Photochemical reaction mechanism of benzophenone protected guanosine at N7 position
-
* Corresponding author.
E-mail address: majiani@snnu.edu.cn (J. Ma).
Citation:
Yan Guo, Hongtao Bian, Le Yu, Jiani Ma, Yu Fang. Photochemical reaction mechanism of benzophenone protected guanosine at N7 position[J]. Chinese Chemical Letters,
;2025, 36(3): 109971.
doi:
10.1016/j.cclet.2024.109971

The overuse of antibiotic has led to the emergence of drug-resistant bacteria [1]. As a result, the development of new antimicrobial drugs and vaccines has attracted the increasing attention of scientists [2]. 3-Deoxy-D-manno-oct-2-ulosonic acid (Kdo) is an eight-carbon monosaccharide widely distributed in bacterial lipopolysaccharides (LPS) and capsule polysaccharides (CPS) [3,4]. In the biosynthesis of LPS, several sequential enzymatic reactions are necessary for the synthesis of Kdo from D-ribulose-5-phosphate and attaching it to lipid A [5]. In this biosynthetic pathway, CMP-Kdo plays a crucial role as a key intermediate [5]. Its derivatives have the potential to act as inhibitors of CMP-Kdo synthase (CKS), thereby obstructing the LPS biosynthesis pathway and ultimately disrupting the production of the bacterial outer membrane [6]. Thus, analogs of Kdo have the potential to act as CKS inhibitors and serve as novel antimicrobial agents.
As a deoxy sugar [7,8], Kdo glycosylation is one of the most challenging glycosylation reactions because of the low reactivity, the uncontrolled stereoselectivity and the formation of 2,3-ene byproducts [9-14]. Therefore, the highly efficient and stereoselective synthesis of Kdo glycosides remains an unmet need in glycosylation methodology, which limits the investigation of the biological activities of Kdo-containing oligosaccharides. Currently, Kdo glycosylation methods can be broadly divided into direct and indirect methods [15]. Using the direct methods, the target glycosidic bonds can be achieved in a single step by utilizing steric hinderance effect [16-31], special intermediate formation [32-34], remote participation effect [35], solvent and ion effect [36,37] and side chain conformation (Scheme 1A) [38]. The selection of protective groups in the direct methods is intricate, often resulting in the formation of additional 2,3-ene byproducts. Using the indirect methods, an auxiliary group (such as S [39], I [40-43], O [44,45]) is added at Kdo's C3 position to modulate the anomeric stereoselectivity (Scheme 1B). After glycosylation, the auxiliary group can be removed by additional steps. Compared with direct methods, indirect methods simplify protecting group operations and enhance reaction efficiency while preserving complete stereoselectivity and generating less 2,3-enes [15].
Our team has previously developed a strategy for Kdo α-glycosylation utilizing a C3-p-tolylthio-substituted Kdo phosphite donor 1 via the classical neighboring group participation effect (Scheme 1B) [15]. The sulfur-positive tricyclic intermediate restricted the acceptor to attacking from the α side of the sugar ring only. Consequently, single α-stereoselective Kdo glycosides were produced without the formation of 2,3-enes. However, the high reactivity of the modified phosphite donor 1, which must be used immediately after preparation, is a drawback of this strategy. In this study, we investigated the reaction of the C3-p-tolylthio-substituted Kdo fluoride donor 2 and demonstrated its increased stability (Scheme 1C). The complete α-stereoselectivity and broad substrate scope proved the superiority of introducing a thioether at the C3 position of Kdo donors. In addition, we synthesized a series of Kdo O/C/S/N-glycosides via fluoride donor 2, which have the potential to act as important precursors of candidate molecules for CKS inhibition.
According to the previous work [15], the C3-p-tolylthio-substituted Kdo hydroxy sugar S1 was synthesized with 2,3-ene by a two-step reaction of addition and hydrolysis. Subsequently, the α-Kdo fluoride donor 2 was prepared by fluorination of S1 in a satisfactory yield of 89% (Scheme S1 in Supporting information). Notably, donor 2 maintained stability for more than one year at room temperature, which was considerably more stable than donor 1.
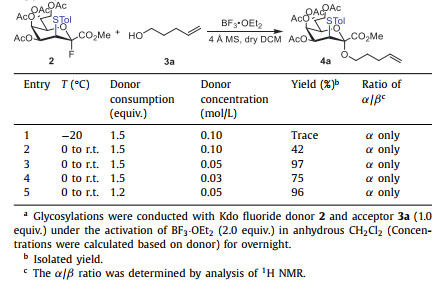
Under the classical conditions for the activation of glycosyl fluoride [41-43], the glycosylation of donor 2 (1.2 equiv.) was conducted with 4-penten-1-ol 3a (1.0 equiv.) in the presence of BF3·OEt2 (2.0 equiv.), 4 Å molecular sieves (MS) at 0 ℃ to room temperature in anhydrous CH2Cl2 (Table 1, entry 2, 0.10 mol/L, calculated based on donor). Surprisingly, the coupling reaction between 2 and 3a provided the Kdo glycoside 4a in a moderate 42% yield as the sole α-isomer without the formation of 2,3-ene byproduct. Similar to the phosphite donor 1, the axial orientation of the thio group at C3 of the fluoride donor 2 prevented the elimination reactions, while efficiently enhanced α-stereoselectivity via neighboring group participation effects [15]. The stereoconfigurations of Kdo glycosides was confirmed via the non-decoupling 13C NMR (3JC1, H3ax < 1.0 Hz) [27]. Considering that the adsorption of hydrogen fluoride formed during the reaction by molecular sieves can drive this reaction [41-43], we adjusted the ratio of molecular sieve and donor by modifying the concentration of the reaction solution (MS: 100 mg/mL). The experiment demonstrated that the reaction afforded glycoside 4a in 96% yield with outstanding α-stereoselectivity when the donor concentration reached 0.05 mol/L (Table 1, entry 5).
|
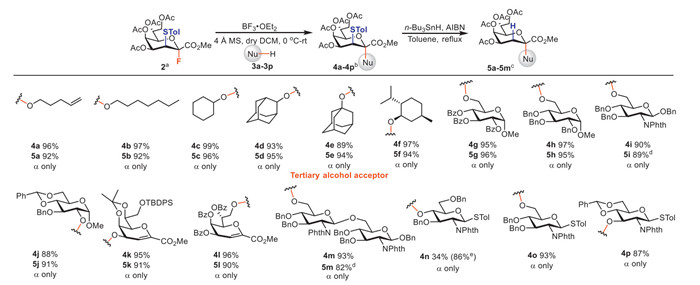
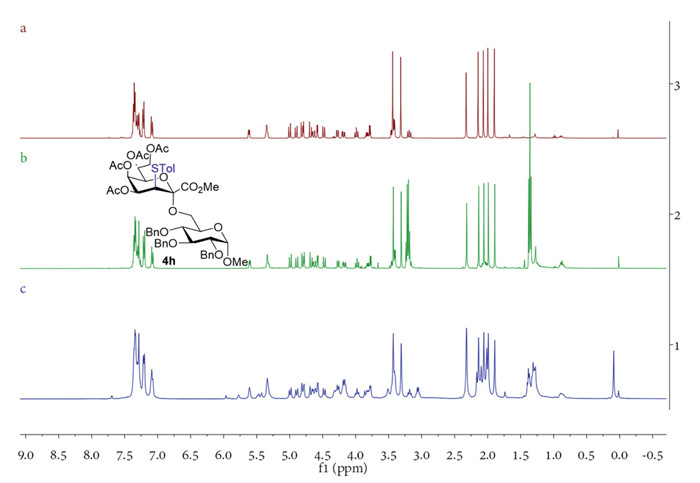
Subsequently, we investigated the O-glycosylation scope of donor 2 (1.2 equiv.) with various acceptors (3a-3p) under the optimal conditions (Scheme 2, donor: 1.2 equiv., acceptor: 1.0 equiv., BF3·OEt2: 2.0 equiv., CH2Cl2: 0.05 mol/L calculated based on donor, 4 Å MS: 100 mg/mL calculated based on solvent, 0 ℃→r.t.). To our satisfaction, we were able to synthesize the fully α-stereoselective Kdo glycosides 4a-4f in excellent yields of 89%−99% without the formation of 2,3-enes through glycosylation of donor 2 with simple alcohols, including primary alcohols (3a: 4-penten-1-ol, 3b: n-heptanol), secondary alcohols (3c: cyclohexanol, 3d: 2-adamantanol, 3f: ʟ-menthol), and tertiary alcohol (3e: 1-adamantanol). For the reacions with glycosyl acceptors, the glycosylation process between donor 2 and acceptor 3g-3m produced sole α-isomer Kdo oligosaccharides 4g-4m with yields ranging from 88% to 97%. It was noteworthy that the fluoride donor 2 reacted with Kdo glycal acceptors 3k and 3l, resulting in the successful formation of di-Kdo saccharides 4k (95%) and 4l (96%) in excellent yields through α-(2→4)- and α-(2→8) linkages. In particular, the Kdo-α-(2→6)-GlcN-β-(1→6)-GlcN trisaccharide 4m is the essential component of several bacterial LPS structures [19,22]. Under the optimized free radical reduction conditions developed by our team [15], the thioether groups of the glycosides 4a-4m were successfully removed, leading to the formation of 5a-5m in high yields of 82%−96%. In addition, the glucosamine thioglycosides acceptors 3n-3p coupled with donor 2 and afforded the desired Kdo-containing disaccharides 4n (34%), 4o (93%) and 4p (87%) without activation of the thioglycosides. Among them, the reaction between inactive 4-OH acceptor 3n and 2 was incomplete with reactants remained, and only 34% of the disaccharide 4n was obtained (86% yield based on the consumed acceptors). Overall, the glycosylation efficiency of fluoride donor 2 with acceptors that are moderately or highly active is comparable to that of phosphite donor 1 [15]. For inactive acceptors, reaction yields of donor 2 can be improved through recycling reactants. In another experiment, acceptor 3h was coupled with donor 1 and 2 separately, and the reaction solutions were filtered to remove molecular sieves, concentrated without column chromatography separation, and analyzed by NMR (Fig. 1). The 1H NMR spectra of the crude products reveals that the reaction system of fluoride glycosylation was very clean (Fig. 1b).
CMP-Kdo is a key intermediate in LPS biosynthesis [4]. Therefore, Kdo derivatives have the potential to be developed as CKS inhibitors, which can block bacterial cell wall synthesis. Additionally, C/S/N-glycosides possess distinct properties compared to O-glycosides, and exhibit resistance to enzymatic degradation while preserving the structural integrity of the glycosides [46]. Notably, some of the CKS inhibitors being investigated are Kdo carboglycoside or thioglycoside derivatives [6]. Thus, we attempted to broaden the structural type of Kdo derivatives by coupling Kdo fluoride donor 2 with common nucleophilic reagents 6a-6k (Scheme 3).
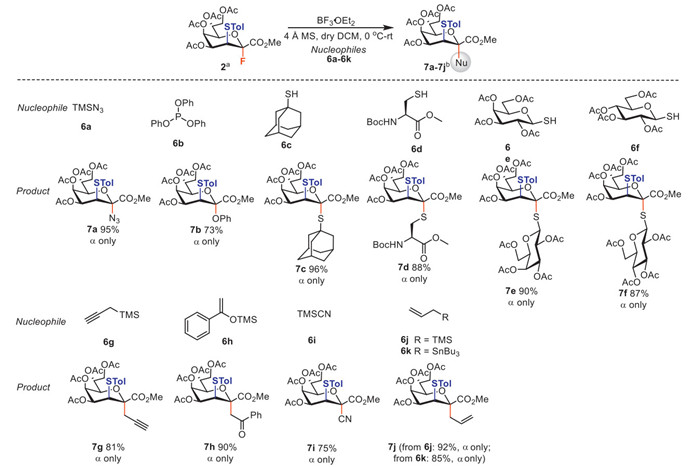
Under standard glycosylation conditions, donor 2 was coupled with TMSN3, resulting in 95% yield of Kdo azide 7a. Subsequently, protected ʟ-propargylglycine 8 was reacted with 7a under click reaction conditions to produce glycosylated unnatural amino acid 9 in 51% yield (Scheme 4). Compound 9 has the potential to be used in glycopeptide synthesis and serves as an intermediate for Kdo derivatization and bioactivity research [47-49]. Interestingly, donor 2 reacted with 6b to yield phenolic glycoside 7b, which is different from the phosphoglycoside products obtained from the reaction of fluoride with 6b as reported [50]. It is possible that the reaction underwent the following process. Under acidic conditions, triphenyl phosphite 6b is easily hydrolyzed to form phosphoric acid and phenol. Molecular sieves can absorb the resulting phosphoric acid and the fluoride 2 is subsequently activated in the presence of boron trifluoride ether and preferentially undergoes glycosylation process with the phenol to produce 7b rather than Arbuzov reaction. In addition, donor 2 reacted with several sulfur nucleophilic reagents (6c-6f) to produce the corresponding thioglycoside derivatives 7c-7f in yields ranging from 87% to 96%. The carboglycoside products 7g-7j were obtained in high yields (75%−92%) by using carbo nucleophilic reagents 6g-6k and donor 2. Moreover, the alkyne group in compound 7g could undergo further derivatization via click reaction. It is worth noting that all of the aforementioned Kdo derivatives exhibit complete α-stereoselectivity. The construction of compound libraries containing Kdo derivatives and the evaluation of their bioactivity are currently in progress.
Scheme 5 shows the proposed reaction mechanism of glycosylation with C3-p-tolylthio-substituted Kdo fluoride donor 2 promoted by BF3·OEt2. Boron trifluoride activates the fluoride donor 2, breaking the carbon-fluorine bond and forming a ternary cyclic episulfonium intermediate. Due to the neighboring group participation effect, the episulfonium blocks the β-side of the Kdo sugar ring, allowing the nucleophilic reagent to attack from the α-side only. After proton dissociation, complete α-stereoselective of Kdo glycosides are formed.
In summary, we synthesized C3-p-tolylthio-substituted fluoride donor 2 to increase the stability of the Kdo phosphite donor 1 and investigated the reactions of donor 2 in Kdo α-glycosylation. The results of this study along with previous reports support that the Kdo glycosylation reaction can be treated as two categories: (1) For acceptors with medium to high reactivity, the glycosylation reaction can be performed more conveniently by using the more stable fluoride donor 2, resulting in cleaner reactions. (2) For low-reactivity acceptors, more reactive phosphite donor 1 can be used for efficient glycosylation reactions. These two methods demonstrated the superiority of introducing a thioether at the C3 position of the Kdo donors. Furthermore, the substrate range was further expanded by utilizing donor 2 to react with various C/S/N-nucleophilic reagents, resulting in high yields and complete α-stereoselectivity of Kdo derivatives. These Kdo derivatives represent new structural types for designing CKS inhibitors.
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Ao Sun: Writing – original draft, Visualization, Methodology, Investigation, Formal analysis, Data curation, Conceptualization. Zipeng Li: Validation, Investigation. Shuchun Li: Writing – review & editing, Project administration. Xiangbao Meng: Writing – review & editing. Zhongtang Li: Writing – review & editing. Zhongjun Li: Writing – review & editing, Supervision, Resources, Project administration, Methodology, Funding acquisition, Conceptualization.
This project was supported by the National Key R&D Program of China (Nos. 2022YFF1203005, 2022YFC2303700), and supported by the National Natural Science Foundation of China (Nos. 81930097, 82151223). We thank Dr. De-Cai Xiong, Peking University, for helpful discussion.
Supplementary material associated with this article can be found, in the online version, at doi:
G. Mayer, A. Heckel, Angew. Chem. Int. Ed. 45 (2006) 4900–4921.
doi: 10.1002/anie.200600387
S. Jia, E.M. Sletten, ACS Chem. Biol. 17 (2022) 3255–3269.
doi: 10.1021/acschembio.1c00518
S.K. Jia, S.X. Yang, H.M. Ji, et al., Chin. Chem. Lett. 31 (2020) 1104–1108.
doi: 10.1016/j.cclet.2019.10.021
Y.J. Li, M.L. Wang, F. Wang, S. Lu, X.Q. Chen, Smart Mol. 1 (2023) e20220003.
doi: 10.1002/smo.20220003
V.M. Lechner, M. Nappi, P.J. Deneny, et al., Chem. Rev. 122 (2022) 1752–1829.
doi: 10.1021/acs.chemrev.1c00357
N. Ankenbruck, T. Courtney, Y. Naro, A. Deiters, Angew. Chem. Int. Ed. 57 (2018) 2768–2798.
doi: 10.1002/anie.201700171
P. Klán, T. Šolomek, C.G. Bochet, et al., Chem. Rev. 113 (2013) 119–191.
doi: 10.1021/cr300177k
R. Weinstain, T. Slanina, D. Kand, P. Klán, Chem. Rev. 120 (2020) 13135–13272.
doi: 10.1021/acs.chemrev.0c00663
Z.C. Situ, W.B. Chen, S.R. Yang, et al., J. Phys. Chem. B 126 (2022) 3338–3346.
doi: 10.1021/acs.jpcb.2c01440
J.M. Govan, R. Uprety, J. Hemphill, M.O. Lively, A. Deiters, ACS Chem. Biol. 7 (2012) 1247–1256.
doi: 10.1021/cb300161r
K. Ohno, D. Sugiyama, L. Takeshita, et al., Bioorg. Med. Chem. 25 (2017) 6007–6015.
doi: 10.1016/j.bmc.2017.09.032
S. Boháčová, Z. Vaníková, L.P. Slavětínská, M. Hocek, Org. Biomol. Chem. 16 (2018) 5427–5432.
doi: 10.1039/c8ob01106k
F. Debart, C. Dupouy, J.J. Vasseur, J. Org. Chem. 14 (2018) 436–469.
doi: 10.3762/bjoc.14.32
V. Dhamodharan, Y. Nomura, M. Dwidar, Y. Yokobayashi, Chem. Commun. 54 (2018) 6181–6183.
doi: 10.1039/c8cc02290a
H. Lusic, R. Uprety, A. Deiters, Org. Lett. 12 (2010) 916–919.
doi: 10.1021/ol902807q
F. Schäfer, K.B. Joshi, M.A.H. Fichte, et al., Org. Lett. 13 (2011) 1450–1453.
doi: 10.1021/ol200141v
S. Boháčová, L. Ludvíková, L. Poštová Slavětínská, et al., Org. Biomol. Chem. 16 (2018) 1527–1535.
doi: 10.1039/c8ob00160j
C. Menge, A. Heckel, Org. Lett. 13 (2011) 4620–4623.
doi: 10.1021/ol201842x
P. Seyfried, M. Heinz, G. Pintér, et al., Chem. Eur. J. 24 (2018) 17568–17576.
doi: 10.1002/chem.201804040
D.Y. Zhang, C.Y. Zhou, K.N. Busby, S.C. Alexander, N.K. Devaraj, Angew. Chem. Int. Ed. 57 (2018) 2822–2826.
doi: 10.1002/anie.201710917
D.Y. Zhang, S.J. Jin, X.J. Piao, N.K. Devaraj, ACS Chem. Biol. 15 (2020) 1773–1779.
doi: 10.1021/acschembio.0c00205
S.Z. Tang, J. Cannon, K. Yang, et al., J. Org. Chem. 85 (2020) 2945–2955.
doi: 10.1021/acs.joc.9b02617
S. Hikage, Y. Sasaki, T. Hisai, et al., J. Photochem. Photobiol. A 331 (2016) 175–183.
doi: 10.1016/j.jphotochem.2016.01.007
L. Anhäuser, N. Klöcker, F. Muttach, et al., Angew. Chem. Int. Ed. 59 (2020) 3161–3165.
doi: 10.1002/anie.201914573
M.C. Cuquerella, V. Lhiaubet-Vallet, J. Cadet, M.A. Miranda, Acc. Chem. Res. 45 (2012) 1558–1570.
doi: 10.1021/ar300054e
W. Adam, M.A. Arnold, W.M. Nau, U. Pischel, C.R. Saha-Möller, J. Am. Chem. Soc. 124 (2002) 3893–3904.
doi: 10.1021/ja017600y
R. Gao, D.P. Yan, Chem. Sci. 8 (2017) 590–599.
doi: 10.1039/C6SC03515A
Y.S. Yang, K.Z. Wang, D.P. Yan, Chem. Commun. 53 (2017) 7752–7755.
doi: 10.1039/C7CC04356B
B. Zhou, D.P. Yan, Adv. Funct. Mater. 29 (2019) 1807599.
doi: 10.1002/adfm.201807599
H.Y. Lin, X.P. Chang, D.P. Yan, W.H. Fang, G.L. Cui, Chem. Sci. 8 (2017) 2086–2090.
doi: 10.1039/C6SC04354B
B. Zhou, Q. Zhao, L.C. Tang, D.P. Yan, Chem. Commun. 56 (2020) 7698–7701.
doi: 10.1039/d0cc02730h
P.F. McGarry, C.E. Doubleday, C.H. Wu, H.A. Staab, N.J. Turro, J. Photochem. Photobiol. A 77 (1994) 109–117.
doi: 10.1016/1010-6030(94)80033-2
Y.A. Wang, C. Wang, J.R. Zhang, et al., Chin. Chem. Lett. 34 (2023) 108062.
doi: 10.1016/j.cclet.2022.108062
J.N. Ma, X.T. Zhang, D.L. Phillips, Acc. Chem. Res. 52 (2019) 726–737.
doi: 10.1021/acs.accounts.8b00619
V. Lhiaubet-Vallet, N. Belmadoui, M.J. Climent, M.A. Miranda, J. Phys. Chem. B 111 (2007) 8277–8282.
doi: 10.1021/jp071524p
I. Andreu, F. Palumbo, F. Tilocca, et al., Org. Lett. 13 (2011) 4096–4099.
doi: 10.1021/ol2016059
S. Jockusch, N.J. Turro, J. Am. Chem. Soc. 121 (1999) 3921–3925.
doi: 10.1021/ja9837194
D. Hristova-Neeley, D. Neshchadin, G. Gescheidt, J. Phys. Chem. B 119 (2015) 13883–13887.
doi: 10.1021/acs.jpcb.5b04263
I.G. Gut, P.D. Wood, R.W. Redmond, J. Am. Chem. Soc. 118 (1996) 2366–2373.
doi: 10.1021/ja9519344
A.A. Lamola, M. Gueron, T. Yamane, J. Eisinger, R.G. Shulman, J. Chem. Phys. 47 (1967) 2210–2217.
doi: 10.1063/1.1703293
M.C. Cuquerella, V. Lhiaubet-Vallet, F. Bosca, M.A. Miranda, Chem. Sci. 2 (2011) 1219–1232.
doi: 10.1039/c1sc00088h
J.W. Longworth, R.O. Rahn, R.G. Shulman, J. Chem. Phys. 45 (1966) 2930–2939.
doi: 10.1063/1.1728048
W.M. Kwok, C.S. Ma, D.L. Phillips, J. Am. Chem. Soc. 130 (2008) 5131–5139.
doi: 10.1021/ja077831q
T. Climent, I. González-Ramírez, R. González-Luque, M. Merchán, L. Serrano-Andrés, J. Phys. Chem. Lett. 1 (2010) 2072–2076.
doi: 10.1021/jz100601p
G. Mayer, A. Heckel, Angew. Chem. Int. Ed. 45 (2006) 4900–4921.
doi: 10.1002/anie.200600387
S. Jia, E.M. Sletten, ACS Chem. Biol. 17 (2022) 3255–3269.
doi: 10.1021/acschembio.1c00518
S.K. Jia, S.X. Yang, H.M. Ji, et al., Chin. Chem. Lett. 31 (2020) 1104–1108.
doi: 10.1016/j.cclet.2019.10.021
Y.J. Li, M.L. Wang, F. Wang, S. Lu, X.Q. Chen, Smart Mol. 1 (2023) e20220003.
doi: 10.1002/smo.20220003
V.M. Lechner, M. Nappi, P.J. Deneny, et al., Chem. Rev. 122 (2022) 1752–1829.
doi: 10.1021/acs.chemrev.1c00357
N. Ankenbruck, T. Courtney, Y. Naro, A. Deiters, Angew. Chem. Int. Ed. 57 (2018) 2768–2798.
doi: 10.1002/anie.201700171
P. Klán, T. Šolomek, C.G. Bochet, et al., Chem. Rev. 113 (2013) 119–191.
doi: 10.1021/cr300177k
R. Weinstain, T. Slanina, D. Kand, P. Klán, Chem. Rev. 120 (2020) 13135–13272.
doi: 10.1021/acs.chemrev.0c00663
Z.C. Situ, W.B. Chen, S.R. Yang, et al., J. Phys. Chem. B 126 (2022) 3338–3346.
doi: 10.1021/acs.jpcb.2c01440
J.M. Govan, R. Uprety, J. Hemphill, M.O. Lively, A. Deiters, ACS Chem. Biol. 7 (2012) 1247–1256.
doi: 10.1021/cb300161r
K. Ohno, D. Sugiyama, L. Takeshita, et al., Bioorg. Med. Chem. 25 (2017) 6007–6015.
doi: 10.1016/j.bmc.2017.09.032
S. Boháčová, Z. Vaníková, L.P. Slavětínská, M. Hocek, Org. Biomol. Chem. 16 (2018) 5427–5432.
doi: 10.1039/c8ob01106k
F. Debart, C. Dupouy, J.J. Vasseur, J. Org. Chem. 14 (2018) 436–469.
doi: 10.3762/bjoc.14.32
V. Dhamodharan, Y. Nomura, M. Dwidar, Y. Yokobayashi, Chem. Commun. 54 (2018) 6181–6183.
doi: 10.1039/c8cc02290a
H. Lusic, R. Uprety, A. Deiters, Org. Lett. 12 (2010) 916–919.
doi: 10.1021/ol902807q
F. Schäfer, K.B. Joshi, M.A.H. Fichte, et al., Org. Lett. 13 (2011) 1450–1453.
doi: 10.1021/ol200141v
S. Boháčová, L. Ludvíková, L. Poštová Slavětínská, et al., Org. Biomol. Chem. 16 (2018) 1527–1535.
doi: 10.1039/c8ob00160j
C. Menge, A. Heckel, Org. Lett. 13 (2011) 4620–4623.
doi: 10.1021/ol201842x
P. Seyfried, M. Heinz, G. Pintér, et al., Chem. Eur. J. 24 (2018) 17568–17576.
doi: 10.1002/chem.201804040
D.Y. Zhang, C.Y. Zhou, K.N. Busby, S.C. Alexander, N.K. Devaraj, Angew. Chem. Int. Ed. 57 (2018) 2822–2826.
doi: 10.1002/anie.201710917
D.Y. Zhang, S.J. Jin, X.J. Piao, N.K. Devaraj, ACS Chem. Biol. 15 (2020) 1773–1779.
doi: 10.1021/acschembio.0c00205
S.Z. Tang, J. Cannon, K. Yang, et al., J. Org. Chem. 85 (2020) 2945–2955.
doi: 10.1021/acs.joc.9b02617
S. Hikage, Y. Sasaki, T. Hisai, et al., J. Photochem. Photobiol. A 331 (2016) 175–183.
doi: 10.1016/j.jphotochem.2016.01.007
L. Anhäuser, N. Klöcker, F. Muttach, et al., Angew. Chem. Int. Ed. 59 (2020) 3161–3165.
doi: 10.1002/anie.201914573
M.C. Cuquerella, V. Lhiaubet-Vallet, J. Cadet, M.A. Miranda, Acc. Chem. Res. 45 (2012) 1558–1570.
doi: 10.1021/ar300054e
W. Adam, M.A. Arnold, W.M. Nau, U. Pischel, C.R. Saha-Möller, J. Am. Chem. Soc. 124 (2002) 3893–3904.
doi: 10.1021/ja017600y
R. Gao, D.P. Yan, Chem. Sci. 8 (2017) 590–599.
doi: 10.1039/C6SC03515A
Y.S. Yang, K.Z. Wang, D.P. Yan, Chem. Commun. 53 (2017) 7752–7755.
doi: 10.1039/C7CC04356B
B. Zhou, D.P. Yan, Adv. Funct. Mater. 29 (2019) 1807599.
doi: 10.1002/adfm.201807599
H.Y. Lin, X.P. Chang, D.P. Yan, W.H. Fang, G.L. Cui, Chem. Sci. 8 (2017) 2086–2090.
doi: 10.1039/C6SC04354B
B. Zhou, Q. Zhao, L.C. Tang, D.P. Yan, Chem. Commun. 56 (2020) 7698–7701.
doi: 10.1039/d0cc02730h
P.F. McGarry, C.E. Doubleday, C.H. Wu, H.A. Staab, N.J. Turro, J. Photochem. Photobiol. A 77 (1994) 109–117.
doi: 10.1016/1010-6030(94)80033-2
Y.A. Wang, C. Wang, J.R. Zhang, et al., Chin. Chem. Lett. 34 (2023) 108062.
doi: 10.1016/j.cclet.2022.108062
J.N. Ma, X.T. Zhang, D.L. Phillips, Acc. Chem. Res. 52 (2019) 726–737.
doi: 10.1021/acs.accounts.8b00619
V. Lhiaubet-Vallet, N. Belmadoui, M.J. Climent, M.A. Miranda, J. Phys. Chem. B 111 (2007) 8277–8282.
doi: 10.1021/jp071524p
I. Andreu, F. Palumbo, F. Tilocca, et al., Org. Lett. 13 (2011) 4096–4099.
doi: 10.1021/ol2016059
S. Jockusch, N.J. Turro, J. Am. Chem. Soc. 121 (1999) 3921–3925.
doi: 10.1021/ja9837194
D. Hristova-Neeley, D. Neshchadin, G. Gescheidt, J. Phys. Chem. B 119 (2015) 13883–13887.
doi: 10.1021/acs.jpcb.5b04263
I.G. Gut, P.D. Wood, R.W. Redmond, J. Am. Chem. Soc. 118 (1996) 2366–2373.
doi: 10.1021/ja9519344
A.A. Lamola, M. Gueron, T. Yamane, J. Eisinger, R.G. Shulman, J. Chem. Phys. 47 (1967) 2210–2217.
doi: 10.1063/1.1703293
M.C. Cuquerella, V. Lhiaubet-Vallet, F. Bosca, M.A. Miranda, Chem. Sci. 2 (2011) 1219–1232.
doi: 10.1039/c1sc00088h
J.W. Longworth, R.O. Rahn, R.G. Shulman, J. Chem. Phys. 45 (1966) 2930–2939.
doi: 10.1063/1.1728048
W.M. Kwok, C.S. Ma, D.L. Phillips, J. Am. Chem. Soc. 130 (2008) 5131–5139.
doi: 10.1021/ja077831q
T. Climent, I. González-Ramírez, R. González-Luque, M. Merchán, L. Serrano-Andrés, J. Phys. Chem. Lett. 1 (2010) 2072–2076.
doi: 10.1021/jz100601p

Dan-Ying Xing , Xiao-Dan Zhao , Chuan-Shu He , Bo Lai . Kinetic study and DFT calculation on the tetracycline abatement by peracetic acid. Chinese Chemical Letters, 2024, 35(9): 109436-. doi: 10.1016/j.cclet.2023.109436
Haibin Yang , Duowen Ma , Yang Li , Qinghe Zhao , Feng Pan , Shisheng Zheng , Zirui Lou . Mo doped Ru-based cluster to promote alkaline hydrogen evolution with ultra-low Ru loading. Chinese Journal of Structural Chemistry, 2023, 42(11): 100031-100031. doi: 10.1016/j.cjsc.2023.100031
Jing Wang , Zhongliao Wang , Jinfeng Zhang , Kai Dai . Single-layer crystalline triazine-based organic framework photocatalysts with different linking groups for H2O2 production. Chinese Journal of Structural Chemistry, 2023, 42(12): 100202-100202. doi: 10.1016/j.cjsc.2023.100202
Lihua Ma , Song Guo , Zhi-Ming Zhang , Jin-Zhong Wang , Tong-Bu Lu , Xian-Shun Zeng . Sensitizing photoactive metal–organic frameworks via chromophore for significantly boosting photosynthesis. Chinese Chemical Letters, 2024, 35(5): 108661-. doi: 10.1016/j.cclet.2023.108661
Manlin Lu , Sheng Liao , Jiayu Li , Zidong Yu , Ningjiu Zhao , Zuoti Xie , Shunli Chen , Li Dang , Ming-De Li . Face-to-face π-π interactions and electron communication boosting efficient reverse intersystem crossing in through-space charge transfer molecules. Chinese Chemical Letters, 2025, 36(6): 110066-. doi: 10.1016/j.cclet.2024.110066
Linshan Peng , Qihang Peng , Tianxiang Jin , Zhirong Liu , Yong Qian . Highly efficient capture of thorium ion by citric acid-modified chitosan gels from aqueous solution. Chinese Chemical Letters, 2024, 35(5): 108891-. doi: 10.1016/j.cclet.2023.108891
Ya-Nan Yang , Zi-Sheng Li , Sourav Mondal , Lei Qiao , Cui-Cui Wang , Wen-Juan Tian , Zhong-Ming Sun , John E. McGrady . Metal-metal bonds in Zintl clusters: Synthesis, structure and bonding in [Fe2Sn4Bi8]3– and [Cr2Sb12]3–. Chinese Chemical Letters, 2024, 35(8): 109048-. doi: 10.1016/j.cclet.2023.109048
Guangchang Yang , Shenglong Yang , Jinlian Yu , Yishun Xie , Chunlei Tan , Feiyan Lai , Qianqian Jin , Hongqiang Wang , Xiaohui Zhang . Regulating local chemical environment in O3-type layered sodium oxides by dual-site Mg2+/B3+ substitution achieves durable and high-rate cathode. Chinese Chemical Letters, 2024, 35(9): 109722-. doi: 10.1016/j.cclet.2024.109722
Lian Sun , Honglei Wang , Ming Ma , Tingting Cao , Leilei Zhang , Xingui Zhou . Shape and composition evolution of Pt and Pt3M nanocrystals under HCl chemical etching. Chinese Chemical Letters, 2024, 35(9): 109188-. doi: 10.1016/j.cclet.2023.109188
Ruonan Yang , Jiajia Li , Dongmei Zhang , Xiuqi Zhang , Xia Li , Han Yu , Zhanhu Guo , Chuanxin Hou , Gang Lian , Feng Dang . Grain-refining Co0.85Se@CNT cathode catalyst with promoted Li2O2 growth kinetics for lithium-oxygen batteries. Chinese Chemical Letters, 2024, 35(12): 109595-. doi: 10.1016/j.cclet.2024.109595
Xiangyu Chen , Aihao Xu , Dong Wei , Fang Huang , Junjie Ma , Huibing He , Jing Xu . Atomic cerium-doped CuOx catalysts for efficient electrocatalytic CO2 reduction to CH4. Chinese Chemical Letters, 2025, 36(1): 110175-. doi: 10.1016/j.cclet.2024.110175
Yanan Zhou , Li Sheng , Lanlan Chen , Wenhua Zhang , Jinlong Yang . Axial coordinated iron-nitrogen-carbon as efficient electrocatalysts for hydrogen evolution and oxygen redox reactions. Chinese Chemical Letters, 2025, 36(1): 109588-. doi: 10.1016/j.cclet.2024.109588
He Yao , Wenhao Ji , Yi Feng , Chunbo Qian , Chengguang Yue , Yue Wang , Shouying Huang , Mei-Yan Wang , Xinbin Ma . Copper-catalyzed and biphosphine ligand controlled 3,4-boracarboxylation of 1,3-dienes with carbon dioxide. Chinese Chemical Letters, 2025, 36(4): 110076-. doi: 10.1016/j.cclet.2024.110076
Quan Xu , Ye-Qing Du , Pan-Pan Chen , Yili Sun , Ze-Nan Yang , Hui Zhang , Bencan Tang , Hong Wang , Jia Li , Yue-Wei Guo , Xu-Wen Li . Computation assisted chemical study of photo-induced late-stage skeleton transformation of marine natural products towards new scaffolds with biological functions. Chinese Chemical Letters, 2025, 36(5): 110141-. doi: 10.1016/j.cclet.2024.110141
Zhongchao Zhou , Jian Song , Yinghao Xie , Yuqian Ma , Hong Hu , Hui Li , Lei Zhang , Charles H. Lawrie . DFT calculation for organic semiconductor-based gas sensors: Sensing mechanism, dynamic response and sensing materials. Chinese Chemical Letters, 2025, 36(6): 110906-. doi: 10.1016/j.cclet.2025.110906
Qian Huang , Zhaowei Li , Jianing Zhao , Ao Yu . Quantum Chemical Calculations Reveal the Details Below the Experimental Phenomenon. University Chemistry, 2024, 39(3): 395-400. doi: 10.3866/PKU.DXHX202309018
Chunyan Yang , Qiuyu Rong , Fengyin Shi , Menghan Cao , Guie Li , Yanjun Xin , Wen Zhang , Guangshan Zhang . Rationally designed S-scheme heterojunction of BiOCl/g-C3N4 for photodegradation of sulfamerazine: Mechanism insights, degradation pathways and DFT calculation. Chinese Chemical Letters, 2024, 35(12): 109767-. doi: 10.1016/j.cclet.2024.109767
Manyu Zhu , Fei Liang , Lie Wu , Zihao Li , Chen Wang , Shule Liu , Xiue Jiang . Revealing the difference of Stark tuning rate between interface and bulk by surface-enhanced infrared absorption spectroscopy. Chinese Chemical Letters, 2025, 36(2): 109962-. doi: 10.1016/j.cclet.2024.109962
Hui Liu , Xiangyang Tang , Zhuang Cheng , Yin Hu , Yan Yan , Yangze Xu , Zihan Su , Futong Liu , Ping Lu . Constructing multifunctional deep-blue emitters with weak charge transfer excited state for high-performance non-doped blue OLEDs and single-emissive-layer hybrid white OLEDs. Chinese Chemical Letters, 2024, 35(10): 109809-. doi: 10.1016/j.cclet.2024.109809
Jiayin Zhou , Depeng Liu , Longqiang Li , Min Qi , Guangqiang Yin , Tao Chen . Responsive organic room-temperature phosphorescence materials for spatial-time-resolved anti-counterfeiting. Chinese Chemical Letters, 2024, 35(11): 109929-. doi: 10.1016/j.cclet.2024.109929