Stereoselective synthesis of α-3-deoxy-D-manno-oct-2-ulosonic acid (α-Kdo) derivatives using a C3-p-tolylthio-substituted Kdo fluoride donor
-
* Corresponding author.
E-mail address: zjli@bjmu.edu.cn (Z. Li).
Citation:
Ao Sun, Zipeng Li, Shuchun Li, Xiangbao Meng, Zhongtang Li, Zhongjun Li. Stereoselective synthesis of α-3-deoxy-D-manno-oct-2-ulosonic acid (α-Kdo) derivatives using a C3-p-tolylthio-substituted Kdo fluoride donor[J]. Chinese Chemical Letters,
;2025, 36(3): 109972.
doi:
10.1016/j.cclet.2024.109972

The overuse of antibiotic has led to the emergence of drug-resistant bacteria [1]. As a result, the development of new antimicrobial drugs and vaccines has attracted the increasing attention of scientists [2]. 3-Deoxy-D-manno-oct-2-ulosonic acid (Kdo) is an eight-carbon monosaccharide widely distributed in bacterial lipopolysaccharides (LPS) and capsule polysaccharides (CPS) [3,4]. In the biosynthesis of LPS, several sequential enzymatic reactions are necessary for the synthesis of Kdo from D-ribulose-5-phosphate and attaching it to lipid A [5]. In this biosynthetic pathway, CMP-Kdo plays a crucial role as a key intermediate [5]. Its derivatives have the potential to act as inhibitors of CMP-Kdo synthase (CKS), thereby obstructing the LPS biosynthesis pathway and ultimately disrupting the production of the bacterial outer membrane [6]. Thus, analogs of Kdo have the potential to act as CKS inhibitors and serve as novel antimicrobial agents.
As a deoxy sugar [7,8], Kdo glycosylation is one of the most challenging glycosylation reactions because of the low reactivity, the uncontrolled stereoselectivity and the formation of 2,3-ene byproducts [9-14]. Therefore, the highly efficient and stereoselective synthesis of Kdo glycosides remains an unmet need in glycosylation methodology, which limits the investigation of the biological activities of Kdo-containing oligosaccharides. Currently, Kdo glycosylation methods can be broadly divided into direct and indirect methods [15]. Using the direct methods, the target glycosidic bonds can be achieved in a single step by utilizing steric hinderance effect [16-31], special intermediate formation [32-34], remote participation effect [35], solvent and ion effect [36,37] and side chain conformation (Scheme 1A) [38]. The selection of protective groups in the direct methods is intricate, often resulting in the formation of additional 2,3-ene byproducts. Using the indirect methods, an auxiliary group (such as S [39], I [40-43], O [44,45]) is added at Kdo's C3 position to modulate the anomeric stereoselectivity (Scheme 1B). After glycosylation, the auxiliary group can be removed by additional steps. Compared with direct methods, indirect methods simplify protecting group operations and enhance reaction efficiency while preserving complete stereoselectivity and generating less 2,3-enes [15].
Our team has previously developed a strategy for Kdo α-glycosylation utilizing a C3-p-tolylthio-substituted Kdo phosphite donor 1 via the classical neighboring group participation effect (Scheme 1B) [15]. The sulfur-positive tricyclic intermediate restricted the acceptor to attacking from the α side of the sugar ring only. Consequently, single α-stereoselective Kdo glycosides were produced without the formation of 2,3-enes. However, the high reactivity of the modified phosphite donor 1, which must be used immediately after preparation, is a drawback of this strategy. In this study, we investigated the reaction of the C3-p-tolylthio-substituted Kdo fluoride donor 2 and demonstrated its increased stability (Scheme 1C). The complete α-stereoselectivity and broad substrate scope proved the superiority of introducing a thioether at the C3 position of Kdo donors. In addition, we synthesized a series of Kdo O/C/S/N-glycosides via fluoride donor 2, which have the potential to act as important precursors of candidate molecules for CKS inhibition.
According to the previous work [15], the C3-p-tolylthio-substituted Kdo hydroxy sugar S1 was synthesized with 2,3-ene by a two-step reaction of addition and hydrolysis. Subsequently, the α-Kdo fluoride donor 2 was prepared by fluorination of S1 in a satisfactory yield of 89% (Scheme S1 in Supporting information). Notably, donor 2 maintained stability for more than one year at room temperature, which was considerably more stable than donor 1.
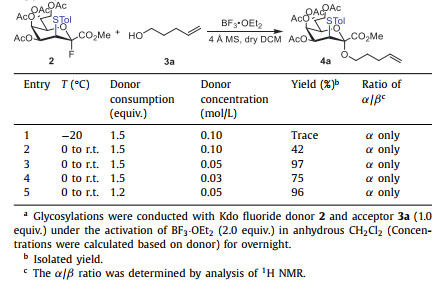
Under the classical conditions for the activation of glycosyl fluoride [41-43], the glycosylation of donor 2 (1.2 equiv.) was conducted with 4-penten-1-ol 3a (1.0 equiv.) in the presence of BF3·OEt2 (2.0 equiv.), 4 Å molecular sieves (MS) at 0 ℃ to room temperature in anhydrous CH2Cl2 (Table 1, entry 2, 0.10 mol/L, calculated based on donor). Surprisingly, the coupling reaction between 2 and 3a provided the Kdo glycoside 4a in a moderate 42% yield as the sole α-isomer without the formation of 2,3-ene byproduct. Similar to the phosphite donor 1, the axial orientation of the thio group at C3 of the fluoride donor 2 prevented the elimination reactions, while efficiently enhanced α-stereoselectivity via neighboring group participation effects [15]. The stereoconfigurations of Kdo glycosides was confirmed via the non-decoupling 13C NMR (3JC1, H3ax < 1.0 Hz) [27]. Considering that the adsorption of hydrogen fluoride formed during the reaction by molecular sieves can drive this reaction [41-43], we adjusted the ratio of molecular sieve and donor by modifying the concentration of the reaction solution (MS: 100 mg/mL). The experiment demonstrated that the reaction afforded glycoside 4a in 96% yield with outstanding α-stereoselectivity when the donor concentration reached 0.05 mol/L (Table 1, entry 5).
|
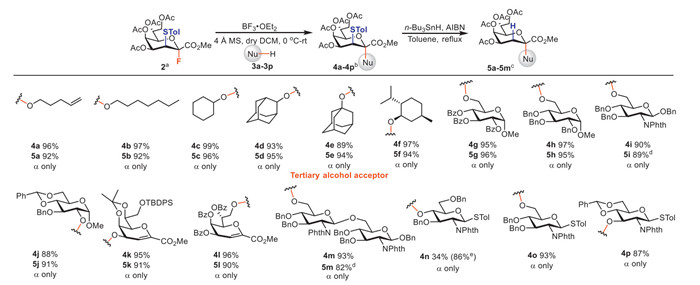
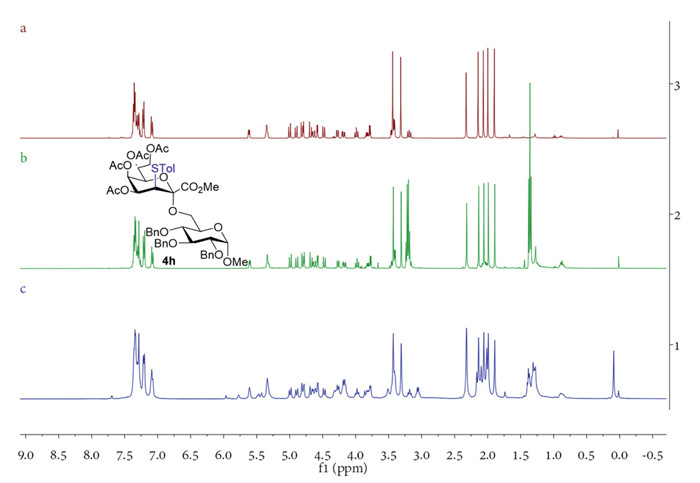
Subsequently, we investigated the O-glycosylation scope of donor 2 (1.2 equiv.) with various acceptors (3a-3p) under the optimal conditions (Scheme 2, donor: 1.2 equiv., acceptor: 1.0 equiv., BF3·OEt2: 2.0 equiv., CH2Cl2: 0.05 mol/L calculated based on donor, 4 Å MS: 100 mg/mL calculated based on solvent, 0 ℃→r.t.). To our satisfaction, we were able to synthesize the fully α-stereoselective Kdo glycosides 4a-4f in excellent yields of 89%−99% without the formation of 2,3-enes through glycosylation of donor 2 with simple alcohols, including primary alcohols (3a: 4-penten-1-ol, 3b: n-heptanol), secondary alcohols (3c: cyclohexanol, 3d: 2-adamantanol, 3f: ʟ-menthol), and tertiary alcohol (3e: 1-adamantanol). For the reacions with glycosyl acceptors, the glycosylation process between donor 2 and acceptor 3g-3m produced sole α-isomer Kdo oligosaccharides 4g-4m with yields ranging from 88% to 97%. It was noteworthy that the fluoride donor 2 reacted with Kdo glycal acceptors 3k and 3l, resulting in the successful formation of di-Kdo saccharides 4k (95%) and 4l (96%) in excellent yields through α-(2→4)- and α-(2→8) linkages. In particular, the Kdo-α-(2→6)-GlcN-β-(1→6)-GlcN trisaccharide 4m is the essential component of several bacterial LPS structures [19,22]. Under the optimized free radical reduction conditions developed by our team [15], the thioether groups of the glycosides 4a-4m were successfully removed, leading to the formation of 5a-5m in high yields of 82%−96%. In addition, the glucosamine thioglycosides acceptors 3n-3p coupled with donor 2 and afforded the desired Kdo-containing disaccharides 4n (34%), 4o (93%) and 4p (87%) without activation of the thioglycosides. Among them, the reaction between inactive 4-OH acceptor 3n and 2 was incomplete with reactants remained, and only 34% of the disaccharide 4n was obtained (86% yield based on the consumed acceptors). Overall, the glycosylation efficiency of fluoride donor 2 with acceptors that are moderately or highly active is comparable to that of phosphite donor 1 [15]. For inactive acceptors, reaction yields of donor 2 can be improved through recycling reactants. In another experiment, acceptor 3h was coupled with donor 1 and 2 separately, and the reaction solutions were filtered to remove molecular sieves, concentrated without column chromatography separation, and analyzed by NMR (Fig. 1). The 1H NMR spectra of the crude products reveals that the reaction system of fluoride glycosylation was very clean (Fig. 1b).
CMP-Kdo is a key intermediate in LPS biosynthesis [4]. Therefore, Kdo derivatives have the potential to be developed as CKS inhibitors, which can block bacterial cell wall synthesis. Additionally, C/S/N-glycosides possess distinct properties compared to O-glycosides, and exhibit resistance to enzymatic degradation while preserving the structural integrity of the glycosides [46]. Notably, some of the CKS inhibitors being investigated are Kdo carboglycoside or thioglycoside derivatives [6]. Thus, we attempted to broaden the structural type of Kdo derivatives by coupling Kdo fluoride donor 2 with common nucleophilic reagents 6a-6k (Scheme 3).
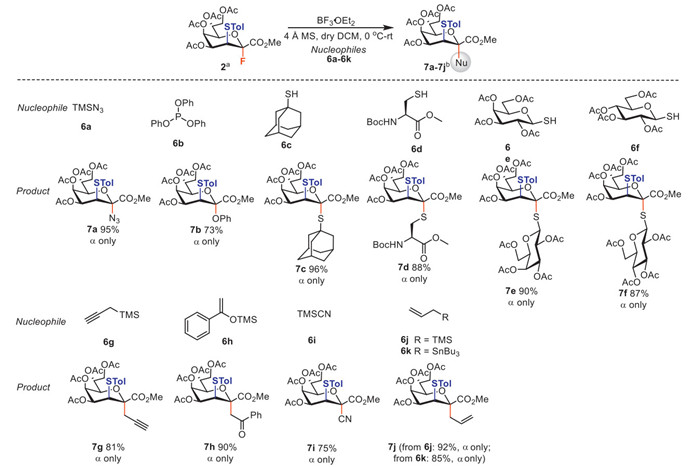
Under standard glycosylation conditions, donor 2 was coupled with TMSN3, resulting in 95% yield of Kdo azide 7a. Subsequently, protected ʟ-propargylglycine 8 was reacted with 7a under click reaction conditions to produce glycosylated unnatural amino acid 9 in 51% yield (Scheme 4). Compound 9 has the potential to be used in glycopeptide synthesis and serves as an intermediate for Kdo derivatization and bioactivity research [47-49]. Interestingly, donor 2 reacted with 6b to yield phenolic glycoside 7b, which is different from the phosphoglycoside products obtained from the reaction of fluoride with 6b as reported [50]. It is possible that the reaction underwent the following process. Under acidic conditions, triphenyl phosphite 6b is easily hydrolyzed to form phosphoric acid and phenol. Molecular sieves can absorb the resulting phosphoric acid and the fluoride 2 is subsequently activated in the presence of boron trifluoride ether and preferentially undergoes glycosylation process with the phenol to produce 7b rather than Arbuzov reaction. In addition, donor 2 reacted with several sulfur nucleophilic reagents (6c-6f) to produce the corresponding thioglycoside derivatives 7c-7f in yields ranging from 87% to 96%. The carboglycoside products 7g-7j were obtained in high yields (75%−92%) by using carbo nucleophilic reagents 6g-6k and donor 2. Moreover, the alkyne group in compound 7g could undergo further derivatization via click reaction. It is worth noting that all of the aforementioned Kdo derivatives exhibit complete α-stereoselectivity. The construction of compound libraries containing Kdo derivatives and the evaluation of their bioactivity are currently in progress.
Scheme 5 shows the proposed reaction mechanism of glycosylation with C3-p-tolylthio-substituted Kdo fluoride donor 2 promoted by BF3·OEt2. Boron trifluoride activates the fluoride donor 2, breaking the carbon-fluorine bond and forming a ternary cyclic episulfonium intermediate. Due to the neighboring group participation effect, the episulfonium blocks the β-side of the Kdo sugar ring, allowing the nucleophilic reagent to attack from the α-side only. After proton dissociation, complete α-stereoselective of Kdo glycosides are formed.
In summary, we synthesized C3-p-tolylthio-substituted fluoride donor 2 to increase the stability of the Kdo phosphite donor 1 and investigated the reactions of donor 2 in Kdo α-glycosylation. The results of this study along with previous reports support that the Kdo glycosylation reaction can be treated as two categories: (1) For acceptors with medium to high reactivity, the glycosylation reaction can be performed more conveniently by using the more stable fluoride donor 2, resulting in cleaner reactions. (2) For low-reactivity acceptors, more reactive phosphite donor 1 can be used for efficient glycosylation reactions. These two methods demonstrated the superiority of introducing a thioether at the C3 position of the Kdo donors. Furthermore, the substrate range was further expanded by utilizing donor 2 to react with various C/S/N-nucleophilic reagents, resulting in high yields and complete α-stereoselectivity of Kdo derivatives. These Kdo derivatives represent new structural types for designing CKS inhibitors.
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Ao Sun: Writing – original draft, Visualization, Methodology, Investigation, Formal analysis, Data curation, Conceptualization. Zipeng Li: Validation, Investigation. Shuchun Li: Writing – review & editing, Project administration. Xiangbao Meng: Writing – review & editing. Zhongtang Li: Writing – review & editing. Zhongjun Li: Writing – review & editing, Supervision, Resources, Project administration, Methodology, Funding acquisition, Conceptualization.
This project was supported by the National Key R&D Program of China (Nos. 2022YFF1203005, 2022YFC2303700), and supported by the National Natural Science Foundation of China (Nos. 81930097, 82151223). We thank Dr. De-Cai Xiong, Peking University, for helpful discussion.
Supplementary material associated with this article can be found, in the online version, at doi:
N. Mobarki, B. Almerabi, A. Hattan, Int. J. Med. Dev. Ctries. 40 (2019) 561–564.
doi: 10.24911/IJMDC.51-1549060699
H.S. Gold, R.C. Moellering Jr, N. Engl. J. Med. 335 (1996) 1445–1453.
doi: 10.1056/NEJM199611073351907
F.M. Unger, Adv. Carbohydr. Chem. Biochem. 38 (1981) 323–388.
L. Cipolla, L. Gabrielli, D. Bini, L. Russo, N. Shaikh, Nat. Prod. Rep. 27 (2010) 1618–1629.
doi: 10.1039/c004750n
J. Lodowska, D. Wolny, L. Węglarz, Can. J. Microbiol. 59 (2013) 645–655.
doi: 10.1139/cjm-2013-0490
L. Cipolla, A. Polissi, C. Airoldi, et al., Curr. Med. Chem. 18 (2011) 830–852.
doi: 10.2174/092986711794927676
C.L. Bennett, M.C. Galan, Chem. Rev. 118 (2018) 7931–7985.
doi: 10.1021/acs.chemrev.7b00731
S. Meng, W. Zhong, W. Yao, Z. Li, Org. Lett. 22 (2020) 2981–2986.
doi: 10.1021/acs.orglett.0c00732
J. Hansson, S. Oscarson, Curr. Org. Chem. 4 (2000) 535–546.
doi: 10.2174/1385272003376184
S. Oscarson, Carbohydr. Chem. 45 (2012) 40–60.
T.K. Pradhan, K.K.T. Mong, Isr. J. Chem. 55 (2015) 285–296.
doi: 10.1002/ijch.201400145
P. Kosma, Tetrahedron Lett. 57 (2016) 2133–2142.
doi: 10.1016/j.tetlet.2016.04.005
P. Kosma, Carbohydr. Chem. 42 (2017) 116–164.
T.K. Pradhan, Eur. J. Org. Chem. 26 (2023) e202300146.
doi: 10.1002/ejoc.202300146
A. Sun, L.Z.Y. Wang, et al., Angew. Chem. Int. Ed. 63 (2024) e202313985.
doi: 10.1002/anie.202313985
H. Yoshizaki, N. Fukuda, K. Sato, et al., Angew. Chem. Int. Ed. 40 (2001) 1475–1480.
doi: 10.1002/1521-3773(20010417)40:8<1475::AID-ANIE1475>3.0.CO;2-V
Y. Fujimoto, M. Iwata, N. Imakita, et al., Tetrahedron Lett. 48 (2007) 6577–6581.
doi: 10.1016/j.tetlet.2007.07.036
K. Mannerstedt, K. Ekelçf, S. Oscarson, Carbohydr. Res. 342 (2007) 631–637.
doi: 10.1016/j.carres.2006.08.021
Y. Zhang, J. Gaekwad, M.A. Wolfert, G.J. Boons, Chem. Eur. J. 14 (2008) 558–569.
doi: 10.1002/chem.200701165
T. Ichiyanagi, M. Fukunaga, R. Tagashira, et al., Tetrahedron 67 (2011) 5964–5971.
doi: 10.1016/j.tet.2011.06.039
A. Shimoyama, Y. Fujimoto, K. Fukase, Synlett 2011 (2011) 2359–2362.
doi: 10.1055/s-0030-1260313
A. Shimoyama, A. Saeki, N. Tanimura, et al., Chem. Eur. J. 17 (2011) 14464–14474.
doi: 10.1002/chem.201003581
T.J. Boltje, W. Zhong, J. Park, et al., J. Am. Chem. Soc. 134 (2012) 14255–14262.
doi: 10.1021/ja306274v
R. Yi, A. Ogaki, M. Fukunaga, H. Nakajima, T. Ichiyanagi, Tetrahedron 70 (2014) 3675–3682.
doi: 10.1016/j.tet.2014.04.024
E. Mancuso, C. Romanò, N. Trattnig, et al., Chem. Eur. J. 27 (2021) 7099–7102.
doi: 10.1002/chem.202100837
T. Ichiyanagi, H. Narimoto, N. Ohtani, Tetrahedron 96 (2021) 132397.
doi: 10.1016/j.tet.2021.132397
J.S. Huang, W. Huang, X. Meng, et al., Angew. Chem. Int. Ed. 54 (2015) 10894–10898.
doi: 10.1002/anie.201505176
X.Y. Zhou, P. Yang, S. Luo, J.S. Yang, Chem. Asian J. 14 (2019) 454–461.
doi: 10.1002/asia.201801779
X.Y. Zhou, L.X. Li, Z. Zhang, et al., Angew. Chem. Int. Ed. 61 (2022) e202204420.
doi: 10.1002/anie.202204420
S. Hamajima, N. Komura, H.N. Tanaka, et al., Org. Lett. 24 (2022) 8672–8676.
doi: 10.1021/acs.orglett.2c03542
S. Hamajima, N. Komura, H.N. Tanaka, et al., Molecules 28 (2023) 102.
doi: 10.3390/molecules28010102
X. Mi, Q. Lou, W. Fan, L. Zhuang, Y. Yang, Carbohydr. Res. 448 (2017) 161–165.
doi: 10.1016/j.carres.2017.04.021
Q. Lou, Q. Hua, L. Zhang, Y. Yang, Org. Lett. 22 (2020) 981–985.
doi: 10.1021/acs.orglett.9b04509
J. Zhang, X. Gao, S. Liu, et al., Org. Lett. 25 (2023) 4150–4155.
doi: 10.1021/acs.orglett.3c01430
W. Huang, Y.Y. Zhou, X.L. Pan, et al., J. Am. Chem. Soc. 140 (2018) 3574–3582.
doi: 10.1021/jacs.7b09461
R.R. Schmidt, A. Esswein, Angew. Chem. Int. Ed. 27 (1988) 1178–1180.
doi: 10.1002/anie.198811781
A. Esswein, H. Rembold, R.R. Schmidt, Carbohydr. Res. 200 (1990) 287–305.
doi: 10.1016/0008-6215(90)84198-4
P. Ngoje, D. Crich, J. Am. Chem. Soc. 142 (2020) 7760–7764.
doi: 10.1021/jacs.0c03215
K. Ikeda, S. Akamatsu, K. Achiwa, Chem. Pharm. Bull. 38 (1990) 279–281.
doi: 10.1248/cpb.38.279
P. Kosma, H. Sekljic, G.J. Balint, J. Carbohydr. Chem. 15 (1996) 701–714.
doi: 10.1080/07328309608005686
B. Pokorny, P. Kosma, Chem. Eur. J. 21 (2015) 305–313.
doi: 10.1002/chem.201405424
B. Pokorny, P. Kosma, ChemOpen 4 (2015), 722–728.
doi: 10.1002/open.201500126
B. Pokorny, P. Kosma, Org. Lett. 17 (2015) 110–113.
doi: 10.1021/ol5033128
M. Reiner, R.R. Schmidt, Tetrahedron Asymmetry 11 (2000) 319–335.
doi: 10.1016/S0957-4166(99)00557-1
K.K.T. Mong, T.K. Pradhan, C.H. Chiu, et al., Org. Chem. Front. 7 (2020) 2179–2186.
doi: 10.1039/D0QO00630K
Y. Yang, B. Yu, Chem. Rev. 117 (2017) 12281–12356.
doi: 10.1021/acs.chemrev.7b00234
G.Z. Tian, X.L. Wang, J. Hu, et al., Chin. Chem. Lett. 26 (2015) 922–930.
doi: 10.1016/j.cclet.2015.04.026
D. Liu, S. Liu, F. Hu, Z. Li, Z. Li, Chin. Chem. Lett. 35 (2024) 108762.
doi: 10.1016/j.cclet.2023.108762
K. Wang, T. Zhang, M. Liu, et al. Chin. Chem. Lett. 34 (2023) 108065.
doi: 10.1016/j.cclet.2022.108065
X. Zhou, H. Ding, P. Chen, et al., Angew. Chem. Int. Ed. 59 (2020) 4138–4144.
doi: 10.1002/anie.201914557
N. Mobarki, B. Almerabi, A. Hattan, Int. J. Med. Dev. Ctries. 40 (2019) 561–564.
doi: 10.24911/IJMDC.51-1549060699
H.S. Gold, R.C. Moellering Jr, N. Engl. J. Med. 335 (1996) 1445–1453.
doi: 10.1056/NEJM199611073351907
F.M. Unger, Adv. Carbohydr. Chem. Biochem. 38 (1981) 323–388.
L. Cipolla, L. Gabrielli, D. Bini, L. Russo, N. Shaikh, Nat. Prod. Rep. 27 (2010) 1618–1629.
doi: 10.1039/c004750n
J. Lodowska, D. Wolny, L. Węglarz, Can. J. Microbiol. 59 (2013) 645–655.
doi: 10.1139/cjm-2013-0490
L. Cipolla, A. Polissi, C. Airoldi, et al., Curr. Med. Chem. 18 (2011) 830–852.
doi: 10.2174/092986711794927676
C.L. Bennett, M.C. Galan, Chem. Rev. 118 (2018) 7931–7985.
doi: 10.1021/acs.chemrev.7b00731
S. Meng, W. Zhong, W. Yao, Z. Li, Org. Lett. 22 (2020) 2981–2986.
doi: 10.1021/acs.orglett.0c00732
J. Hansson, S. Oscarson, Curr. Org. Chem. 4 (2000) 535–546.
doi: 10.2174/1385272003376184
S. Oscarson, Carbohydr. Chem. 45 (2012) 40–60.
T.K. Pradhan, K.K.T. Mong, Isr. J. Chem. 55 (2015) 285–296.
doi: 10.1002/ijch.201400145
P. Kosma, Tetrahedron Lett. 57 (2016) 2133–2142.
doi: 10.1016/j.tetlet.2016.04.005
P. Kosma, Carbohydr. Chem. 42 (2017) 116–164.
T.K. Pradhan, Eur. J. Org. Chem. 26 (2023) e202300146.
doi: 10.1002/ejoc.202300146
A. Sun, L.Z.Y. Wang, et al., Angew. Chem. Int. Ed. 63 (2024) e202313985.
doi: 10.1002/anie.202313985
H. Yoshizaki, N. Fukuda, K. Sato, et al., Angew. Chem. Int. Ed. 40 (2001) 1475–1480.
doi: 10.1002/1521-3773(20010417)40:8<1475::AID-ANIE1475>3.0.CO;2-V
Y. Fujimoto, M. Iwata, N. Imakita, et al., Tetrahedron Lett. 48 (2007) 6577–6581.
doi: 10.1016/j.tetlet.2007.07.036
K. Mannerstedt, K. Ekelçf, S. Oscarson, Carbohydr. Res. 342 (2007) 631–637.
doi: 10.1016/j.carres.2006.08.021
Y. Zhang, J. Gaekwad, M.A. Wolfert, G.J. Boons, Chem. Eur. J. 14 (2008) 558–569.
doi: 10.1002/chem.200701165
T. Ichiyanagi, M. Fukunaga, R. Tagashira, et al., Tetrahedron 67 (2011) 5964–5971.
doi: 10.1016/j.tet.2011.06.039
A. Shimoyama, Y. Fujimoto, K. Fukase, Synlett 2011 (2011) 2359–2362.
doi: 10.1055/s-0030-1260313
A. Shimoyama, A. Saeki, N. Tanimura, et al., Chem. Eur. J. 17 (2011) 14464–14474.
doi: 10.1002/chem.201003581
T.J. Boltje, W. Zhong, J. Park, et al., J. Am. Chem. Soc. 134 (2012) 14255–14262.
doi: 10.1021/ja306274v
R. Yi, A. Ogaki, M. Fukunaga, H. Nakajima, T. Ichiyanagi, Tetrahedron 70 (2014) 3675–3682.
doi: 10.1016/j.tet.2014.04.024
E. Mancuso, C. Romanò, N. Trattnig, et al., Chem. Eur. J. 27 (2021) 7099–7102.
doi: 10.1002/chem.202100837
T. Ichiyanagi, H. Narimoto, N. Ohtani, Tetrahedron 96 (2021) 132397.
doi: 10.1016/j.tet.2021.132397
J.S. Huang, W. Huang, X. Meng, et al., Angew. Chem. Int. Ed. 54 (2015) 10894–10898.
doi: 10.1002/anie.201505176
X.Y. Zhou, P. Yang, S. Luo, J.S. Yang, Chem. Asian J. 14 (2019) 454–461.
doi: 10.1002/asia.201801779
X.Y. Zhou, L.X. Li, Z. Zhang, et al., Angew. Chem. Int. Ed. 61 (2022) e202204420.
doi: 10.1002/anie.202204420
S. Hamajima, N. Komura, H.N. Tanaka, et al., Org. Lett. 24 (2022) 8672–8676.
doi: 10.1021/acs.orglett.2c03542
S. Hamajima, N. Komura, H.N. Tanaka, et al., Molecules 28 (2023) 102.
doi: 10.3390/molecules28010102
X. Mi, Q. Lou, W. Fan, L. Zhuang, Y. Yang, Carbohydr. Res. 448 (2017) 161–165.
doi: 10.1016/j.carres.2017.04.021
Q. Lou, Q. Hua, L. Zhang, Y. Yang, Org. Lett. 22 (2020) 981–985.
doi: 10.1021/acs.orglett.9b04509
J. Zhang, X. Gao, S. Liu, et al., Org. Lett. 25 (2023) 4150–4155.
doi: 10.1021/acs.orglett.3c01430
W. Huang, Y.Y. Zhou, X.L. Pan, et al., J. Am. Chem. Soc. 140 (2018) 3574–3582.
doi: 10.1021/jacs.7b09461
R.R. Schmidt, A. Esswein, Angew. Chem. Int. Ed. 27 (1988) 1178–1180.
doi: 10.1002/anie.198811781
A. Esswein, H. Rembold, R.R. Schmidt, Carbohydr. Res. 200 (1990) 287–305.
doi: 10.1016/0008-6215(90)84198-4
P. Ngoje, D. Crich, J. Am. Chem. Soc. 142 (2020) 7760–7764.
doi: 10.1021/jacs.0c03215
K. Ikeda, S. Akamatsu, K. Achiwa, Chem. Pharm. Bull. 38 (1990) 279–281.
doi: 10.1248/cpb.38.279
P. Kosma, H. Sekljic, G.J. Balint, J. Carbohydr. Chem. 15 (1996) 701–714.
doi: 10.1080/07328309608005686
B. Pokorny, P. Kosma, Chem. Eur. J. 21 (2015) 305–313.
doi: 10.1002/chem.201405424
B. Pokorny, P. Kosma, ChemOpen 4 (2015), 722–728.
doi: 10.1002/open.201500126
B. Pokorny, P. Kosma, Org. Lett. 17 (2015) 110–113.
doi: 10.1021/ol5033128
M. Reiner, R.R. Schmidt, Tetrahedron Asymmetry 11 (2000) 319–335.
doi: 10.1016/S0957-4166(99)00557-1
K.K.T. Mong, T.K. Pradhan, C.H. Chiu, et al., Org. Chem. Front. 7 (2020) 2179–2186.
doi: 10.1039/D0QO00630K
Y. Yang, B. Yu, Chem. Rev. 117 (2017) 12281–12356.
doi: 10.1021/acs.chemrev.7b00234
G.Z. Tian, X.L. Wang, J. Hu, et al., Chin. Chem. Lett. 26 (2015) 922–930.
doi: 10.1016/j.cclet.2015.04.026
D. Liu, S. Liu, F. Hu, Z. Li, Z. Li, Chin. Chem. Lett. 35 (2024) 108762.
doi: 10.1016/j.cclet.2023.108762
K. Wang, T. Zhang, M. Liu, et al. Chin. Chem. Lett. 34 (2023) 108065.
doi: 10.1016/j.cclet.2022.108065
X. Zhou, H. Ding, P. Chen, et al., Angew. Chem. Int. Ed. 59 (2020) 4138–4144.
doi: 10.1002/anie.201914557

Hongjin Shi , Guoyin Yin , Xi Lu , Yangyang Li . Stereoselective synthesis of 2-deoxy-α-C-glycosides from glycals. Chinese Chemical Letters, 2024, 35(12): 109674-. doi: 10.1016/j.cclet.2024.109674
Chen Li , Ziyuan Zhao , Shouyun Yu . Photoredox-catalyzed C-glycosylation of peptides with glycosyl bromides. Chinese Chemical Letters, 2024, 35(6): 109128-. doi: 10.1016/j.cclet.2023.109128
Zhigang Zeng , Changzhou Liao , Lei Yu . Molecules for COVID-19 treatment. Chinese Chemical Letters, 2024, 35(7): 109349-. doi: 10.1016/j.cclet.2023.109349
Dake Liu , Shuyan Liu , Fanlei Hu , Zhongtang Li , Zhongjun Li . N-Glycosylated type Ⅱ collagen peptides as therapeutic saccharide vaccines for rheumatoid arthritis. Chinese Chemical Letters, 2024, 35(5): 108762-. doi: 10.1016/j.cclet.2023.108762
Yimin Guo , Yiting Luo , Shuwen Hua , Chuan-Fan Ding , Yinghua Yan . Application of magnetic nanomaterials in peptidomics: A review in the past decade. Chinese Chemical Letters, 2025, 36(6): 110070-. doi: 10.1016/j.cclet.2024.110070
Hong Lu , Yidie Zhai , Xingxing Cheng , Yujia Gao , Qing Wei , Hao Wei . Advancements and Expansions in the Proline-Catalyzed Asymmetric Aldol Reaction. University Chemistry, 2024, 39(5): 154-162. doi: 10.3866/PKU.DXHX202310074
Lijia Xu , Tong Zhong , Wei Zhao , Bing Yao , Lin Ding , Huangxian Ju . Chemoselective labeling-based spermatozoa glycan imaging reveals abnormal glycosylation in oligoasthenotspermia. Chinese Chemical Letters, 2024, 35(4): 108760-. doi: 10.1016/j.cclet.2023.108760
Shehla Khalid , Muhammad Bilal , Nasir Rasool , Muhammad Imran . Photochemical reactions as synthetic tool for pharmaceutical industries. Chinese Chemical Letters, 2024, 35(9): 109498-. doi: 10.1016/j.cclet.2024.109498
Zhiwei Zhong , Yanbin Huang , Wantai Yang . A simple photochemical method for surface fluorination using perfluoroketones. Chinese Chemical Letters, 2024, 35(5): 109339-. doi: 10.1016/j.cclet.2023.109339
Yujie Li , Ya-Nan Wang , Yin-Gen Luo , Hongcai Yang , Jinrui Ren , Xiao Li . Advances in synthetic biology-based drug delivery systems for disease treatment. Chinese Chemical Letters, 2024, 35(11): 109576-. doi: 10.1016/j.cclet.2024.109576
Erzhuo Cheng , Yunyi Li , Wei Yuan , Wei Gong , Yanjun Cai , Yuan Gu , Yong Jiang , Yu Chen , Jingxi Zhang , Guangquan Mo , Bin Yang . Galvanostatic method assembled ZIFs nanostructure as novel nanozyme for the glucose oxidation and biosensing. Chinese Chemical Letters, 2024, 35(9): 109386-. doi: 10.1016/j.cclet.2023.109386
Keyang Li , Yanan Wang , Yatao Xu , Guohua Shi , Sixian Wei , Xue Zhang , Baomei Zhang , Qiang Jia , Huanhua Xu , Liangmin Yu , Jun Wu , Zhiyu He . Flash nanocomplexation (FNC): A new microvolume mixing method for nanomedicine formulation. Chinese Chemical Letters, 2024, 35(10): 109511-. doi: 10.1016/j.cclet.2024.109511
Wenxuan Yang , Long Shang , Xiaomeng Liu , Sihan Zhang , Haixia Li , Zhenhua Yan , Jun Chen . Ultrafast synthesis of nanocrystalline spinel oxides by Joule-heating method. Chinese Chemical Letters, 2024, 35(11): 109501-. doi: 10.1016/j.cclet.2024.109501
Chen Chen , Jinzhou Zheng , Chaoqin Chu , Qinkun Xiao , Chaozheng He , Xi Fu . An effective method for generating crystal structures based on the variational autoencoder and the diffusion model. Chinese Chemical Letters, 2025, 36(4): 109739-. doi: 10.1016/j.cclet.2024.109739
Xue-Zhi Wang , Yi-Tong Liu , Chuang-Wei Zhou , Bei Wang , Dong Luo , Mo Xie , Meng-Ying Sun , Yong-Liang Huang , Jie Luo , Yan Wu , Shuixing Zhang , Xiao-Ping Zhou , Dan Li . Amplified circularly polarized luminescence of chiral metal-organic frameworks via post-synthetic installing pillars. Chinese Chemical Letters, 2024, 35(10): 109380-. doi: 10.1016/j.cclet.2023.109380
Hao Wang , Meng-Qi Pan , Ya-Fei Wang , Chao Chen , Jian Xu , Yuan-Yuan Gao , Chuan-Song Qi , Wei Li , Xian-He Bu . Post-synthetic modifications of MOFs by different bolt ligands for controllable release of cargoes. Chinese Chemical Letters, 2024, 35(10): 109581-. doi: 10.1016/j.cclet.2024.109581
Qiang Wu , Baofeng Wang . Exploring synthetic strategy for stabilizing nickel-rich layered oxide cathodes through structural design. Chinese Chemical Letters, 2024, 35(12): 110089-. doi: 10.1016/j.cclet.2024.110089
Junyi Yu , Yin Cheng , Anhong Cai , Xianfeng Huang , Qingrui Zhang . Synthetic Cu(Ⅲ) from copper plating wastewater for onsite decomplexation of Cu(Ⅱ)- and Ni(Ⅱ)-organic complexes. Chinese Chemical Letters, 2025, 36(2): 110549-. doi: 10.1016/j.cclet.2024.110549
Ying Li , Long-Jie Wang , Yong-Kang Zhou , Jun Liang , Bin Xiao , Ji-Shen Zheng . An improved installation of 2-hydroxy-4-methoxybenzyl (iHmb) method for chemical protein synthesis. Chinese Chemical Letters, 2024, 35(5): 109033-. doi: 10.1016/j.cclet.2023.109033
Peng Zhou , Ziang Jiang , Yang Li , Peng Xiao , Feixiang Wu . Sulphur-template method for facile manufacturing porous silicon electrodes with enhanced electrochemical performance. Chinese Chemical Letters, 2024, 35(8): 109467-. doi: 10.1016/j.cclet.2023.109467