Elevated degradation of di-n–butyl phthalate by activating peroxymonosulfate over GO–CoFe2O4 composites: Synergistic effects and mechanisms
-
* Corresponding author.
E-mail address: yzhao16@hit.edu.cn (Y. Zhao).
Citation:
Qingliang Liu, Hang Qie, Zhiqiang Sun, Yufei Zhen, Liying Wu, Ying Zhao, Jun Ma. Elevated degradation of di-n–butyl phthalate by activating peroxymonosulfate over GO–CoFe2O4 composites: Synergistic effects and mechanisms[J]. Chinese Chemical Letters,
;2023, 34(12): 108397.
doi:
10.1016/j.cclet.2023.108397

Porphyrin arrays are organic functional molecules with large π-conjugated systems and have potential applications in optoelectronic devices [1-11], sensors [12-15] and photodynamic therapy (PDT) [16-18]. In the last decade, porphyrin arrays with alkynes [19, 20], benzene [21] or heterocycles (such as thiophene [22], pyridine [23], pyrrole [24, 25]) as bridging units have been intensively studied. Porphyrin dimers with a single carbon or heteroatom bridging unit have received much attention due to their unique photophysical properties, chemical properties, and characteristic electronic delocalization [26-37]. In 2006, Arnold et al. reported the first isolation of meso-meso nitrogen-bridged diporphyrinylamine 1, which showed a broadened Soret band and red shift Q bands, indicating substantial electronic interaction between the porphyrins [27]. Ruppert et al. reported meso-meso, β-meso, β-β-nitrogen-bridged diporphyrinylamines [29], which were all synthesized by Buchwald-Hartwig amination. Later, Osuka et al. reported that meso-meso nitrogen-bridged Ni(Ⅱ) porphyrin dimer was cleanly converted into aminyl radical 2 and nitrenium cation 3 by oxidation with PbO2 and tris(4-bromophenyl)aminiumyl hexachloroantimonate (Magic Blue), respectively (Fig. 1) [34]. As an extension, we report here the synthesis of nitrogen-atom bridged Ni(Ⅱ) porphyrin trimers.
First we attempted to synthesize linear NH-bridged porphyrin trimer 4Ni-2H by the similar Buchwald-Hartwig amination of 5, 15-dibromo Ni(Ⅱ)porphyrin 7Ni with 5-amino Ni(Ⅱ)porphyrin 6Ni [34]. A 4:1 solution of 6Ni and 7Ni in toluene was heated at 100 ℃ for 12 h in the presence of 0.4 equiv. Pd(OAc)2, 0.4 equiv. BINAP, and 7 equiv. t-BuOK (Scheme 1). To our surprise, only a linear trimer 4Ni bearing a central quinodiimine-type porphyrinoid unit was obtained in 38% yield. The matrix-assisted laser desorption ionization time-of-flight (MALDI-TOF) mass spectrum showed the parent ion of 4Ni at m/z 2627.3453 [M]+ (calcd. for (C172H192N14Ni3)+ = 2627.3509) (Fig. S13 in Supporting information), which is smaller by two than the expected parent ion peak of 4Ni-2H. The structure of 4Ni has been revealed by X-ray diffraction structural analysis (Fig. 2 and Fig. S17 in Supporting information). The bond lengths of C2meso-N (1.300(6) Å and 1.304(6) Å) are distinctly shorter than those of C1meso-N (1.412(6) Å and 1.395(7) Å). The 1H NMR spectrum of 4Ni showed broadened signals at room temperature in CDCl3 (Fig. S3 in Supporting information), which gradually changed to sharp peaks upon cooling down to −60 ℃ (Fig. S4 in Supporting information) [38], suggesting conformational motions at room temperature, which are comparable or faster than 1H NMR timescale. It is noteworthy that four doublets due to the b-protons of the central quinodiimine unit were observed in the up-field shifted region at 7.77, 6.76, 5.70 and 3.99 ppm.
Similarly, Buchwald-Hartwig amination of 5, 10-dibromo Ni(Ⅱ)porphyrin 8Ni with 6Ni afforded l-shaped bent trimer 5Ni in 25% yield. The quinodiimine structure of 5Ni has been also confirmed by X-ray analysis. 5Ni shows that the bond lengths of C2meso-N (1.299(5) Å and 1.302(6) Å) are shorter than those of C1meso-N bonds (1.399(5) Å and 1.413(6) Å) (Fig. 2 and Fig. S18 in Supporting information). The 1H NMR spectrum of 5Ni showed broadened signals at room temperature that became sharp and complicated signals at −60 ℃ in CDCl3 (Figs. S5 and S6 in Supporting information). In line with the quinodiimine structure, the corresponding β-protons were observed in the high field at 7.07, 6.73, 6.42, 6.33, 5.66, 4.33, and 3.74 ppm.
The structural data of 4Ni shows that lengths of C1meso-N bonds (1.412(6) Å and 1.395(7) Å) bond to the terminal porphyrin units are longer than C2meso-N (1.300(6) Å and 1.304(6) Å) attached to the central quinodiimine units. Similarly, 5Ni shows that lengths of C1meso-N bonds (1.399(5) Å and 1.413(6) Å) bond to the terminal porphyrin units are longer than C2meso-N (1.299(5) Å and 1.302(6) Å) attached to the central quinodiimine units. The observed short C2meso-N bond lengths in 4Ni and 5Ni indicated its double bond characters significantly [34], which further proved the structure of 4Ni and 5Ni to be N-bridged (rather than NH-bridged) porphyrin trimer. The dihedral angles between the terminal porphyrins and terminal porphyrin, terminal porphyrin and central quinodiimine are 66.81(3)°, 56.34(3)° and 58.06(3)° in 4Ni, and 6.83(3)°, 42.67(3)° and 39.34(3)° in 5Ni (Fig. 2 and Figs. S17 and S18 in Supporting information).
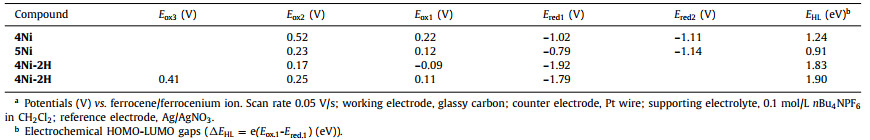
Electrochemical properties of 4Ni and 5Ni were examined by cyclic voltammetry and differential-pulse voltammetry in CH2Cl2 against a ferrocene/ferrocenium ion couple (Table 1 and Table S4 in Supporting information). Reversible oxidation waves were recorded at 0.22 and 0.52 V for 4Ni, and at 0.12 and 0.23 V for 5Ni. Reversible reduction waves were observed at −1.02 and −1.11 V for 4Ni, and at −0.79 and −1.14 V for 5Ni (Figs. S20 and S21 in Supporting information). As a result, the electrochemical HOMO-LUMO gaps of 4Ni and 5Ni were determined to be 1.24 and 0.91 eV, respectively. The observed reversible reduction waves of 4Ni and 5Ni encouraged us to examine their chemical reduction. After many attempts, we found that reduction of 5Ni with aqueous hydrazine in CH2Cl2 afforded 5Ni-2H quantitatively (Scheme 2). Curiously, 4Ni was not reduced with aqueous hydrazine but was reduced quantitatively to give 4Ni-2H with NaBH4 and Pd/C in CH2Cl2/CH3OH. 1H NMR spectra of both 4Ni-2H and 5Ni-2H are very simple, reflecting their symmetric structures with signals of the β-protons appearing in the range of 8–9 ppm (Fig. 3 and Figs. S7 and S8 in Supporting information). The structure of 5Ni-2H has been confirmed by single crystal X-ray diffraction analysis (Fig. 4 and Fig. S19 in Supporting information). In 5Ni-2H, the bond lengths of the C2meso-N bond and the C1meso-N bond are similar, being 1.409(8) Å, 1.406(8) Å and 1.393(7) Å, 1.434(11) Å, respectively, in line with the assigned structures. In addition, the dihedral angles between the terminal porphyrins and the central porphyrin are 58.29(7)° and 58.15(7)°, which are larger than those on 5Ni (42.67(3)° and 39.34(3)°).
|
The unexpected formation of 4Ni and 5Ni may be ascribed to the facile oxidation of 4Ni-2H and 5Ni-2H under the amination reaction conditions. These trimers have the central electron-rich Ni(Ⅱ) porphyrin bearing 5, 15 or 5, 10-aminoporphyrin units. Thus, we examined the electrochemical properties of 4Ni-2H and 5Ni-2H (Table 1 and Table S4 in Supporting information). Actually, the reversible oxidation waves were observed at −0.09 and 0.17 V for 4Ni-2H, and at 0.11, 0.25 and 0.41 V for 5Ni-2H (Figs. S22 and S23 in Supporting information). It is thus conceivable that 4Ni-2H and 5Ni-2H are oxidized under the amination conditions with air. So, when we try to oxidized them with PbO2 and Magic Blue, neither aminyl radical nor nitrenium cation was found. The possible reason may be that the quinodiimine unit is more stable than other species.
The UV–vis-NIR absorption spectra of 4Ni, 5Ni, 4Ni-2H and 5Ni-2H in CH2Cl2 are shown in Fig. 5. 4Ni shows two split Soret bands at 426 and 472 nm, a Q-band at 537 nm, and a broadened Q-like band at 915 nm. 5Ni shows a Soret band at 429 nm, Q-bands at 540 and 581 nm, and a broadened Q-like band at 892 nm. Both 4Ni and 5Ni exhibit characteristic absorption spectra of quinonoidal porphyrinoid arrays [39-42]. 4Ni-2H shows a Soret band at 423 nm, and a Q-band at 627 nm. Similarly to 4Ni-2H, the absorption spectrum of 5Ni-2H shows a Soret band at 418 nm, and a Q-band at 664 nm. In particular, 4Ni and 5Ni display the lowest energy band reaching to 1200 nm and 1400 nm, respectively.
Density functional theory (DFT) calculations clearly indicated that both the HOMO of 4Ni and HOMO-1 5Ni were localized at terminal porphyrin units, whereas both LUMOs of 4Ni and 5Ni were localized at the central quinodiimine units (Figs. S28 and S29 in Supporting information). Time-dependent density functional theory (TD-DFT) calculations indicated that the absorption bands around 1000 nm of trimers 4Ni and 5Ni resulted from the transition from HOMO to LUMO of 4Ni and HOMO-1 to LUMO of 5Ni, respectively (Figs. S24 and S25 in Supporting information). These results show that both absorption bands around 1000 nm of 4Ni and 5Ni could be assigned to charge transfer (CT) band.
In summary, we synthesized N-bridged porphyrinoid trimers 4Ni and 5Ni having the central quinodiimine through Buchwald-Hartwig amination, under which the oxidations of the NH-bridged porphyrin trimers 4Ni-2H and 5Ni-2H proceeded smoothly. The trimer 4Ni-2H was obtained by reduction with NaBH4 and Pd/C, while 5Ni-2H was obtained by reduction with aqueous hydrazine. The structures of 4Ni, 5Ni and 5Ni-2H were determined by X-ray diffraction analysis. The UV–vis-NIR absorption spectra showed that the trimers 4Ni and 5Ni have the lowest energy band reaching to 1200 nm and 1400 nm, respectively. These N-bridged porphyrinoid trimers exhibited interesting spectral properties. Further exploration of cyclic or larger N-bridged porphyrinoid arrays is ongoing in our laboratory.
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
The work at Hunan Normal University was supported by the National Natural Science Foundation of China (Nos. 21772036, 22071052, 21602058, 21702057), the Science and Technology Planning Project of Hunan Province (No. 2018TP1017), and the Scientific Research Fund of Hunan Provincial Education Department (No. 19A331), and Hunan Provincial Innovation Foundation for Postgraduate (No. CX20210473).
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.cclet.2022.01.061.
K. Fent, A.A. Weston, Aquat. Toxicol. 76 (2006) 122–159.
doi: 10.1016/j.aquatox.2005.09.009
O. Bajt, G. Mailhot, M. Bolte, Appl. Catal. B: Environ. 33 (2001) 239–248.
doi: 10.1016/S0926-3373(01)00179-5
B. Wang, H.X. Wang, W. Zhou, et al., Environ. Sci. Technol. 49 (2015) 1120–1129.
doi: 10.1021/es504455a
J. Lee, U.V. Gunten, J.H. Kim, Environ. Sci. Technol. 54 (2020) 3064–3081.
doi: 10.1021/acs.est.9b07082
W.D. Oh, Z. Dong, T.T. Lim, Appl. Catal. B: Environ. 194 (2016) 169–201.
doi: 10.1016/j.apcatb.2016.04.003
J.L. Wang, S.Z. Wang, Chem. Eng. J. 334 (2018) 1502–1517.
doi: 10.1016/j.cej.2017.11.059
P.D. Hu, M.C. Long, Appl. Catal. B: Environ. 181 (2016) 103–117.
doi: 10.1016/j.apcatb.2015.07.024
J. Deng, Y. Shao, N. Gao, et al., J. Hazard. Mater. 262 (2013) 836–844.
doi: 10.1016/j.jhazmat.2013.09.049
K. Zhang, D. Sun, C. Ma, et al., Chemosphere 241 (2020) 125021.
doi: 10.1016/j.chemosphere.2019.125021
Y.M. Ren, L.Q. Lin, J. Ma, et al., Appl. Catal. B: Environ. 165 (2015) 572–578.
doi: 10.1016/j.apcatb.2014.10.051
L.J. Xu, W. Chu, L. Gan, Chem. Eng. J. 263 (2015) 435–443.
doi: 10.1016/j.cej.2014.11.065
A.T. Smith, A.M. LaChance, S. Zeng, B. Liu, L. Sun, Nano Mater. Sci. 1 (2019) 31–47.
doi: 10.1016/j.nanoms.2019.02.004
J.P. Zhou, H.L. Liu, J. Luo, et al., ACS Appl. Mater. Interfaces 8 (2016) 18140–18149.
doi: 10.1021/acsami.6b05895
Z.Q. Cai, A.D. Dwivedi, W.N. Lee, et al., Environ. Sci. -Nano 5 (2018) 27–47.
doi: 10.1039/C7EN00644F
L. Tang, X. Meng, D. Deng, X. Bao, Adv. Mater. 31 (2019) 1901996.
doi: 10.1002/adma.201901996
W. Choi, I. Lahiri, R. Seelaboyina, Y.S. Kang, Crit. Rev. Solid State 35 (2010) 52–71.
doi: 10.1080/10408430903505036
X. Zhu, Y. Zhu, S. Murali, M.D. Stoller, R.S. Ruoff, ACS Nano 5 (2011) 3333–3338.
doi: 10.1021/nn200493r
C. Chen, W. Cai, M. Long, et al., ACS Nano 4 (2010) 6425–6432.
doi: 10.1021/nn102130m
X.R. You, C.Y. Huang, W. Huang, et al., Environ. Sci. : Nano 7 (2020) 554–570.
doi: 10.1039/C9EN01163C
J. Deng, L.W. Xiao, S.J. Yuan, et al., Sep. Purif. Technol. 255 (2021) 117685.
doi: 10.1016/j.seppur.2020.117685
R. Tabit, O. Amadine, Y. Essamlali, et al., RSC Adv. 8 (2018) 1351–1360.
doi: 10.1039/C7RA09949E
W.S. Hung, S.M. Chang, R.L.G. Lecaros, et al., Carbon 117 (2017) 112–119.
doi: 10.1016/j.carbon.2017.02.088
N. Meng, R.C.E. Priestley, Y. Zhang, H.T. Wang, X.W. Zhang, J. Membrane Sci. 501 (2016) 169–178.
doi: 10.1016/j.memsci.2015.12.004
D.R. Yang, J. Feng, L.L. Jiang, et al., Adv. Funct. Mater. 25 (2015) 7080–7087.
doi: 10.1002/adfm.201502970
N. Li, Z.F. Geng, M.H. Cao, et al., Carbon 54 (2013) 124–132.
doi: 10.1016/j.carbon.2012.11.009
M. Seredych, T.J. Bandosz, J. Mater. Chem. 22 (2012) 23525–23533.
doi: 10.1039/c2jm34294d
M. Kim, C. Lee, J. Jang, Adv. Funct. Mater. 24 (2014) 2489–2499.
doi: 10.1002/adfm.201303282
K. Ashwini, A.D. Mashkoor, S. Poorva, V. Dinesh, AIP Conf. Proc. 1148 (2014) 1148–1150.
T. Lin, L. Yu, M. Sun, et al., Chem. Eng. J. 286 (2016) 114–121.
doi: 10.1016/j.cej.2015.09.024
Y. Zhao, H.Z. An, G.J. Dong, et al., App. Surf. Sci. 505 (2020) 144476.
doi: 10.1016/j.apsusc.2019.144476
Y. Zhao, S. Wang, T. Wei, Y.M. Ren, T.Z. Luan, J. Environ. Chem. Eng. 10 (2022) 107241.
doi: 10.1016/j.jece.2022.107241
J. Liu, Z.W. Zhao, P.H. Shao, F.Y. Cui, Chem. Eng. J. 262 (2015) 854–861.
doi: 10.1016/j.cej.2014.10.043
Z.L. Wu, Z.K. Xiong, R. Liu, et al., J. Hazard. Mater. 427 (2022) 128204.
doi: 10.1016/j.jhazmat.2021.128204
R.H. Zhang, M.L. Chen, Z.K. Xiong, Y. Guo, B. Lai, Chin. Chem. Lett. 33 (2022) 948–952.
doi: 10.1016/j.cclet.2021.07.029
Z. Wang, R.T. Bush, L.A. Sullivan, C.C. Chen, J.S. Liu, Environ. Sci. Technol. 48 (2014) 3978–3985.
doi: 10.1021/es405143u
S. Wang, M.Y. Zhang, J. Feng, et al., Chem. Eng. J. 430 (2022) 133175.
doi: 10.1016/j.cej.2021.133175
Z. Liu, H. Ding, C. Zhao, et al., Water Res. 159 (2019) 111–121.
doi: 10.1016/j.watres.2019.04.052
Y. Zhao, H.Z. An, J. Feng, Y.M. Ren, J. Ma, Environ. Sci. Technol. 53 (2019) 4500–4510.
doi: 10.1021/acs.est.9b00658
Z.C. Yang, J.S. Qian, A.Q. Yu, B.C. Pan, Proc. Natl. Acad. Sci. U. S. A. 116 (2019) 6659–6664.
doi: 10.1073/pnas.1819382116
E.T. Yun, J.H. Lee, J. Kim, H.D. Park, J. Lee, Environ. Sci. Technol. 52 (2018) 7032–7042.
doi: 10.1021/acs.est.8b00959
J.J. You, C.Y. Zhang, Z.L. Wu, et al., Chem. Eng. J. 415 (2021) 128890.
doi: 10.1016/j.cej.2021.128890
J.E. Yang, Y. Lin, H.H. Peng, et al., Appl. Catal. B: Environ. 268 (2020) 118549.
doi: 10.1016/j.apcatb.2019.118549
L.P. Wu, B. Li, Y. Li, et al., ACS Catal. 11 (2021) 5532–5543.
doi: 10.1021/acscatal.1c00701
A.H. Mady, M.L. Baynosa, D. Tuma, J.J. Shim, Appl. Catal. B: Environ. 244 (2019) 946–956.
doi: 10.1016/j.apcatb.2018.11.086
X.B. Hu, B.Z. Liu, Y.H. Deng, et al., Appl. Catal. B: Environ. 107 (2011) 274–283.
doi: 10.1016/j.apcatb.2011.07.025
X.T. Li, J. Wang, X.G. Duan, et al., ACS Catal. 11 (2021) 4848–4861.
doi: 10.1021/acscatal.0c05089
C. Liu, S. Liu, L. Liu, et al., Chem. Eng. J. 379 (2020) 122274.
doi: 10.1016/j.cej.2019.122274
L.Y. Wu, Z.Y. Li, P.T. Cheng, et al., Water Res. 223 (2022) 119013.
doi: 10.1016/j.watres.2022.119013
J. Ye, J.D. Dai, D.Y. Yang, C.X. Li, Y.S. Yan, J. Environ. Chem. Eng. 9 (2021) 106076.
doi: 10.1016/j.jece.2021.106076
K. Fent, A.A. Weston, Aquat. Toxicol. 76 (2006) 122–159.
doi: 10.1016/j.aquatox.2005.09.009
O. Bajt, G. Mailhot, M. Bolte, Appl. Catal. B: Environ. 33 (2001) 239–248.
doi: 10.1016/S0926-3373(01)00179-5
B. Wang, H.X. Wang, W. Zhou, et al., Environ. Sci. Technol. 49 (2015) 1120–1129.
doi: 10.1021/es504455a
J. Lee, U.V. Gunten, J.H. Kim, Environ. Sci. Technol. 54 (2020) 3064–3081.
doi: 10.1021/acs.est.9b07082
W.D. Oh, Z. Dong, T.T. Lim, Appl. Catal. B: Environ. 194 (2016) 169–201.
doi: 10.1016/j.apcatb.2016.04.003
J.L. Wang, S.Z. Wang, Chem. Eng. J. 334 (2018) 1502–1517.
doi: 10.1016/j.cej.2017.11.059
P.D. Hu, M.C. Long, Appl. Catal. B: Environ. 181 (2016) 103–117.
doi: 10.1016/j.apcatb.2015.07.024
J. Deng, Y. Shao, N. Gao, et al., J. Hazard. Mater. 262 (2013) 836–844.
doi: 10.1016/j.jhazmat.2013.09.049
K. Zhang, D. Sun, C. Ma, et al., Chemosphere 241 (2020) 125021.
doi: 10.1016/j.chemosphere.2019.125021
Y.M. Ren, L.Q. Lin, J. Ma, et al., Appl. Catal. B: Environ. 165 (2015) 572–578.
doi: 10.1016/j.apcatb.2014.10.051
L.J. Xu, W. Chu, L. Gan, Chem. Eng. J. 263 (2015) 435–443.
doi: 10.1016/j.cej.2014.11.065
A.T. Smith, A.M. LaChance, S. Zeng, B. Liu, L. Sun, Nano Mater. Sci. 1 (2019) 31–47.
doi: 10.1016/j.nanoms.2019.02.004
J.P. Zhou, H.L. Liu, J. Luo, et al., ACS Appl. Mater. Interfaces 8 (2016) 18140–18149.
doi: 10.1021/acsami.6b05895
Z.Q. Cai, A.D. Dwivedi, W.N. Lee, et al., Environ. Sci. -Nano 5 (2018) 27–47.
doi: 10.1039/C7EN00644F
L. Tang, X. Meng, D. Deng, X. Bao, Adv. Mater. 31 (2019) 1901996.
doi: 10.1002/adma.201901996
W. Choi, I. Lahiri, R. Seelaboyina, Y.S. Kang, Crit. Rev. Solid State 35 (2010) 52–71.
doi: 10.1080/10408430903505036
X. Zhu, Y. Zhu, S. Murali, M.D. Stoller, R.S. Ruoff, ACS Nano 5 (2011) 3333–3338.
doi: 10.1021/nn200493r
C. Chen, W. Cai, M. Long, et al., ACS Nano 4 (2010) 6425–6432.
doi: 10.1021/nn102130m
X.R. You, C.Y. Huang, W. Huang, et al., Environ. Sci. : Nano 7 (2020) 554–570.
doi: 10.1039/C9EN01163C
J. Deng, L.W. Xiao, S.J. Yuan, et al., Sep. Purif. Technol. 255 (2021) 117685.
doi: 10.1016/j.seppur.2020.117685
R. Tabit, O. Amadine, Y. Essamlali, et al., RSC Adv. 8 (2018) 1351–1360.
doi: 10.1039/C7RA09949E
W.S. Hung, S.M. Chang, R.L.G. Lecaros, et al., Carbon 117 (2017) 112–119.
doi: 10.1016/j.carbon.2017.02.088
N. Meng, R.C.E. Priestley, Y. Zhang, H.T. Wang, X.W. Zhang, J. Membrane Sci. 501 (2016) 169–178.
doi: 10.1016/j.memsci.2015.12.004
D.R. Yang, J. Feng, L.L. Jiang, et al., Adv. Funct. Mater. 25 (2015) 7080–7087.
doi: 10.1002/adfm.201502970
N. Li, Z.F. Geng, M.H. Cao, et al., Carbon 54 (2013) 124–132.
doi: 10.1016/j.carbon.2012.11.009
M. Seredych, T.J. Bandosz, J. Mater. Chem. 22 (2012) 23525–23533.
doi: 10.1039/c2jm34294d
M. Kim, C. Lee, J. Jang, Adv. Funct. Mater. 24 (2014) 2489–2499.
doi: 10.1002/adfm.201303282
K. Ashwini, A.D. Mashkoor, S. Poorva, V. Dinesh, AIP Conf. Proc. 1148 (2014) 1148–1150.
T. Lin, L. Yu, M. Sun, et al., Chem. Eng. J. 286 (2016) 114–121.
doi: 10.1016/j.cej.2015.09.024
Y. Zhao, H.Z. An, G.J. Dong, et al., App. Surf. Sci. 505 (2020) 144476.
doi: 10.1016/j.apsusc.2019.144476
Y. Zhao, S. Wang, T. Wei, Y.M. Ren, T.Z. Luan, J. Environ. Chem. Eng. 10 (2022) 107241.
doi: 10.1016/j.jece.2022.107241
J. Liu, Z.W. Zhao, P.H. Shao, F.Y. Cui, Chem. Eng. J. 262 (2015) 854–861.
doi: 10.1016/j.cej.2014.10.043
Z.L. Wu, Z.K. Xiong, R. Liu, et al., J. Hazard. Mater. 427 (2022) 128204.
doi: 10.1016/j.jhazmat.2021.128204
R.H. Zhang, M.L. Chen, Z.K. Xiong, Y. Guo, B. Lai, Chin. Chem. Lett. 33 (2022) 948–952.
doi: 10.1016/j.cclet.2021.07.029
Z. Wang, R.T. Bush, L.A. Sullivan, C.C. Chen, J.S. Liu, Environ. Sci. Technol. 48 (2014) 3978–3985.
doi: 10.1021/es405143u
S. Wang, M.Y. Zhang, J. Feng, et al., Chem. Eng. J. 430 (2022) 133175.
doi: 10.1016/j.cej.2021.133175
Z. Liu, H. Ding, C. Zhao, et al., Water Res. 159 (2019) 111–121.
doi: 10.1016/j.watres.2019.04.052
Y. Zhao, H.Z. An, J. Feng, Y.M. Ren, J. Ma, Environ. Sci. Technol. 53 (2019) 4500–4510.
doi: 10.1021/acs.est.9b00658
Z.C. Yang, J.S. Qian, A.Q. Yu, B.C. Pan, Proc. Natl. Acad. Sci. U. S. A. 116 (2019) 6659–6664.
doi: 10.1073/pnas.1819382116
E.T. Yun, J.H. Lee, J. Kim, H.D. Park, J. Lee, Environ. Sci. Technol. 52 (2018) 7032–7042.
doi: 10.1021/acs.est.8b00959
J.J. You, C.Y. Zhang, Z.L. Wu, et al., Chem. Eng. J. 415 (2021) 128890.
doi: 10.1016/j.cej.2021.128890
J.E. Yang, Y. Lin, H.H. Peng, et al., Appl. Catal. B: Environ. 268 (2020) 118549.
doi: 10.1016/j.apcatb.2019.118549
L.P. Wu, B. Li, Y. Li, et al., ACS Catal. 11 (2021) 5532–5543.
doi: 10.1021/acscatal.1c00701
A.H. Mady, M.L. Baynosa, D. Tuma, J.J. Shim, Appl. Catal. B: Environ. 244 (2019) 946–956.
doi: 10.1016/j.apcatb.2018.11.086
X.B. Hu, B.Z. Liu, Y.H. Deng, et al., Appl. Catal. B: Environ. 107 (2011) 274–283.
doi: 10.1016/j.apcatb.2011.07.025
X.T. Li, J. Wang, X.G. Duan, et al., ACS Catal. 11 (2021) 4848–4861.
doi: 10.1021/acscatal.0c05089
C. Liu, S. Liu, L. Liu, et al., Chem. Eng. J. 379 (2020) 122274.
doi: 10.1016/j.cej.2019.122274
L.Y. Wu, Z.Y. Li, P.T. Cheng, et al., Water Res. 223 (2022) 119013.
doi: 10.1016/j.watres.2022.119013
J. Ye, J.D. Dai, D.Y. Yang, C.X. Li, Y.S. Yan, J. Environ. Chem. Eng. 9 (2021) 106076.
doi: 10.1016/j.jece.2021.106076

Mengmeng Ao , Jian Wei , Chuan-Shu He , Heng Zhang , Zhaokun Xiong , Yonghui Song , Bo Lai . Insight into the activation of peroxymonosulfate by N-doped copper-based carbon for efficient degradation of organic pollutants: Synergy of nonradicals. Chinese Chemical Letters, 2025, 36(1): 109882-. doi: 10.1016/j.cclet.2024.109882
Yun-Xin Huang , Lin-Qian Yu , Ke-Yu Chen , Hao Wang , Shou-Yan Zhao , Bao-Cheng Huang , Ren-Cun Jin . Biochar with self-doped N to activate peroxymonosulfate for bisphenol-A degradation via electron transfer mechanism: The active edge graphitic N site. Chinese Chemical Letters, 2024, 35(9): 109437-. doi: 10.1016/j.cclet.2023.109437
Wenhao Feng , Chunli Liu , Zheng Liu , Huan Pang . In-situ growth of N-doped graphene-like carbon/MOF nanocomposites for high-performance supercapacitor. Chinese Chemical Letters, 2024, 35(12): 109552-. doi: 10.1016/j.cclet.2024.109552
Ying Chen , Li Li , Junyao Zhang , Tongrui Sun , Xuan Zhang , Shiqi Zhang , Jia Huang , Yidong Zou . Tailored ionically conductive graphene oxide-encased metal ions for ultrasensitive cadaverine sensor. Chinese Chemical Letters, 2024, 35(8): 109102-. doi: 10.1016/j.cclet.2023.109102
Linping Li , Junhui Su , Yanping Qiu , Yangqin Gao , Ning Li , Lei Ge . Design and fabrication of ternary Au/Co3O4/ZnCdS spherical composite photocatalyst for facilitating efficient photocatalytic hydrogen production. Chinese Journal of Structural Chemistry, 2024, 43(12): 100472-100472. doi: 10.1016/j.cjsc.2024.100472
Zimo Peng , Quan Zhang , Gaocan Qi , Hao Zhang , Qian Liu , Guangzhi Hu , Jun Luo , Xijun Liu . Nanostructured Pt@RuOx catalyst for boosting overall acidic seawater splitting. Chinese Journal of Structural Chemistry, 2024, 43(1): 100191-100191. doi: 10.1016/j.cjsc.2023.100191
Jiayi Guo , Liangxiong Ling , Qinwei Lu , Yi Zhou , Xubiao Luo , Yanbo Zhou . Degradation of chloroxylenol by CoSx activated peroxomonosulfate: Role of cobalt-sulfur ratio. Chinese Chemical Letters, 2025, 36(4): 110380-. doi: 10.1016/j.cclet.2024.110380
Dong Cheng , Youyou Feng , Bingxi Feng , Ke Wang , Guoxin Song , Gen Wang , Xiaoli Cheng , Yonghui Deng , Jing Wei . Polyphenol-mediated interfacial deposition strategy for supported manganese oxide catalysts with excellent pollutant degradation performance. Chinese Chemical Letters, 2024, 35(5): 108623-. doi: 10.1016/j.cclet.2023.108623
Yu Yao , Jinqiang Zhang , Yantao Wang , Kunsheng Hu , Yangyang Yang , Zhongshuai Zhu , Shuang Zhong , Huayang Zhang , Shaobin Wang , Xiaoguang Duan . Nitrogen-rich carbon for catalytic activation of peroxymonosulfate towards green synthesis. Chinese Chemical Letters, 2024, 35(11): 109633-. doi: 10.1016/j.cclet.2024.109633
Yinyin Xu , Yuanyuan Li , Jingbo Feng , Chen Wang , Yan Zhang , Yukun Wang , Xiuwen Cheng . Covalent organic frameworks doped with manganese-metal organic framework for peroxymonosulfate activation. Chinese Chemical Letters, 2024, 35(4): 108838-. doi: 10.1016/j.cclet.2023.108838
Shimei Wu , Yining Li , Lantao Chen , Yufei Zhang , Lingxing Zeng , Haosen Fan . Hexapod cobalt phosphosulfide nanorods encapsulating into multiple hetero-atom doped carbon frameworks for advanced sodium/potassium ion battery anodes. Chinese Chemical Letters, 2025, 36(4): 109796-. doi: 10.1016/j.cclet.2024.109796
Rui Liu , Yue Yu , Lu Deng , Maoxia Xu , Haorong Ren , Wenjie Luo , Xudong Cai , Zhenyu Li , Jingyu Chen , Hua Yu . The synergistic effect of A-site cation engineering and phase regulation enables efficient and stable Ruddlesden-Popper perovskite solar cells. Chinese Chemical Letters, 2024, 35(12): 109545-. doi: 10.1016/j.cclet.2024.109545
Fengrui Yang , Debing Wang , Xinying Zhang , Jie Zhang , Zhichao Wu , Qiaoying Wang . Synergistic effects of peroxydisulfate on UV/O3 process for tetracycline degradation: Mechanism and pathways. Chinese Chemical Letters, 2024, 35(10): 109599-. doi: 10.1016/j.cclet.2024.109599
Yuanyi Zhou , Ke Ma , Jinfeng Liu , Zirun Zheng , Bo Hu , Yu Meng , Zhizhong Li , Mingshan Zhu . Is reactive oxygen species the only way for cancer inhibition over single atom nanomedicine? Autophagy regulation also works. Chinese Chemical Letters, 2024, 35(6): 109056-. doi: 10.1016/j.cclet.2023.109056
Ke Gong , Jinghan Liao , Jiangtao Lin , Quan Wang , Zhihua Wu , Liting Wang , Jiali Zhang , Yi Dong , Yourong Duan , Jianhua Chen . Mitochondria-targeted nanoparticles overcome chemoresistance via downregulating BACH1/CD47 axis in ovarian carcinoma. Chinese Chemical Letters, 2024, 35(5): 108888-. doi: 10.1016/j.cclet.2023.108888
Kexin Yin , Jingren Yang , Yanwei Li , Qian Li , Xing Xu . Metal-free diatomaceous carbon-based catalyst for ultrafast and anti-interference Fenton-like oxidation. Chinese Chemical Letters, 2024, 35(12): 109847-. doi: 10.1016/j.cclet.2024.109847
Ming-Zhen Li , Yang Zhang , Kun Li , Ya-Nan Shang , Yi-Zhen Zhang , Yu-Jiao Kan , Zhi-Yang Jiao , Yu-Yuan Han , Xiao-Qiang Cao . In situ regeneration of catalyst for Fenton-like degradation by photogenerated electron transportation: Characterization, performance and mechanism comparison. Chinese Chemical Letters, 2025, 36(1): 109885-. doi: 10.1016/j.cclet.2024.109885
Qingbai Tian , BingLiang Yu , Zhihao Li , Wei Hong , Qian Li , Xing Xu . Versatile catalytic membranes anchored with metal-nitrogen based metal oxides for ultrafast Fenton-like oxidation. Chinese Chemical Letters, 2025, 36(6): 110322-. doi: 10.1016/j.cclet.2024.110322
Liping Zhao , Xixi Guo , Zhimeng Zhang , Xi Lu , Qingxuan Zeng , Tianyun Fan , Xintong Zhang , Fenbei Chen , Mengyi Xu , Min Yuan , Zhenjun Li , Jiandong Jiang , Jing Pang , Xuefu You , Yanxiang Wang , Danqing Song . Novel berberine derivatives as adjuvants in the battle against Acinetobacter baumannii: A promising strategy for combating multi-drug resistance. Chinese Chemical Letters, 2024, 35(10): 109506-. doi: 10.1016/j.cclet.2024.109506
Xuejie Gao , Xinyang Chen , Ming Jiang , Hanyan Wu , Wenfeng Ren , Xiaofei Yang , Runcang Sun . Long-lifespan thin Li anode achieved by dead Li rejuvenation and Li dendrite suppression for all-solid-state lithium batteries. Chinese Chemical Letters, 2024, 35(10): 109448-. doi: 10.1016/j.cclet.2023.109448