However, considering that the ECR process is usually accompanied by the formation of C1 products, C2 products, and H2, it is often difficult to control the high selectivity for the target products with pure Cu catalysts [11]. Therefore, a large number of strategies have been used to enhance the selectivity of Cu-based catalysts for single products, such as heteroatom doping [12–15], modulation of nanoparticle size [16,17], forming bimetallic catalysts [10,18-20], crystal plane control [21,22]. Among all these strategies, the formation of bimetallic catalysts is one of the most frequently used strategies for the engineering of vacancies and defects [23]. Bimetallic catalysts occupy a unique position because their specific electronic properties differ from those of the corresponding constituent metals [24], while the abundance of defects in the vicinity of vacancies allows chemisorption and stabilization of critical reaction intermediates for the subsequent generation of target products [25,26]. However, the O vacancy has the disadvantage of being unstable, and the degree of defect is difficult to regulate, which makes it difficult to achieve efficient and selective conversion of target products. It is worth noting that if the defect degree of O vacancies can be effectively controlled, selective regulation of reaction products can be achieved.
In this study, we demonstrate the synthesis of a simple CuO-CdO bimetallic electrocatalyst with a large-scale and intimate contact interface. Electrochemical tests show that the resulting product contains CO, CH4, C2H4, and H2. Selective conversion of the product can be achieved by adjusting the amount of CdO added and controlling the extent of defective O vacancies. The Cu–Cd bimetallic catalyst in 0.1 mol/L KHCO3 electrolyte has the best ethylene selectivity at a Cu/Cd molar ratio of 11/1 (CuO2.75-CdO0.25), with FEC reaching 55.4% at −1.2 V (vs. reversible hydrogen electrode (RHE)), pure CdO exhibiting more than 88% FECO, and a series of Cu-Cd bimetallic catalysts only. This work provides a new and feasible idea for future selective conversion of CO2 to a single product by adjusting the degree of defect in the O vacancy.
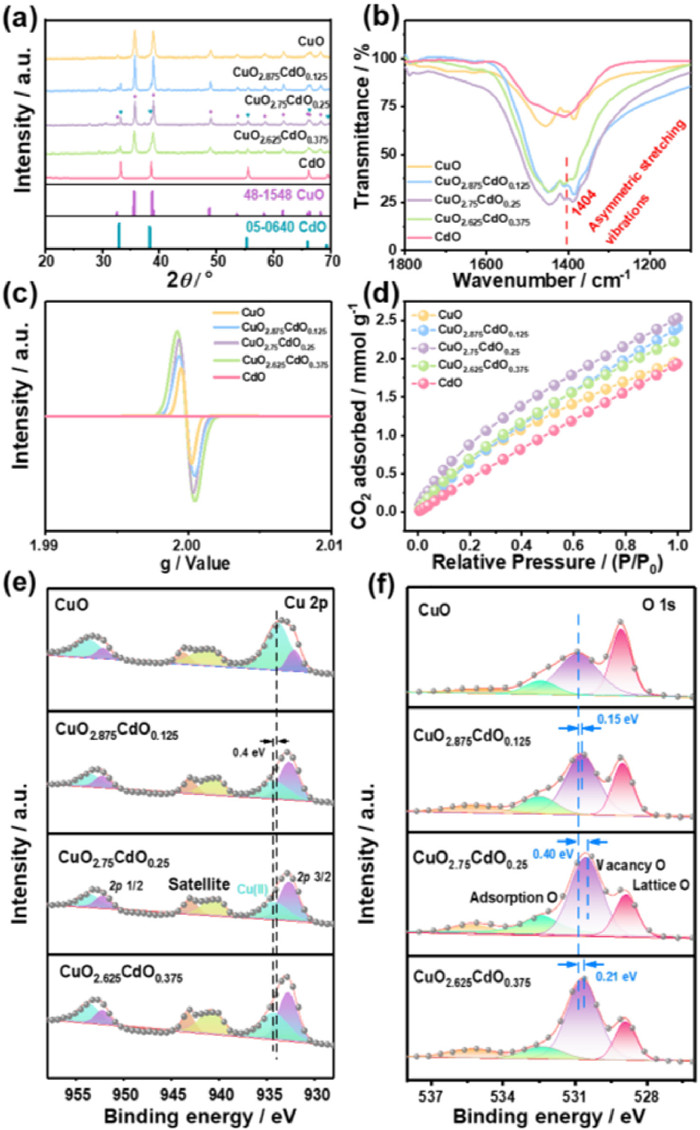
X-ray diffraction measurements were carried out to investigate the phases of the products (Fig. 1a). The bimetallic catalysts consisted mainly of CuO and CdO, which was confirmed by peaks at 32.5°, 35.4°, 35.5°, 38.7°, 38.9°, 48.7°, 53.5°, 58.3°, 61.5°, 65.8°, 66.2° and 68.1° for the CuO(110), (002), (111), (111), (200), (202), (020), (202), (113), (311) and (220) planes (JCPDS No. 48-1548) and peaks at 33.0°, 38.3°, 55.3°, 65.9° and 69.3° for the CdO (111), (200), (220), (311), and (222) planes (JCPDS No. 05-0640), respectively.
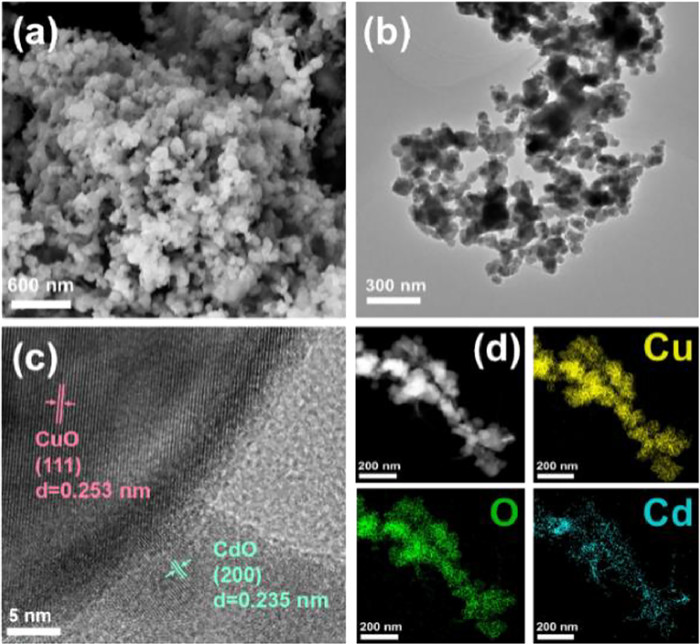
To clearly distinguish between several catalysts in the series, scanning electron microscopy (SEM) (Fig. 2a and Figs. S4a-d in Supporting information) and transmission electron microscopy (TEM) imaging (Fig. 2b) were performed on Cu-Cd bimetallic catalysts. There is no major difference in the morphological structure of the Cu–Cd bimetallic catalysts, with regularly sized nanoparticles in a closely arranged massive structure and a rough surface. High-resolution TEM (HR-TEM) (Fig. 2c) revealed that CuO and CdO are highly crystalline with ordered lattice fringes. Further HR-TEM images (Fig. S5 in Supporting information) show a clear interfacial connection between CuO and CdO, indicating the formation of a heterogeneous structure between CuO and CdO. This corresponds in part to the XRD results and the energy dispersive X-ray spectroscopy (EDS) elemental mapping (Fig. 2d) showing that Cu, O, and Cd elements are evenly distributed on the pellet, which also indicates that no alloy structure is formed between Cu and Cd. The molar ratios of the Cu-Cd bimetallic catalysts were obtained by ICP-OES (Table S1 in Supporting information) and showed that the molar ratios matched the feed ratios, which is consistent with the EDS elemental mapping results (Fig. 2d).
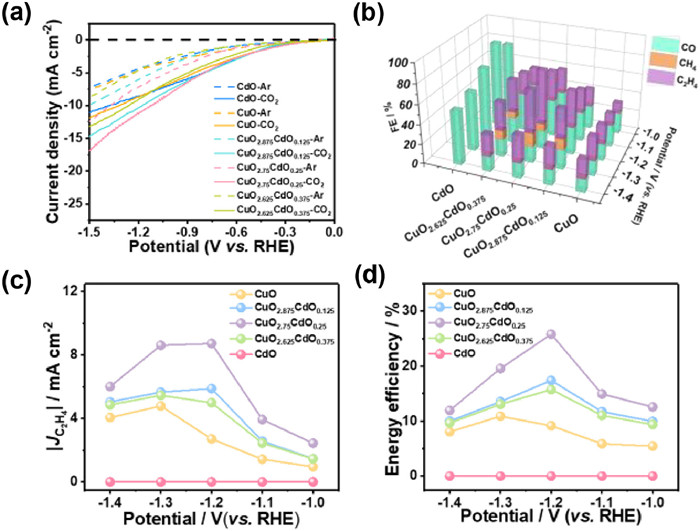
The electrochemical performance of Cu-Cd bimetallic catalysts was investigated in a three-electrode H-type closed electrolyzer containing 0.1 mol/L KHCO3 (Fig. S6 in Supporting information). Linear scanning voltammetry (LSV) results (Fig. 3a) have shown that the current density in the CO2 environment is greater than that in the Ar environment within a voltage range of 0 V to −1.5 V (vs. RHE). Notably, CuO2.75–CdO0.25 obtained the highest current densities compared to the other investigated materials across the entire tested potential range. Gas chromatography (GC) and nuclear magnetic resonance (NMR) were used to detect the gas phase and liquid phase products of the Cu–Cd bimetallic catalyst after 1 h constant voltage electrochemical test. The gas phase products mainly include H2, CO, CH4, and C2H4. The yields of the liquid phase products are negligibly small (Fig. S7 in Supporting information). Pure CuO exhibited a certain ability to produce C2H4, but its FE was low and FEC increased with increasing CdO content, reaching a maximum at CuO2.75–CdO0.25. A further increase in the amount of CdO leads to a decrease in the ECR activity, most likely due to the addition of large amounts of CdO reducing the surface Cu active site, while the Cd active site produces more C1 products and even pure CdO generates large amounts of CO (Fig. 3b). Combined with the ESR spectroscopy results, we conclude that the selectivity of ECR products is closely related to the degree of O vacancy defects; specifically, a moderate degree of O defects helps to enhance the selectivity of the catalyst for C2H4 during ECR, and either too high or too low O defect ranges are detrimental to the production of C2H4 products. The highest FEC of 55.4% was produced by CuO2.75-CdO0.25 at −1.2 V (vs. RHE) approximately 2.3, 1.5, and 1.7 times than that of CuO, CuO2.875–CdO0.125, and CuO2.625–CdO0.375, respectively. Interestingly, we observed that the addition of CdO reduced the potential to produce the best FEC from −1.3 V to −1.2 V. Figs. 3c and d show the special current density and energy efficiency (EE) of ethylene after optimization of Cu-Cd bimetallic catalysts. CuO2.75–CdO0.25 delivers a maximal EEC of ~25.9% at a current density of 8.8 mA/cm2. Notably, the FEC or low applied potential demonstrated by CuO2.75–CdO0.25 exceeded many of the existing Cu-based materials in similar reports (Fig. S8 and Table S2 in Supporting information) [10,26,40–47]. In addition, CuO2.75–CdO0.25 provides better selectivity (FEC/FEC1 ≈ 3) and C2H4 production rate (maximum ~16.4 µmol mg−1 h−1) of C2H4 products than pure CuO catalysts or other molar ratios of Cu–Cd bimetallic catalysts over a wide voltage range of −1.0 V to −1.4 V (vs. RHE) (Figs. S9 and S10 in Supporting information).
The selective enhancement can be attributed primarily to the varying degrees of O defects formed by the stretching action of CdO on CuO rather than to the commonly assumed change in the electronic properties of the interface. Since the high CdO content (CuO2.625–CdO0.375) has a greater interfacial electronic property change, but its ethylene product selectivity shows a decreasing trend, which is not as convincing as expected, we believe that there is a limit to the O vacancies formed by CdO stretching in enhancing the catalytic performance and that an appropriate degree of defects is favorable for the production of ethylene products.
To obtain a deeper understanding of the activity and mechanism of different catalysts on ECR catalysts, the double-layer capacitance (Cdl) of each catalyst in this study was tested by CV to obtain the electrochemically active surface area (ECSA) of each sample (Figs. S11a-e in Supporting information) [48]. As shown in Fig. S10a, CuO2.75–CdO0.25 exhibits the highest ECSA, suggesting that CuO2.75–CdO0.25 provides more active sites for ECR, increasing the opportunity for intermediates to bind at the interface with the electrolyte [49]. The charge transfer rates between the catalyst and electrolyte interfaces were characterized by electrochemical impedance spectrum (EIS) (Fig. S12 in Supporting information). The results show that the half-circle diameter of Nyquist with CuO2.75–CdO0.25 is less than that with CuO2.875–CdO0.125, CuO2.625–CdO0.375, CuO, and CdO, indicating that it has a faster realization of electron transfer [50,51]. The Tafel slope of the Cu-Cd bimetallic catalyst is shown in Fig. S13 (Supporting information). The Tafel slope was used to measure the reaction kinetics of the four catalysts, with the lowest Tafel slope (340.2 mV/dec) for CuO2.75–CdO0.25, implying the lowest kinetic energy required to activate the CO2. In addition, the continuous ECR experiments at −1.2 V (vs. RHE) resulted in almost no decrease in FEC within 11 h, indicating that the catalyst has good electrochemical stability (Fig. S14 in Supporting information).
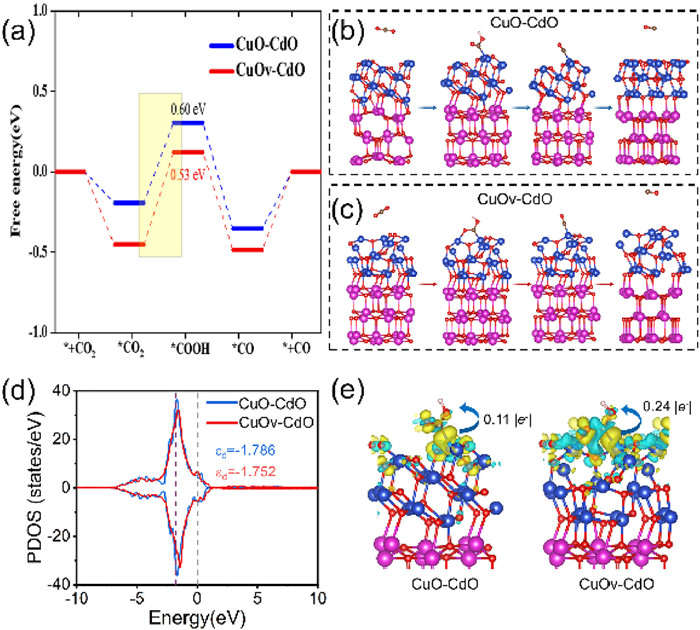
DFT was employed to further explore the potential mechanism of ECR of Cu-Cd bimetallic catalysts. First, based on X-ray diffraction (CuO with JCPDS No. 48-1548 and CdO with JCPDS No. 05-0640) and HRTEM, the unit structures models of CuO and CdO are shown in Fig. S15 (Supporting information). CuO consists of 4 Cu and 4 O atoms with a space group of C2/c and a lattice length of 4.70 Å × 4.70 Å × 4.70 Å. CdO consists of four Cd and four O atoms with a space group of Fm-3 m and a lattice length of 4.69 Å × 3.42 Å × 5.13 Å. The heterojunction CuO–CuO consists of CuO(111) and CdO(200) with a lattice mismatch of less than 5%, demonstrating the excellent suitability of CuO(111) and CdO(200). The O atoms were removed according to the ratio of XPS results from the CuO–CuO surface to represent O vacancy.
To further explore the mechanism of enhanced ethylene production efficiency by CuOv-CdO, we calculated the projected density of states and d-band center of the d orbitals of Cu for CuO–CuO and CuOv–CdO (Fig. 4d). The d orbitals of Cu for CuOv–CdO move toward the Fermi level, indicating the activation of Cu. Generally, the approach of the d-band center to the Fermi level indicates that transition metal catalysts are more likely to exhibit high adsorption activity [53,54]. The d-band center of CuOv-CdO was closer to the Fermi level than that of CuO–CuO (−1.752 vs. −1.786), indicating the adsorption activity of CuOv-CdO. CuOv–CdO showed an increased adsorption activity of Cu, thus improving and facilitating the chemisorption of reaction intermediates on the catalytic surface. The charge density difference and Bader charge were employed to reveal the mechanism of the high adsorption of *COOH on CuOv–CdO. As shown in Fig. 4e, yellow represents charge aggregation and cyan represents charge dissipation. CuOv–CdO showed more significant charge transfer with *COOH than the CuO–CuO surface and transferred more charge (0.24 |e−| vs. 0.11 |e−|), with more charge transfer indicating that the oxygen vacancies increased the adsorption of *COOH and lowered the reaction potential. Therefore, the increasing charge transfer indicates that the oxygen vacancies increase the adsorption of *COOH and lower the reaction potential, thus increasing the yield of ethylene.
To study the stability of the catalysts, the catalysts used were characterized by XPS and TEM, the results of which are shown in Figs. S18-S21 (Supporting information). In general, the species of Cu and Cd did not change significantly, while the ratio of O vacancies was different from that of fresh, which may be due to in situ reduction and air oxidation during the reaction. The good stability of CuO2.75–CdO0.25 was further confirmed by the negligible variation in its TEM and EDS mapping after electrolysis.
In summary, we have demonstrated for the first time a low-cost and easily prepared bimetallic catalyst of CuO and CdO for the electrochemical CO2 reduction to C2H4, which can provide 55.4% of FEC at −1.2 V (vs. RHE). It is 2.3 times more than the pure CuO catalyst prepared by the same method and possesses an electrochemical stability of more than 10 h. The characterization results showed that the CuO2.75–CdO0.25 bimetallic catalyst formed by CdO loading on CuO particles exhibited excellent performance. The experimental results showed that the addition of CdO not only facilitated the adsorption of CO2 by CuO but also CdO could produce abundant O defects in CuO. The degree of defective O vacancy can be controlled by changing the content of CdO to further change the product selectivity. We propose that the degree of O vacancies defects correlates with the selectivity of the products of the ECR process and that a moderate degree of O vacancy defects facilitates the conversion of CO2 to C2H4, while excess O vacancies are detrimental to the adsorption of CO2, leading to a decrease in its selectivity. The DFT results and in-situ Raman spectrum confirmed that the oxygen vacancy of CuOv–CdO leads to a lower reaction potential for the generation of *COOH intermediates and thus increases the efficiency of C2H4 production. This study provides a new and feasible idea for the future selective production of ECR on an industrial scale by regulating the degree of O vacancy defects.
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
This research was funded by the National Natural Science Foundation of Zhejiang Province (Nos. LQ21B030007 and LTGS23B030002), “Leading Goose” R&D Program of Zhejiang (No. 2023C01191); the National Natural Science Foundation of China (No. 22005269), Science and Technological program of Ningbo (No. 2021S136), The Open Research Subject of Zhejiang Key Laboratory of Petrochemical Environmental Pollution Control (No. 2022Z02).
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.cclet.2023.108692.
-
[1]
L. Escobar, P. Ballester, Chem. Rev. 121 (2021) 2445-2514.
doi: 10.1021/acs.chemrev.0c00522
-
[2]
K. Wang, X. Tian, J.H. Jordan, et al., Chin. Chem. Lett. 33 (2022) 89-96.
doi: 10.1016/j.cclet.2021.06.026
-
[3]
K. Wang, M. Zuo, T. Zhang, H. Yue, X.Y. Hu, Chin. Chem. Lett. 34 (2023) 107848.
doi: 10.1016/j.cclet.2022.107848
-
[4]
S. Niu, L.L. Mao, H. Xiao, et al., Chin. Chem. Lett. 33 (2022) 1970-1974.
doi: 10.1016/j.cclet.2021.09.090
-
[5]
Y.X. Yan, Y. Zhang, Y. Chen, Y. Liu, ACS Appl. Polym. Mater. 2 (2020) 5641-5645.
doi: 10.1021/acsapm.0c00958
-
[6]
J.R. Moran, S. Karbach, D.J. Cram, J. Am. Chem. Soc. 104 (1982) 5826-5828.
doi: 10.1021/ja00385a064
-
[7]
D.J. Cram, Science 219 (1983) 1177-1183.
doi: 10.1126/science.219.4589.1177
-
[8]
Q. Sun, L. Escobar, P. Ballester, Angew. Chem. Int. Ed. 60 (2021) 10359-10365.
doi: 10.1002/anie.202101499
-
[9]
L. Escobar, F.A. Arroyave, P. Ballester, Eur. J. Org. Chem. 2018 (2018) 1097-1106.
doi: 10.1002/ejoc.201701602
-
[10]
K. Hermann, D.A. Turner, C.M. Hadad, J.D. Badjic, Chem. Eur. J. 18 (2012) 8301-8305.
doi: 10.1002/chem.201201153
-
[11]
S.E. Border, R.Z. Pavlovic, L. Zhiquan, J.D. Badjic, J. Am. Chem. Soc. 139 (2017) 18496-18499.
doi: 10.1021/jacs.7b11960
-
[12]
S. Mirzaei, V.M. Espinoza Castro, R. H. Sánchez, Chem. Sci. 13 (2022) 2026-2032.
doi: 10.1039/d1sc07041j
-
[13]
H. Erdtman, S. Högberg, S. Abrahamsson, B. Nilsson, Tetrahedron Lett. 9 (1968) 1679-1682.
doi: 10.1016/S0040-4039(01)99028-8
-
[14]
A. Baeyer, Ber. Dtsch. Chem. Ges. 5 (1872) 280-282.
doi: 10.1002/cber.18720050186
-
[15]
J.M. Bourgeois, H. Stoeckli-Evans, Helv. Chim. Acta 88 (2005) 2722-2730.
doi: 10.1002/hlca.200590211
-
[16]
E.R. Abdurakhmanova, P. Cmoch, A. Szumna, Org. Biomol. Chem. 20 (2022) 5095-5103.
doi: 10.1039/d2ob00880g
-
[17]
M. Wehbie, G. Arrachart, I. Karamé, L. Ghannam, S. Pellet-Rostaing, Sep. Purif. Technol. 169 (2016) 17-24.
doi: 10.1016/j.seppur.2016.06.003
-
[18]
A. Mouradzadegun, S. Elahi, F. Abadast, RSC Adv. 4 (2014) 31239-31248.
doi: 10.1039/C4RA03463E
-
[19]
A. Pedrini, F. Bertani, E. Dalcanale, Molecules 23 (2018) 2670.
doi: 10.3390/molecules23102670
-
[20]
N.W. Wu, J. Rebek, Jr., J. Am. Chem. Soc. 138 (2016) 7512-7515.
doi: 10.1021/jacs.6b04278
-
[21]
J.L. Liu, M. Sun, Y.H. Shi, et al., J. Inclus. Phenom. Macrocycl. Chem. 102 (2022) 201-233.
doi: 10.1007/s10847-021-01119-w
-
[22]
T. Heinz, D.M. Rudkevich, J. Rebek, Jr., Nature 394 (1998) 764-766.
doi: 10.1038/29501
-
[23]
D. Ajami, J. Rebek, Jr., Acc. Chem. Res. 46 (2013) 990-999.
doi: 10.1021/ar300038r
-
[24]
J. Jiao, B. Sun, Y. Ding, et al., Org. Lett. 22 (2020) 8984-8988.
doi: 10.1021/acs.orglett.0c03385
-
[25]
M.L. Tan, Q.H. Guo, X.Y. Wang, et al., Angew. Chem. Int. Ed. 59 (2020) 23649-23658.
doi: 10.1002/anie.202013149
-
[26]
L. Turunen, F. Pan, N.K. Beyeh, et al., Cryst. Growth Des. 18 (2017) 513-520.
-
[27]
L. Turunen, F. Pan, N.K. Beyeh, et al., CrystEngComm 19 (2017) 5223-5229.
doi: 10.1039/C7CE01118K
-
[28]
L. Turunen, N.K. Beyeh, F. Pan, A. Valkonen, K. Rissanen, Chem. Commun. 50 (2014) 15920-15923.
doi: 10.1039/C4CC07771G
-
[29]
K. Twum, K. Rissanen, N.K. Beyeh, Chem. Rec. 21 (2021) 386-395.
doi: 10.1002/tcr.202000140
-
[30]
Y.J. Zhu, Y. Gao, M.M. Tang, J. Rebek, Jr., Y. Yu, Chem. Commun. 57 (2021) 1543-1549.
doi: 10.1039/d0cc07784d
-
[31]
R. Pinalli, M. Suman, E. Dalcanale, Eur. J. Org. Chem. 2004 (2004) 451-462.
doi: 10.1002/ejoc.200300430
-
[32]
R. Pinalli, G. Brancatelli, A. Pedrini, et al., J. Am. Chem. Soc. 138 (2016) 8569-8580.
doi: 10.1021/jacs.6b04372
-
[33]
N. Bontempi, E. Biavardi, D. Bordiga, et al., Nanoscale 9 (2017) 8639-8646.
doi: 10.1039/C7NR02491F
-
[34]
J.H. Jordan, H. S. Ashbaugh, J.T. Mague, B.C. Gibb, J. Am. Chem. Soc. 143 (2021) 18605-18616.
doi: 10.1021/jacs.1c08457
-
[35]
H.R. Aziz, W. Yao, J.H. Jordan, B.C. Gibb, Supramol. Chem. 33 (2021) 226-271.
doi: 10.1080/14702436.2021.1888644
-
[36]
A. Gissot, J. Rebek, J. Am. Chem. Soc. 126 (2004) 7424-7425.
doi: 10.1021/ja049074r
-
[37]
H. Konishi, T. Nakamura, K. Ohata, K. Kobayashi, O. Morikawa, Tetrahedron Lett. 37 (1996) 7383-7386.
doi: 10.1016/0040-4039(96)01683-8
-
[38]
C. Naumann, E. Roman, C. Peinador, et al., Chem. Eur. J. 7 (2001) 1637-1645.
doi: 10.1002/1521-3765(20010417)7:8<1637::AID-CHEM16370>3.0.CO;2-X
-
[39]
C. Naumann, S. Place, J.C. Sherman, J. Am. Chem. Soc. 124 (2002) 16-17.
-
[40]
T.H. Shi, S. Tong, M.X. Wang, Angew. Chem. Int. Ed. 59 (2020) 7700-7705.
doi: 10.1002/anie.202002827
-
[41]
Q.H. Guo, Y. Qiu, M.X. Wang, J.F. Stoddart, Nat. Chem. 13 (2021) 402-419.
doi: 10.1038/s41557-021-00671-9
-
[42]
P.D. Sala, C. Gaeta, W. Navarra, et al., J. Org. Chem. 81 (2016) 5726-5731.
doi: 10.1021/acs.joc.6b00803
-
[43]
C. Gaeta, P.D. Sala, C. Talotta, et al., Org. Chem. Front. 3 (2016) 1276-1280.
doi: 10.1039/C6QO00336B
-
[44]
M. Chwastek, A. Szumna, Org. Lett. 22 (2020) 6838-6841.
doi: 10.1021/acs.orglett.0c02357
-
[45]
M. Chwastek, P. Cmoch, A. Szumna, Angew. Chem. Int. Ed. 60 (2021) 4540-4544.
doi: 10.1002/anie.202013105
-
[46]
T. Gottschalk, B. Jaun, F. Diederich, Angew. Chem. Int. Ed. 46 (2007) 260-264.
doi: 10.1002/anie.200603366
-
[47]
I. Pochorovski, M.O. Ebert, J.P. Gisselbrecht, et al., J. Am. Chem. Soc. 134 (2012) 14702-14705.
doi: 10.1021/ja306473x
-
[48]
I. Pochorovski, F. Diederich, Acc. Chem. Res. 47 (2014) 2096-2105.
doi: 10.1021/ar500104k
-
[49]
J.V. Milic, F. Diederich, Chem. Eur. J. 25 (2019) 8440-8452.
doi: 10.1002/chem.201900852
-
[50]
V. Garcia-Lopez, M. Zalibera, N. Trapp, et al., Chem. Eur. J. 26 (2020) 11451-11461.
doi: 10.1002/chem.202001788
-
[51]
L. Pirondini, F. Bertolini, B. Cantadori, et al., Proc. Natl. Acad. Sci. U.S.A. 99 (2002) 4911-4915.
doi: 10.1073/pnas.072612199
-
[52]
R.M. Yebeutchou, E. Dalcanale, J. Am. Chem. Soc. 131 (2009) 2452-2453.
doi: 10.1021/ja809614y
-
[53]
G. Valenti, E. Rampazzo, E. Biavardi, et al., Faraday Discuss. 185 (2015) 299-309.
doi: 10.1039/C5FD00096C
-
[54]
F. Bertani, N. Riboni, F. Bianchi, et al., Chem. Eur. J. 22 (2016) 3312-3319.
doi: 10.1002/chem.201504229
-
[55]
A. Aprile, F. Ciuchi, R. Pinalli, E. Dalcanale, P. Pagliusi, J. Phys. Chem. Lett. 7 (2016) 3022-3026.
doi: 10.1021/acs.jpclett.6b01300
-
[56]
W. Zhong, R.J. Hooley, Acc. Chem. Res. 55 (2022) 1035-1046.
doi: 10.1021/acs.accounts.2c00026
-
[57]
J. Chen, B.L. Hickey, L. Wang, et al., Nat. Chem. 13 (2021) 488-495.
doi: 10.1038/s41557-021-00647-9
-
[58]
Q. Shi, D. Masseroni, J. Rebek, Jr., J. Am. Chem. Soc. 138 (2016) 10846-10848.
doi: 10.1021/jacs.6b06950
-
[59]
S. Mosca, Y. Yu, J.V. Gavette, K.D. Zhang, J. Rebek, Jr., J. Am. Chem. Soc. 137 (2015) 14582-14585.
doi: 10.1021/jacs.5b10028
-
[60]
J.M. Yang, Y. Yu, J. Rebek, Jr., J. Am. Chem. Soc. 143 (2021) 2190-2193.
doi: 10.1021/jacs.0c12302
-
[61]
Y.H. Wan, Y.J. Zhu, J. Rebek, Jr., Y. Yu, Molecules 26 (2021) 1922.
doi: 10.3390/molecules26071922
-
[62]
K. Wang, X. Cai, W. Yao, et al., J. Am. Chem. Soc. 141 (2019) 6740-6747.
doi: 10.1021/jacs.9b02287
-
[63]
K. Wang, J.H. Jordan, B.C. Gibb, Chem. Commun. 55 (2019) 11695-11698.
doi: 10.1039/c9cc06501f
-
[64]
K. Wang, B.C. Gibb, J. Org. Chem. 82 (2017) 4279-4288.
doi: 10.1021/acs.joc.7b00264
-
[65]
F.U. Rahman, D. Tzeli, I.D. Petsalakis, et al., J. Am. Chem. Soc. 142 (2020) 5876-5883.
doi: 10.1021/jacs.0c01290
-
[66]
Y. Yu, J.M. Yang, J. Rebek, Chem 6 (2020) 1265-1274.
doi: 10.1016/j.chempr.2020.04.014
-
[67]
M. Petroselli, Y.Q. Chen, M.K. Zhao, J. Rebek, Y. Yu, Chin. Chem. Lett. 34 (2023) 107834.
doi: 10.1016/j.cclet.2022.107834
-
[68]
Y. Zhu, M. Tang, H. Zhang, et al., J. Am. Chem. Soc. 143 (2021) 12397-12403.
doi: 10.1021/jacs.1c06510
-
[69]
F.U. Rahman, R. Wang, H.B. Zhang, et al., Angew. Chem. Int. Ed. 61 (2022) e202205534.
doi: 10.1002/anie.202205534
-
[70]
K. Kanagaraj, R. Wang, M.K. Zhao, et al., J. Am. Chem. Soc. 145 (2023) 5816–5823.
doi: 10.1021/jacs.2c12907
-
[71]
F.U. Rahman, Y.S. Li, I.D. Petsalakis, et al., Proc. Natl. Acad. Sci. U.S.A. 116 (2019) 17648-17653.
doi: 10.1073/pnas.1909154116
-
[72]
F.U. Rahman, J.M. Yang, Y.H. Wan, et al., Chem. Commun. 56 (2020) 6945-6948.
doi: 10.1039/d0cc02778b
-
[73]
Y.H. Wan, Faiz-Ur Rahman, J. Rebek Jr., Y. Yu, Chin. J. Chem. 39 (2021) 1498-1502.
doi: 10.1002/cjoc.202000709
-
[74]
H.B. Zhang, K. Kanagaraj, J. Rebek, Jr., Y. Yu, J. Org. Chem. 86 (2021) 8873-8881.
doi: 10.1021/acs.joc.1c00794
-
[75]
Y.Q. Chen, H.W. Guan, K. Kanagaraj, J. Rebek, Y. Yu, Chin. Chem. Lett. 33 (2022) 4908-4911.
doi: 10.1016/j.cclet.2022.03.039
-
[76]
J.M. Yang, Y.Q. Chen, Y. Yu, P. Ballester, J. Rebek, Jr., J. Am. Chem. Soc. 143 (2021) 19517-19524.
doi: 10.1021/jacs.1c09226
-
[77]
C.L.D. Gibb, B.C. Gibb, J. Am. Chem. Soc. 126 (2004) 11408-11409.
doi: 10.1021/ja0475611
-
[78]
P. Suating, T.T. Nguyen, N.E. Ernst, et al., Chem. Sci. 11 (2020) 3656-3663.
doi: 10.1039/c9sc06268h
-
[79]
J.W. Barnett, M.R. Sullivan, J.A. Long, et al., Nat. Chem. 12 (2020) 589-594.
doi: 10.1038/s41557-020-0458-8
-
[80]
P. Suating, N.E. Ernst, B.D. Alagbe, et al., J. Phys. Chem. B 126 (2022) 3150-3160.
doi: 10.1021/acs.jpcb.2c00628
-
[81]
P. Jagadesan, S.R. Samanta, R. Choudhury, V. Ramamurthy, J. Phys. Org. Chem. 30 (2017) e3728.
doi: 10.1002/poc.3728
-
[82]
S. Mirzaei, E. Castro, R.H. Sanchez, Chem. Sci. 11 (2020) 8089-8094.
doi: 10.1039/d0sc03384g
-
[83]
E. Castro, S. Mirzaei, R.H. Sanchez, Org. Lett. 23 (2021) 87-92.
doi: 10.1021/acs.orglett.0c03765
-
[84]
S. Mirzaei, E. Castro, R.H. Sanchez, Chem. Eur. J. 27 (2021) 8642-8655.
doi: 10.1002/chem.202005408
-
[85]
A. Lledó, S. Kamioka, A.C. Sather, J. Rebek, Angew. Chem. Int. Ed. 50 (2011) 1299-1301.
doi: 10.1002/anie.201006166
-
[86]
S. Ro, S.J. Rowan, A.R. Pease, D.J. Cram, J.F. Stoddart, Org. Lett. 2 (2000) 2411-2414.
doi: 10.1021/ol005962p
-
[87]
D. Shimoyama, T. Haino, Asian J. Org. Chem. 9 (2020) 1718-1725.
doi: 10.1002/ajoc.202000302
-
[88]
H. Yamada, T. Ikeda, T. Mizuta, T. Haino, Org. Lett. 14 (2012) 4510–4513.
doi: 10.1021/ol301996q
-
[89]
D. Shimoyama, T. Ikeda, R. Sekiya, T. Haino, J. Org. Chem. 82 (2017) 13220-13230.
doi: 10.1021/acs.joc.7b02301
-
[90]
D. Shimoyama, R. Sekiya, H. Kudo, T. Haino, Org. Lett. 22 (2020) 352-356.
doi: 10.1021/acs.orglett.9b03693
-
[91]
D. Shimoyama, H. Yamada, T. Ikeda, R. Sekiya, T. Haino, Eur. J. Org. Chem. 20 (2016) 3300-3303.
doi: 10.1002/ejoc.201600410
-
[92]
C.A. Hunter, H.L. Anderson, Angew. Chem. Int. Ed. 48 (2009) 7488-7499.
doi: 10.1002/anie.200902490
-
[93]
D. Shimoyama, T. Haino, J. Org. Chem. 84 (2019) 13483-13489.
doi: 10.1021/acs.joc.9b01730
-
[94]
D. Shimoyama, R. Sekiya, T. Haino, Chem. Commun. 56 (2020) 3733-3736.
doi: 10.1039/d0cc00933d
-
[95]
H. Fujimoto, D. Shimoyama, K. Katayanagi, et al., Org. Lett. 23 (2021) 6217-6221.
doi: 10.1021/acs.orglett.1c01837
-
[96]
K. Wang, J.H. Jordan, X.Y. Hu, L. Wang, Angew. Chem. Int. Ed. 59 (2020) 13712-13721.
doi: 10.1002/anie.202000045
-
[97]
S. Korom, P. Ballester, J. Am. Chem. Soc. 139 (2017) 12109-12112.
doi: 10.1021/jacs.7b05458
-
[98]
N. Natarajan, E. Brenner, D. Sémeril, D. Matt, J. Harrowfield, Eur. J. Org. Chem. 2017 (2017) 6100-6113.
doi: 10.1002/ejoc.201700725
-
[99]
M.P. Schramm, M. Kanaura, K. Ito, M. Ide, T. Iwasawa, Eur. J. Org. Chem. 2016 (2016) 813-820.
doi: 10.1002/ejoc.201501426
-
[100]
M. Inoue, K. Ugawa, T. Maruyama, T. Iwasawa, Eur. J. Org. Chem. 2018 (2018) 5304-5311.
doi: 10.1002/ejoc.201800948
-
[101]
Y. Matsumoto, Y. Taguchi, N. Yoshida, et al., Supramol. Chem. 33 (2021) 253-265.
doi: 10.1080/10610278.2021.1981323
-
[102]
N. Endo, M. Kanaura, M.P. Schramm, T. Iwasawa, Tetrahedron Lett. 57 (2016) 4754-4757.
doi: 10.1016/j.tetlet.2016.09.039
-
[103]
M. Inoue, S. Kamiguchi, K. Ugawa, et al., Eur. J. Org. Chem. 2019 (2019) 6261-6268.
doi: 10.1002/ejoc.201901058
-
[104]
I. Martin-Torres, G. Ogalla, J.M. Yang, A. Rinaldi, A.M. Echavarren, Angew. Chem. Int. Ed. 60 (2021) 9339-9344.
doi: 10.1002/anie.202017035
-
[105]
D. Vidal, M. Costas, A. Lledó, ACS Catal. 8 (2018) 3667-3672.
doi: 10.1021/acscatal.7b04426
-
[106]
M. Nakamura, K. Kishimoto, Y. Kobori, et al., J. Am. Chem. Soc. 138 (2016) 12564-12577.
doi: 10.1021/jacs.6b07284
-
[107]
N. Nishimura, K. Kobayashi, Angew. Chem. Int. Ed. 47 (2008) 6255-6258.
doi: 10.1002/anie.200802293
-
[108]
K. Kobayashi, M. Yamanaka, Chem. Soc. Rev. 44 (2015) 449-466.
doi: 10.1039/C4CS00153B
-
[109]
X. Liu, R. Warmuth, J. Am. Chem. Soc. 128 (2006) 14120-14127.
doi: 10.1021/ja0644733
-
[110]
S. Collin, A. Parrot, L. Marcelis, et al., Chem. Eur. J. 24 (2018) 17964-17974.
doi: 10.1002/chem.201804768
-
[111]
S. Collin, N. Giraud, E. Dumont, O. Reinaud, Org. Chem. Front. 6 (2019) 1627-1636.
doi: 10.1039/c9qo00263d
-
[112]
L. He, S.C. Wang, L.T. Lin, et al., J. Am. Chem. Soc. 142 (2020) 7134-7144.
doi: 10.1021/jacs.0c01482
-
[113]
R. Pinalli, E. Dalcanale, F. Ugozzoli, C. Massera, CrystEngComm 18 (2016) 5788-5802.
doi: 10.1039/C6CE01010E
-
[114]
W.Y. Pei, G. Xu, J. Yang, et al., J. Am. Chem. Soc. 139 (2017) 7648-7656.
doi: 10.1021/jacs.7b03169
-
[115]
F.F. Wang, Y.Y. Liu, W.Y. Pei, J.F. Ma, Inorg. Chem. 60 (2021) 7329-7336.
doi: 10.1021/acs.inorgchem.1c00497
-
[116]
C.X. Hu, Y.H. Xuan, Z.H. Jiang, et al., CrystEngComm 23 (2021) 7179-7185.
doi: 10.1039/d1ce01069g
-
[117]
Z. Li, Y.W. Yang, Adv. Mater. 34 (2022) e2107401.
doi: 10.1002/adma.202107401
-
[118]
R. Tang, Y. Ye, S. Zhu, et al., Chin. Chem. Lett. 34 (2023) 1007734.
doi: 10.1016/j.cclet.2022.08.014
-
[119]
L.P. Skala, A. Yang, M.J. Klemes, L. Xiao, W.R. Dichtel, J. Am. Chem. Soc. 141 (2019) 13315-13319.
doi: 10.1021/jacs.9b06749

 Login In
Login In





 DownLoad:
DownLoad:

 DownLoad:
DownLoad: