Spintronics is a cutting-edge field of developing new electronic devices by manipulating the electron spin and magnetic moment [1]. Traditional spintronic research mainly focuses on transition metals and inorganic semiconductors, while organic molecules have the advantage of being extremely easy to realize efficient spin control by modifying the specific external conditions for desired electronic structures and magnetic characteristics. Corrole, as a ring-contracted porphyrin, is the aromatic analog of the central macrocycle of vitamin B12. Corrole has a squeezed inner cavity and three inner NHs in its free-base form, making it easier to stabilize high-valent metal ions and thus a promising candidate in spintronics.
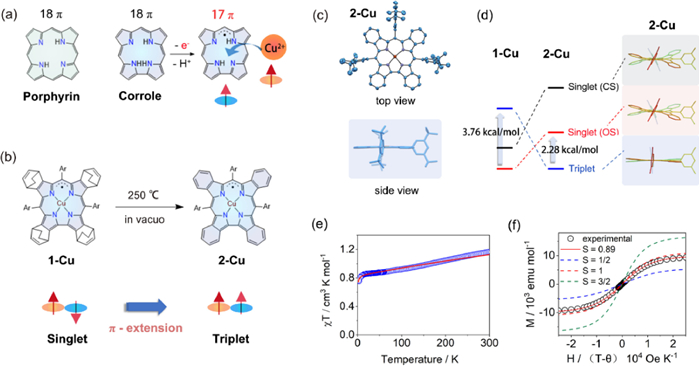
When coordinated to metal ions such as Cu, Co, and Fe, the electron-rich corrole ligand could be partially oxidized to exhibit radical character, making it difficult to determine the exact oxidation states of central metals and ligands. The most controversial debate was the Cu(Ⅱ)/Cu(Ⅲ) dilemma on copper corrole [2]. The compound was initially thought to be a closed-shell Cu(Ⅲ) complex in 2000, but significant experimental and theoretical evidence over the next twenty years progressively revealed its open-shell singlet state ground state comprised of a Cu(Ⅱ) core and partially oxidized radical ligand (Fig. 1a).
Shen Z. and Wu F. from Nanjing University have developed a series of metallocorroles with extended π-conjugation systems, which not only facilitated the formation and stabilization of radical ligands, but also allowed spin configurations of complexes to be easily controlled [3-5]. Recently, Shen, Wu, and co-workers reported that the unambiguous Cu(Ⅱ) corrole with fully oxidized [4n + 1]π radical ligand was obtained through the benzo-fusion at the β-position of corrole ligand [6]. The ground-state conversion of copper corrole radical from singlet to triplet was achieved via a retro-Diels-Alder reaction (Fig. 1b).
The conformations of copper corroles were assumed to be "inherently saddle distorted" owing to the strong d-π interactions of antiferromagnetically coupled Cu(Ⅱ) corrole radicals. When compared to other copper corroles, 2-Cu stood out due to its highly planar macrocycle with a mean plane deviation value of only 0.024 Å (Fig. 1c). The planar structure could perfectly sustain the ferromagnetic coupling (S = 1) between Cu(Ⅱ) and corrole radical.
The research conducted by Shen's group introduces a new approach to the fine-tuning of interactions between metal center and corrole ligand and provides a promising strategy for the creation of stable corrole radical complexes with distinctive high-spin systems. The strategy will further trigger the development of novel functional materials based on corroles and their work will encourage an increasing amount of spintronics research for the use of innovative magnetic and electrical devices.
-
[1]
J. Sastre, F.V. Pallardo, J. Viña, Glutathione, in: T. Grune (Ed.), Reactions, Processes: Oxidants and Antioxidant Defense Systems, Springer Berlin Heidelberg, Berlin, Heidelberg, 2005, pp. 91–108.
-
[2]
G. Teskey, R. Abrahem, R. Cao, et al., Glutathione as a marker for human disease, in: G.S. Makowski (Ed.), Advances in Clinical Chemistry, Elsevier, London, 2018, pp. 141–159.
-
[3]
G.K. Balendiran, R. Dabur, D. Fraser, Cell Biochem. Funct. 22 (2004) 343–352.
doi: 10.1002/cbf.1149
-
[4]
M.P. Gamcsik, M.S. Kasibhatla, S.D. Teeter, et al., Biomarkers 17 (2012) 671–691.
doi: 10.3109/1354750X.2012.715672
-
[5]
Y. Sun, W.F. Li, N.Y. Chen, et al., Lancet Oncol. 17 (2016) 1509–1520.
doi: 10.1016/S1470-2045(16)30410-7
-
[6]
Y.P. Chen, A.T.C. Chan, Q.T. Le, et al., Lancet 394 (2019) 64–80.
doi: 10.1016/S0140-6736(19)30956-0
-
[7]
S. Guan, J. Wei, L. Huang, L. Wu, Eur. J. Med. Chem. 207 (2020) 112758.
doi: 10.1016/j.ejmech.2020.112758
-
[8]
M.A. Fuertes, C. Alonso, J.M. Pérez, Chem. Rev. 103 (2003) 645–662.
doi: 10.1021/cr020010d
-
[9]
J.W. Lv, Z.Y. Qi, G.Q. Zhou, et al., Cancer Sci. 109 (2018) 751–763.
doi: 10.1111/cas.13474
-
[10]
M. Song, M. Cui, K. Liu, Eur. J. Med. Chem. 232 (2022) 114205.
doi: 10.1016/j.ejmech.2022.114205
-
[11]
T. Jin, W.F. Qin, F. Jiang, et al., Transl. Oncol. 12 (2019) 633–639.
doi: 10.1016/j.tranon.2019.01.002
-
[12]
H. Yu, P. Guo, X. Xie, et al., J. Cell. Mol. Med. 21 (2017) 648–657.
doi: 10.1111/jcmm.13008
-
[13]
J. Li, F. Cao, H.L. Yin, et al., Cell. Death Dis. 11 (2020) 88.
doi: 10.1038/s41419-020-2298-2
-
[14]
B. Lu, X.B. Chen, M.D. Ying, et al., Front. Pharmacol. 8 (2017) 992.
-
[15]
X. Xia, X. Fan, M. Zhao, P. Zhu, Curr. Gene Ther. 19 (2019) 117–124.
doi: 10.2174/1566523219666190628152137
-
[16]
Y. Zhao, Y. Li, R. Zhang, et al., Onco Targets Ther. 13 (2020) 5429–5441.
doi: 10.2147/ott.s254995
-
[17]
D. Giustarini, I. Dalle-Donne, A. Milzani, et al., Nat. Protoc. 8 (2013) 1660–1669.
doi: 10.1038/nprot.2013.095
-
[18]
Y. Zhu, J. Wu, K. Wang, J. Xie, et al., Talanta 224 (2021) 121852.
doi: 10.1016/j.talanta.2020.121852
-
[19]
D. Sun, Z. Chen, J. Hu, et al., Chin. Chem. Lett. 33 (2022) 4478–4494.
doi: 10.1016/j.cclet.2021.12.043
-
[20]
D. Chen, Y. Feng, Crit. Rev. Anal. Chem. 52 (2022) 649–666.
doi: 10.1080/10408347.2020.1819193
-
[21]
Z. Xu, T. Qin, X. Zhou, et al., Trends Anal. Chem. 121 (2019) 115672.
doi: 10.1016/j.trac.2019.115672
-
[22]
S. Lee, J. Li, X. Zhou, et al., Coord. Chem. Rev. 366 (2018) 29–68.
doi: 10.1016/j.ccr.2018.03.021
-
[23]
Y. Zhang, J. Zhang, M. Su, C. Li, Biosens. Bioelectron. 175 (2021) 112866.
doi: 10.1016/j.bios.2020.112866
-
[24]
F. Liang, S. Jiao, D. Jin, et al., Spectrochim. Acta A 224 (2020) 117403.
doi: 10.1016/j.saa.2019.117403
-
[25]
Z. Xu, X. Huang, X. Han, et al., Chem. 4 (2018) 1609–1628.
doi: 10.1016/j.chempr.2018.04.003
-
[26]
N. Li, T. Wang, N. Wang, et al., Angew. Chem. Int. Ed. 62 (2023) e202217326.
doi: 10.1002/anie.202217326
-
[27]
W. Shu, J. Yu, H. Wang, et al., Anal. Chim. Acta 1220 (2022) 340081.
doi: 10.1016/j.aca.2022.340081
-
[28]
W. Liu, J. Chen, Q. Qiao, et al., Chin. Chem. Lett. 33 (2022) 4943–4947.
doi: 10.1016/j.cclet.2022.03.121
-
[29]
F. Chen, J. Zhang, W. Qu, et al., Sens. Actuators B Chem. 266 (2018) 528–533.
doi: 10.1016/j.snb.2018.03.162
-
[30]
K. Umezawa, M. Yoshida, M. Kamiya, et al., Nat. Chem. 9 (2017) 279–286.
doi: 10.1038/nchem.2648
-
[31]
D. Gong, S.C. Han, A. Iqbal, et al., Anal. Chem. 89 (2017) 13112–13119.
doi: 10.1021/acs.analchem.7b02311
-
[32]
S. Hou, Y. Wang, Y. Zhang, et al., Anal. Chim. Acta 1214 (2022) 339957.
doi: 10.1016/j.aca.2022.339957
-
[33]
K. Wang, G. Nie, S. Ran, et al., Dyes Pigments 172 (2020) 107837.
doi: 10.1016/j.dyepig.2019.107837
-
[34]
P. Hou, J. Sun, H. Wang, et al., Sens. Actuators B Chem. 304 (2020) 127244.
doi: 10.1016/j.snb.2019.127244
-
[35]
Z. Zheng, Y. Huyan, H. Li, et al., Sens. Actuators B Chem. 301 (2019) 127065.
doi: 10.1016/j.snb.2019.127065
-
[36]
C. Zhang, Y. Qin, C. Deng, et al., Anal. Chim. Acta 1248 (2023) 340933.
doi: 10.1016/j.aca.2023.340933
-
[37]
H.M. Jiang, G.X. Yin, Y.B. Gan, et al., Chin. Chem. Lett. 33 (2022) 1609–1612.
doi: 10.1016/j.cclet.2021.09.036
-
[38]
X.W. Li, C.Y. Liu, N. Gao, et al., Chin. Chem. Lett. 33 (2022) 2527–2531.
doi: 10.1016/j.cclet.2021.11.080
-
[39]
T.X. Jin, M.Y. Cui, D. Wu, et al., Chin. Chem. Lett. 32 (2021) 3899–3902.
doi: 10.1016/j.cclet.2021.06.033
-
[40]
Y. Zou, M. Li, Y. Xing, et al., ACS Sens. 5 (2020) 242–249.
doi: 10.1021/acssensors.9b02118
-
[41]
N. Ahmed, W. Zareen, Y. Ye, Chin. Chem. Lett. 33 (2022) 2765–2772.
doi: 10.1016/j.cclet.2021.12.092
-
[42]
L.R. Jiang, T.H. Chen, E.W. Song, et al., Chem. Eng. J. 427 (2022) 131563.
doi: 10.1016/j.cej.2021.131563
-
[43]
S.T. Cai, Q.C. Liu, C. Liu, et al., J. Mater. Chem. B 10 (2022), 1265–1271.
doi: 10.1039/d1tb02639a
-
[44]
S. Xu, W.J. Pan, T.B. Ren, et al., Chin. J. Chem. 40 (2021) 1073–1082.
doi: 10.1002/cjoc.202100807
-
[45]
C. Duan, M. Won, P. Verwilst, et al., Anal. Chem. 91 (2019) 4172–4178.
doi: 10.1021/acs.analchem.9b00224
-
[46]
J.C. Xu, J. Pan, X.M. Jiang, et al., Biosens. Bioelectron. 77 (2016), 725–732.
doi: 10.1016/j.bios.2015.10.049
-
[47]
Y.Y. Ma, Z.C. Xu, Q. Sun, et al., Spectrochim. Acta. A 247 (2021) 1386–1425.
doi: 10.1049/icp.2022.0265
-
[48]
X.Y. Zhang, W.B. Qu, H. L, et al., Anal. Chim. Acta 1109 (2020) 37–43.
doi: 10.1016/j.aca.2020.02.061
-
[49]
J. Dong, G. Lu, Y. Tu, C. Fan, New J. Chem. 46 (2022) 10995–11020.
doi: 10.1039/d1nj06244a
-
[50]
M.Y. Lucero, J. Chan, Nat. Chem. 13 (2021) 1248–1256.
doi: 10.1038/s41557-021-00804-0
-
[51]
J. Dai, C. Ma, P. Zhang, et al., Dyes Pigments 177 (2020) 108321.
doi: 10.1016/j.dyepig.2020.108321
-
[52]
L.Y. Niu, Y.Z. Chen, H.R. Zheng, et al., Chem. Soc. Rev. 44 (2015) 6143–6160.
doi: 10.1039/C5CS00152H
-
[53]
J. Hu, W. Gu, N. Ma, et al., Br. J. Pharmacol. 179 (2022) 3991–4009.
doi: 10.1111/bph.15834
-
[54]
Q. Cheng, L. Bao, M. Li, et al., J. Obstet. Gynaecol. Res. 47 (2021) 2481–2491.
doi: 10.1111/jog.14779
-
[55]
M. Sato, R. Kusumi, S. Hamashima, et al., Sci. Rep. 8 (2018) 968.
doi: 10.1038/s41598-018-19213-4

 Login In
Login In





 DownLoad:
DownLoad:

 DownLoad:
DownLoad: