activity for eradicating drug-resistant bacteria and promoting wound healing. Chinese Chemical Letters, 2025, 36(4): 110003-. doi: 10.1016/j.cclet.2024.110003
Ning Li , Yu Li , Hulin Zhang . Personal thermoregulatory clothing powered by sunlight. Chinese Journal of Structural Chemistry, 2024, 43(9): 100357-100357. doi: 10.1016/j.cjsc.2024.100357
Qin Yu , Haisheng He , Jianping Qi , Yi Lu , Wei Wu . Oral delivery of insulin by barbed microneedles actuated by intestinal peristalsis. Chinese Chemical Letters, 2024, 35(9): 109888-. doi: 10.1016/j.cclet.2024.109888
Zhenzhong MEI , Hongyu WANG , Xiuqi KANG , Yongliang SHAO , Jinzhong GU . Syntheses and catalytic performances of three coordination polymers with tetracarboxylate ligands. Chinese Journal of Inorganic Chemistry, 2024, 40(9): 1795-1802. doi: 10.11862/CJIC.20240081
Ju Wang , Yongbing Sun , Lingbang Meng , Jianfang Feng , Meng Cheng , Liangxing Tu . Intestinal transporters and oral absorption enhancing strategies based on these transporters. Chinese Chemical Letters, 2025, 36(5): 110529-. doi: 10.1016/j.cclet.2024.110529
Xiaoyu Zhang , Xin Yu . Solar-powered heterogeneous water disinfection nano-system. Chinese Journal of Structural Chemistry, 2025, 44(3): 100439-100439. doi: 10.1016/j.cjsc.2024.100439
Man Wu , Chuandong Jia . A light-powered molecular pump achieving transmembrane concentration gradient. Chinese Journal of Structural Chemistry, 2025, 44(4): 100452-100452. doi: 10.1016/j.cjsc.2024.100452
Si-Hua Liu , Jun-Hao Zhou , Jian-Ke Sun . Interconnecting zero-dimensional porous organic cages into sub-8 nm nanofilm for bio-inspired separation. Chinese Journal of Structural Chemistry, 2024, 43(7): 100312-100312. doi: 10.1016/j.cjsc.2024.100312