Cell mechanics and energetic costs of collective cell migration under confined microchannels
-
* Corresponding authors.
E-mail addresses: binkang@nju.edu.cn (B. Kang), xujj@nju.edu.cn (J.-J. Xu).
Citation:
Xiao-Hong Wang, Yu Liu, Bin Kang, Jing-Juan Xu, Hong-Yuan Chen. Cell mechanics and energetic costs of collective cell migration under confined microchannels[J]. Chinese Chemical Letters,
;2023, 34(5): 107789.
doi:
10.1016/j.cclet.2022.107789

Neurochemicals play vital roles in maintaining homeostasis of brain functions. The occurrence and development of brain diseases are usually accompanied by neurochemical imbalance [1, 2]. Thus, in-situ monitoring of neurochemical levels is essential for accurately understanding brain function, which will promote the development of diagnosis and treatment of brain diseases. As a consequence, there has been growing interest in developing new methods and techniques for in-situ analysis in the brain.
Electrochemical (EC) analysis based on microelectrode is regarded as a powerful tool for in vivo neurochemicals detection owing to its high sensitivity and good temporal-spatial resolution [3, 4]. Photoelectrochemical (PEC) sensing is a relatively new branch of EC analysis that utilizes light instead of external bias as the excitation source, possessing reduced background signal as compared to EC methods [5, 6]. Our recent works revealed that PEC sensors based on chemically modified photoelectrode could resolve the specificity issue of EC analysis via modulating the excitation light by chemical recognition of the target, showing a promising prospect for in vivo monitoring of chemicals [7, 8]. Nonetheless, the efficient excitation of photoactive materials on the photoelectrode in living body is still a challenge. Considering the penetration depth of light in biological tissues, it is imperative to employ near-infrared (NIR) light as excitation source. Regrettably, the vast majority of currently available photoactive materials can only be excited by UV or visible light due to their wide band gaps [9]. Moreover, because of the gradual decay of light intensity in tissues, the analysis in different depths, e.g., different brain regions, is likely distorted. As such, it is of great significance to find alternative strategies for the in vivo excitation of photoelectrode, so as to circumvent the limitation of light penetration in living body.
As a solution, we herein developed an optical fiber (OF)-based microelectrode by integrating the functions of a photoelectrode and a fiber for in vivo PEC analysis, with Cu2+ as the proof-of-concept target (Scheme 1). OFs have found applications in sensing and imaging owing to their advantages including small size, light weight, high robustness and low cost [10-12]. For instance, OF was employed in neuroscience studies for in vivo optogenetic stimulation or collection of fluorescence signal [13-15]. In addition, OFs combined with electrodes of micron or nano scale also have been used as scanning probe in electrochemical microscopy [16]. We conceived that photoactive materials could be excited in situ in the brain by loading them on the surface of an OF, thus addressing the problem of light penetration. To this end, a micron-sized (ca. 150 µm) OF was made of flexible polymethyl methacrylate (PMMA), which enabled close contact with tissues to decrease tissue damage [17]. A gold film was coated on the surface of OF to confer it a good conductivity. Then, CdS@ZnO film as the photoactive material was deposited to obtain the OF-based photoelectrode, which can be excited by visible light (excitation wavelength for CdS) from the tip of OF to produce a rapid photocurrent response. Since CdS can interact with Cu2+ by a chemical displacement reaction to generate CuS as the exciton trapping sites [18, 19], the microelectrode would be able to respond to Cu2+ in the interested region. To endow the electrode with anti-biofouling ability and facilitate it is in vivo use, a cross-linked film of cell membrane and alginate was loaded. The constructed microelectrode was applied to monitor variation of Cu2+ level in a rat model of cerebral ischemia/reperfusion.
Fabrication of optical fiber based microelectrodes: Optical fibers made of PMMA, which is soluble in acetone, (Shenzhen TeNai Optoelectronic Technology Co., Ltd., Shenzhen, China) with a diameter of 250 µm and a length of 5 cm, were immersed in acetone for a period of time to etch down to 150 µm, then successively ultrasonicated in ethanol and water. After being dried at room temperature, the etched fibers were immersed into 1.5 mg/mL dopamine solution for 1 h to facilitate the subsequent growth of gold film [20]. The fibers were then rinsed with water and immersed into a freshly prepared gold nanoparticles solution at 4 ℃ overnight. Subsequently, the prepared optical fibers were incubated in 10 mL of mixed solution containing 60 µL of 1% HAuCl4 and 30 µL of 0.2 mol/L NH2OH and shaken for 20 min. Repeating this growth step for a certain time, then the obtained gold coated optical fiber microelectrodes (GOFMEs) were rinsed with distilled water and dried at 60 ℃. Finally, each GOGME was adhered with a piece of copper wire using silver conductive glue for connecting external circuits.
Next, the GOFME was immersed into 3 µL of ZnO dispersion solution with different concentrations and dried naturally. Subsequently, the ZnO modified GOFME was immersed in 3 µL of 10 mmol/L Cd(NO3)2 and 10 mmol/L Thioacetamide (TAA) mixed solution at room temperature for a period of time for CdS growth. Finally, the prepared CdS@ZnO/GOFME was sequentially soaked in 0.1% chitosan solution and SA-CM solution to obtain the anti-fouling photochemical microsensor (SA-CMCdS@ZnO/GOFME), with the length of effective photoactive material being controlled as 0.5 mm.
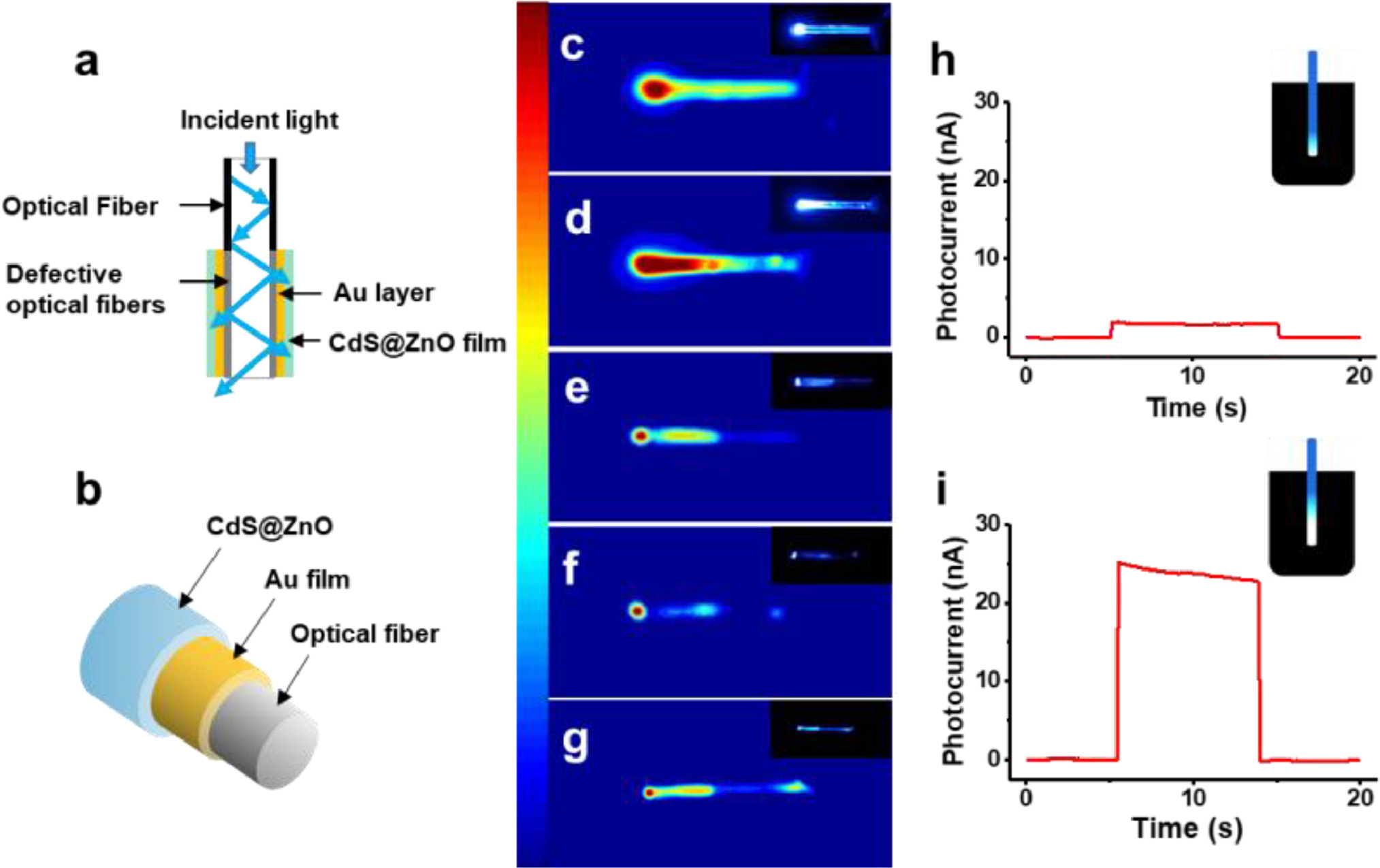
The configuration of the OF-based photoelectrode is depicted in Figs. 1a and b. OFs mainly consist of an inner core and the surrounding cladding with a lower refractive index. Thus, major portion of incident light is guided to the tip section of OFs by the primary mechanism of total internal reflection, as illustrated in Fig. 1a [21]. Our results (Fig. 1c) exhibited that the light was mainly concentrated in a small range of the fiber tip, while weak on the sidewall. With the purpose of improving photocurrent which strongly depends on the effective exciting area of photoelectrode, defects were artificially introduced on the side wall by polishing, resulting in multi-direction emission near the tip of OF (Fig. 1d). Next, the OF surface was coated with a gold layer by electroless deposition to guarantee the conductivity of the microelectrode (denoted as GOFME) [22, 23]. The electrochemical performance of GOFME was tested by cyclic voltammetry (CV). The redox peak current of [Fe(CN)6]3−/4− at GOFME increased along with the growth times of Au film, suggesting the increasing conductivity (Fig. S1 in Supporting information). The result was further confirmed by resistance of the GOFME (Fig. S2 in Supporting information). Afterward, ZnO and CdS were stepwise decorated on the surface of GOFME to produce the OF-based photoelectrode, CdS@ZnO/GOFME, which was characterized by XRD and UV-vis absorption spectroscopy (Figs. S3 and S4 in Supporting information) [24]. Here, CdS functioned as both the photoactive material and the recognition unit for Cu2+, and ZnO was employed to enhance the photocurrent through forming the van der Waals heterojunction with CdS. The transmission electron microscopy (TEM) and high resolution TEM (HRTEM) images presented the microstructure of the composites and interface lattice of CdS/ZnO heterojunction (Fig. S5 in Supporting information). The multi-layer structure of CdS@ZnO/GOFME was also characterized by SEM (Fig. S6 in Supporting information). It is noteworthy that the thickness of the conducting layer, i.e., the growth cycles of Au film, could be critical to the PEC performance because of its double-sided effects. As discovered above, a thicker Au film was beneficial to the conductivity of the electrode. On the other hand, the light transmittance should be taken into consideration since it is also crucial for photocurrent generation. As shown in Figs. 1e and f, the transmittance of GOFME decreased obviously with the thickness of Au layer increasing. To gain an appropriate balance between conductivity and light transmissibility, the PEC signal of CdS@ZnO/GOFME was optimized against thickness of Au film. Experimental results indicated that 6 cycles of Au deposition was optimum to obtain the maximal photocurrent (Fig. S7 in Supporting information). The cyclic voltammetry measurements for 50 cycles exhibited a good stability of the electrode (Fig. S8 in Supporting information). The construction conditions of the photoactive layer, including the concentration of ZnO and deposition time of CdS were also optimized as shown in Fig. S9 (Supporting information). Additionally, the good light transmission of ZnO/GOFME ensures the effective excitation of CdS layer (Fig. 1g). For a comparison, we also studied the photocurrent obtained from CdS@ZnO/GOFME with/without defects on the side wall. As shown in Figs. 1h and i, when excited with the same LED (3 W, 460–480 nm), the photocurrent of the photoelectrode with artificially introduced defects was 14.6 times higher than that without defects.
The implantation of microelectrode into biological tissues will inevitably be subject to biofouling of electrode surface such as nonspecific adsorption of proteins, which impairs sensitivity and accuracy of assays. Therefore, anti-biofouling modification of the electrode is meaningful. Reported anti-biofouling materials include natural biomaterials such as cell membranes (CM) containing phospholipids with hydrophilic ends facing outside [25, 26], and biocompatible polymers preferentially absorbing water molecules to form a hydration layer that is highly resistant to protein adsorption [27, 28]. Herein, we prepared a cross-linked alginate (sodium alginate, SA)-CM film as the antifouling layer to fabricate the working electrode (SA-CM/CdS@ZnO/GOFME), which was characterized with SEM (Fig. S10 in Supporting information). We firstly compared the photocurrent reserving capacity of differently modified microelectrodes after immersing them in bovine serum albumin (BSA) solution for 2 h. As shown in Fig. 2a, the unprotected electrode CdS@ZnO/GOFME underwent an obvious loss of photocurrent signal (~40%) after the immersion. While both the modification with SA and CM enhanced the photocurrent reservation, the chemically linked SA-CM complex provided further elevated anti-biofouling ability, and the photocurrent of SA-CM/CdS@ZnO/GOFME can be retained by 93.8% ± 3.9% (with a SA-to-CM ratio of 1:1). Further evidence can be found in the cyclic voltammograms of the SA-CM masked and unmasked GOFME electrodes before and after their implantation in the brain of a living rat for 2 h. The redox peak current of [Fe(CN)6]3−/4− at SA-CM/GOFME retained approximately 88.2% ± 4.8% of its initial value, while that of unprotected GOFME lost more than a half (Fig. 2b and Fig. S11 in Supporting information). Additionally, the influence of SA-CM membrane toward electrochemical performance of the GOFME was investigated. As shown in Fig. S12 (Supporting information), there is no obvious change for the shape of the CV curve before and after SA-CM masking, which indicated that the SA-CM membrane does not impede either electron transport or mass transport during the electrochemical process. After 50 CV cycles, the curve overlays almost exactly with the initial one (Fig. S13 in Supporting information), demonstrating the good stability of SA-CM/GOFME. We also investigated the adsorption of protein on different microelectrodes through fluorescence imaging. After the microelectrodes were incubated in a solution of BSA labeled with fluorescein isothiocyanate (FITC-BSA) for 2 h, CdS@ZnO/GOFME exhibited bright fluorescence (Fig. 2c) because of the adsorption of FITC-BSA. The fluorescence signals of CdS@ZnO/GOFME coated with SA (Fig. 2d), CM (Fig. 2e), and SA-CM (Fig. 2f) decreased in turn, which suggested the increasing anti-biofouling ability of these microelectrodes and was in accordance with the above PEC measurements (Fig. 2a). Such anti-biofouling ability can also be confirmed by the formation of hydrophilic interface [29, 30]. As shown in Figs. 2g–j, the changes of water contact angle on four ITO electrodes were consistent with the photocurrent reservation and the FITC-BSA adsorption.

With the anti-biofouling OF-based microelectrode in hand, we evaluated its response to Cu2+ under visible light excitation (460–480 nm LED). As shown in Fig. 3a, ZnO/GOFME did not generate an obvious signal since ZnO with a band gap of 3.37 eV hardly respond to visible light. Compared with CdS alone, CdS@ZnO/GOFME presented a dramatically increased photocurrent response, due to the well-matched band alignment between ZnO and CdS. Which is beneficial to the fast separation of photogenerated carriers and hence inhibits their recombination. Then, the photocurrent response slightly reduced after the coating of the anti-biofouling layer. Upon the introduction of Cu2+, the photocurrent intensity of SA-CM/CdS@ZnO/GOFME decreased remarkably, which can be ascribed to the formation of CuS and accordingly the suppression of electron transfer from the photoactive material to the electrode, following the mechanism depicted in Fig. 3b [31, 32]. Note that the given sensor is not reusable in this case since the ion replacement reaction between Cu2+ and CdS to form CuS is not reversible. It is worth pointing out that Cu+ was also reported to react with CdS to form Cu2S, which would be detrimental to Cu2+ detection [33]. Our experiments showed that Cu+ indeed reduced the photocurrent signal of SA-CM/CdS@ZnO/GOFME as well. But the large difference between the kinetics of the two reactions still enabled the sensing of Cu2+ without interference from Cu+. As shown in Figs. S14 and S15 (Supporting information), the photocurrent signal of the microsensor did not change until 10 min for Cu+, whereas for Cu2+ it was completed within 5 min and reached to a plateau.
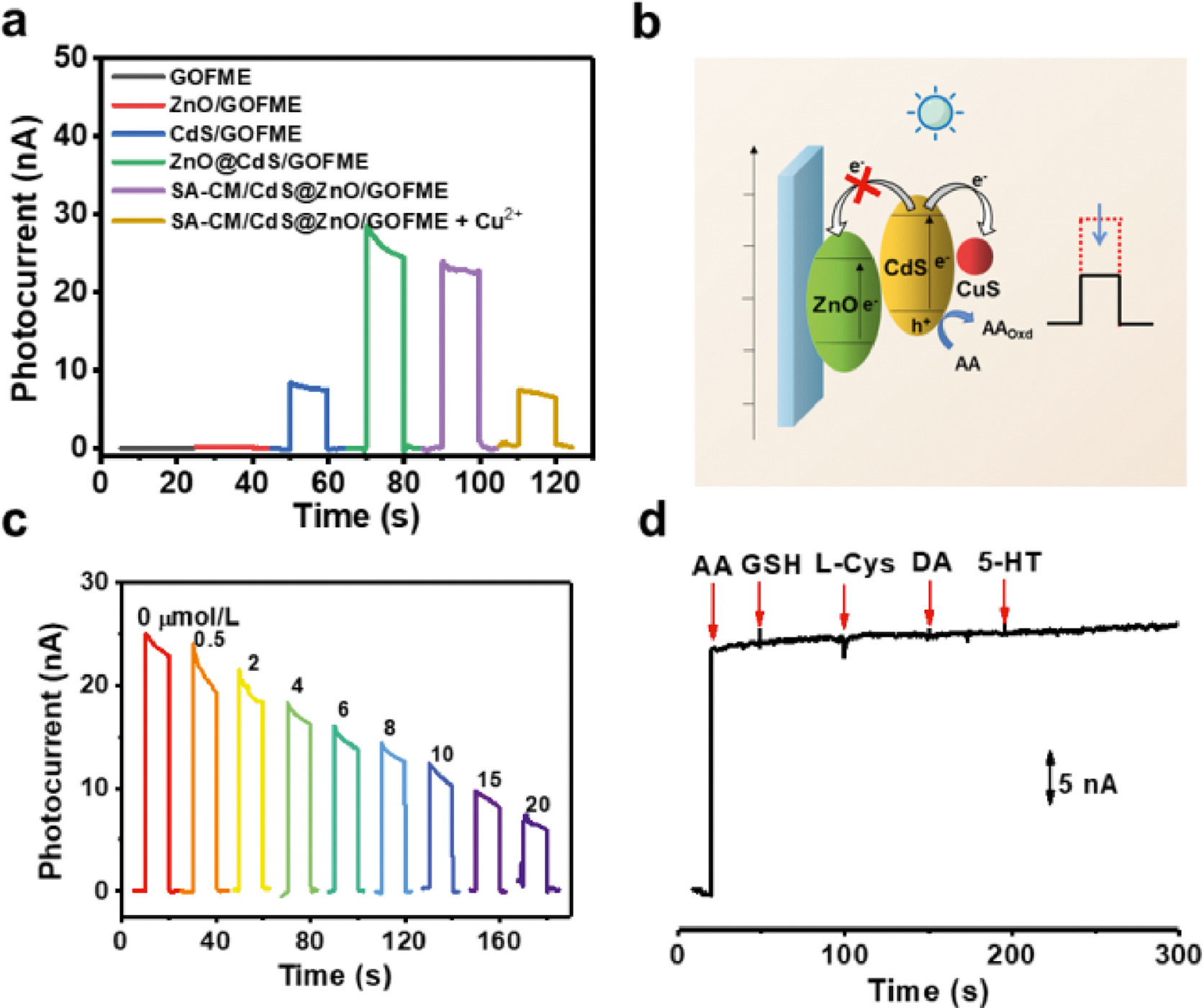
Solution assay of Cu2+ exhibited a concentration-dependent decrease of photocurrent (Fig. 3c), from which a good linearity can be obtained in the range of 0.5–15 µmol/L with a correlation coefficient (R2) of 0.9934 (Fig. S16 in Supporting information), and the detection limit was estimated to be 0.46 µmol/L (data from independent experiments are presented as mean ± standard deviation, n = 5). Such linear range is just suitable for the in vivo analysis of extracellular brain Cu2+, which is reported to be 2–10 µmol/L [34]. The selectivity of the PEC microsensor against other metal ions and small molecules potentially coexisting in biological environments was evaluated (Fig. S17 in Supporting information). There was no detectable difference in photocurrent before and after addition of the interfering species, even when some interferents were at a concentration much higher than that in the body. As known, ascorbic acid (AA) usually acts as the electron donor in PEC sensing [7]. Therefore, the influence of AA concentration on PEC signal needs to be examined. As shown in Fig. S18 (Supporting information), the photocurrent increased with AA concentration in the range of 0–100 µmol/L, whereas it remained constant at higher concentrations (100–600 µmol/L). Since the physiological level of AA in the brain is significantly higher than 100 µmol/L [34], the signal of this PEC sensor would not be disturbed by possible variation of AA concentration. In addition, we also confirmed that other endogenous reductive species including glutathione (GSH), cysteine (Cys), dopamine (DA) and 5-hydroxytryptamine (5-HT) did not induce perceptible changes of photocurrent in the presence of AA (Fig. 3d), since AA has lower oxidation potential than these reductive species (Fig. S19 in Supporting information). Beside, the signal of this PEC sensor was insensitive to pH in the range of 5.0–9.0 (Fig. S20 in Supporting information). The stability of the sensor was inspected by monitoring the variation of photocurrent. The microsensor exhibited rapid and stable response in artificial cerebrospinal fluid (aCSF) containing 200 µmol/L AA during 10 continuous cycles of irradiation (Fig. S21 in Supporting information). Reproducibility is also a key element of the electrode performance, and the results in Fig. S22 (Supporting information) indicated a good reproducibility (the relative standard deviation, RSD < 5.6%) of the microelectrodes. We also examined the performance of SA-CM/CdS@ZnO/GOFME in vivo before proceeding to analysis in the brains. Under 10 continuous on-off-on cycles of irradiation, the photocurrent signal remained unchanged in the rat brain (Fig. S23 in Supporting information). Furthermore, the standard deviation for five microelectrodes tested in the same rat brain was < 6.0% (Fig. S24 in Supporting information). These results indicated a satisfactory reproducibility and stability of the as-prepared microsensor both in vitro and in vivo. Moreover, the cytotoxicity of the SA-CM/CdS@ZnO film was assessed by culturing U87 cells on its surface. As verified by fluorescence staining (Fig. S25 in Supporting information), the cells growing on the SA-CM/CdS@ZnO surface showed high viability. Photothermal effect of the microelectrode was investigated by an infrared thermal imaging camera. The results showed no change in temperature after irradiation for 10 s (Fig. S26 in Supporting information), precluding photodamage to bio-tissues. All these results indicated a good biocompatibility of the sensing materials. Lastly, to validate the feasibility of this sensor for in vivo assay, exogenous Cu2+ added by local microinjection was detected in rat brain. As shown in Fig. S27 (Supporting information), the photocurrent decreased obviously with the increase of Cu2+ concentration.
Cu2+ is known to participate in a variety of neurochemical processes. Insufficient or excessive Cu2+ in the central nervous system (CNS) would both cause neurological disorders [35-37]. Herein, the PEC microsensor was applied for sensing the changes of extracellular Cu2+ in separate rat brains during middle cerebra artery occlusion (MCAO) and reperfusion. 2, 3, 5-Triphenyltetrazolium chloride (TTC) staining was performed to validate the cerebral ischemia injury after MCAO surgery, where the infarct volume progressively expanded with the prolongation of ischemia duration (Fig. S28 in Supporting information). By virtue of the lossless and zero-distance in-situ light excitation, the microelectrode would be capable of detecting arbitrarily brain regions, particularly the deep brain regions that are hard to reach with external light as excitation source. Therefore, we detected three different brain regions with different depths (Fig. 4a): Primary motor cortex (M1), hippocampal CA1 (CA1) and striatum caudate-putamen (CPu). Firstly, we found Cu2+ ions in the three regions at normal state were at the same level (the sham group in Figs. 4b and c), which corroborated previous findings [38]. After ischemia for 1 h, the photocurrent from M1, CA1 and CPu significantly decreased by approximately 37.8%, 39.4% and 42.1% compared to sham, respectively, implying the accumulation of Cu2+ during early ischemia. After that, the photocurrent maintained almost unchanged with ischemia extended to 1.5 h and 2 h, indicating the steady state of Cu2+ in the later stage of ischemia. These results were in accordance with previous reports [39]. Our results also revealed the amounts of Cu2+ in the three brain regions do not have significant difference at all stages of ischemia.
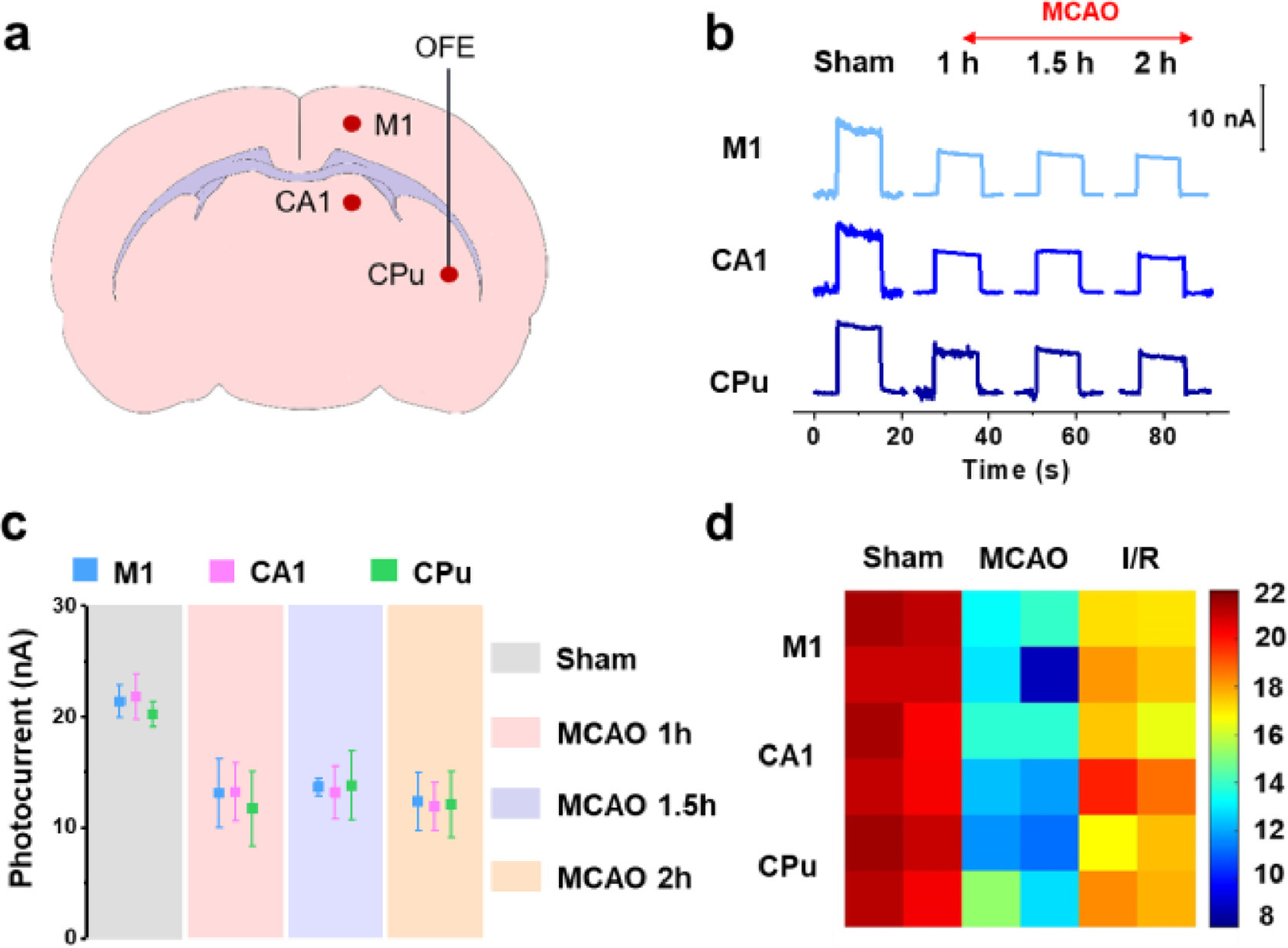
After 2 h of ischemia, reperfusion was achieved by drawing back the thread in the middle cerebral artery. The Cu2+ concentrations were detected 2 h post-reperfusion. Our results showed that the photocurrent intensities of all three brain regions markedly recovered (Fig. 4d), suggesting decreased amounts of Cu2+. In order to further confirm the regulation of Cu2+ during cerebral ischemia/reperfusion, the Cu2+ contents in tissues were measured by F-Cu2+, a reported fluorescent probe for Cu2+ which was synthesized and characterized (Figs. S29 and S30 in Supporting information). As shown in Fig. S31 (Supporting information), Cu2+ can convert this non-fluorescent probe into a fluorescent product, and the fluorescence intensity increased with increasing Cu2+ concentration (Fig. S32 in Supporting information). With the prolonged period of ischemia, the fluorescence intensities of F-Cu2+ in all three brain regions were enhanced, and as expected, they declined after the reperfusion (Fig. S33 in Supporting information). The results of fluorescence imaging also showed that Cu2+ concentrations in the three brain regions do not have significant difference, which was consistent to the PEC measurements, again verifying that the implantable OF-based photoelectrode is free of signal variation caused by depth-dependent light attenuation in vivo.
In summary, we fabricated an optical fiber-based microelectrode for in vivo PEC sensing, which integrated the function of a photoelectrode and an optical fiber. By implanting the microelectrode into the brain, the in-situ excitation of photoactive material was achieved, circumventing the limitation of penetration depth of light in living bodies. The OF-based PEC microsensor was successfully applied to monitor Cu2+ levels in rat brains of cerebral ischemia/reperfusion. In this way, PEC analysis for deep brain regions can be realized. Moreover, since a light at any region (UV to NIR) is applicable, extensive photoactive materials are available for PEC analysis, which will greatly promote the in vivo application of PEC sensing.
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
This work was financially supported by the National Natural Science Foundation of China (Nos. 21625503 and 21906047) and Natural Science Fund for Creative Research Groups of Hubei Province of China (No. 2020CFA035).
Supplementary material associated with this article can be found, in the online version, at doi:
D.E. Ingber, FASEB J. 20 (2006) 811–827.
doi: 10.1096/fj.05-5424rev
B. Ladoux, R.M. Mège, Nat. Rev. Mol. Cell Biol. 18 (2017) 743–757.
doi: 10.1038/nrm.2017.98
P.K. Viji Babu, C. Rianna, U. Mirastschijski, et al., Sci. Rep. 9 (2019) 12317.
doi: 10.1038/s41598-019-48566-7
M. Gómez-González, E. Latorre, M. Arroyo, et al., Nat. Rev. Phys. 2 (2020) 300–317.
doi: 10.1038/s42254-020-0184-6
X. Hu, F.M. Margadant, M. Yao, et al., Protein Sci. 26 (2017) 1337–1351.
doi: 10.1002/pro.3188
E.A.R. Morris, S. Bodin, B. Delaval, et al., Bio. Cell 109 (2017) 210–221.
doi: 10.1111/boc.201700001
S. Jain, V.M.L. Cachoux, G.H.N.S. Narayana, et al., Nat. Rev. Phys. 16 (2020) 802–809.
doi: 10.1038/s41567-020-0875-z
Y. Inoue, M. Suzuki, T. Watanabe, et al., Biomech. Model. Mech. 15 (2016) 1733–1746.
doi: 10.1007/s10237-016-0794-1
J.W. Song, L.L. Munn, Proc. Natl. Acad. Sci. U. S. A. 108 (2011) 15342–15347.
doi: 10.1073/pnas.1105316108
H. Zang, X. Li, Phys. Rev. E 101 (2020) 032406.
doi: 10.1103/PhysRevE.101.032406
G.M. Allen, K.C. Lee, E.L. Barnhart, et al., Cell Syst. 11 (2020) 286–299.
doi: 10.1016/j.cels.2020.08.008
S. Yokoyama, T.S. Matsui, S. Deguchi, Biochem. Bioph. Res. Co. 482 (2017) 975–979.
doi: 10.1016/j.bbrc.2016.11.142
S.R.K. Vedula, H. Hirata, M.H. Nai, et al., Nat. Mater. 13 (2014) 87–96.
doi: 10.1038/nmat3814
E. Bazellières, V. Conte, A. Elosegui-Artola, et al., Nat. Cell Biol. 17 (2015) 409–420.
doi: 10.1038/ncb3135
Z. Liu, J.L. Tan, D.M. Cohen, C.S. Chen, Proc. Natl. Acad. Sci. U. S. A. 107 (2010) 9944–9949.
doi: 10.1073/pnas.0914547107
G. Charras, A.S. Yap, Curr. Biol. 28 (2018) R445–R457.
doi: 10.1016/j.cub.2018.02.003
A. Ray, O. Lee, Z. Win, et al., Nat. Commun. 8 (2017) 14923.
doi: 10.1038/ncomms14923
C.M. Nelson, R.P. Jean, J.L. Tan, et al., Proc. Natl. Acad. Sci. U. S. A. 102 (2005) 11594–11599.
doi: 10.1073/pnas.0502575102
J.T. Parsons, A.R. Horwitz, M.A. Schwartz, Nat. Rev. Mol. Cell Biol. 11 (2010) 633–643.
doi: 10.1038/nrm2957
X.H. Wang, F. Yang, J.B. Pan, et al., Anal. Chem. 92 (2020) 16180–16187.
doi: 10.1021/acs.analchem.0c03935
B. Zhao, C. O'Brien, A.P.K.K.K. Mudiyanselage, et al., J. Am. Chem. Soc. 139 (2017) 18182–18185.
doi: 10.1021/jacs.7b11176
Y. Chang, Z. Liu, Y. Zhang, et al., J. Am. Chem. Soc. 138 (2016) 2901–2904.
doi: 10.1021/jacs.5b11602
V.P.Y. Ma, K. Salaita, Small 15 (2019) 1900961.
doi: 10.1002/smll.201900961
S.R.K. Vedula, M.C. Leong, T.L. Lai, et al., Proc. Natl. Acad. Sci. U. S. A. 109 (2012) 12974–12979.
doi: 10.1073/pnas.1119313109
W. Xi, S. Sonam, T. B. Saw, et al., Nat. Commun. 8 (2017) 1517.
doi: 10.1038/s41467-017-01390-x
R. Mayor, S. Etienne-Manneville, Nat. Rev. Mol. Cell Biol. 17 (2016) 97–109.
doi: 10.1038/nrm.2015.14
J.G. Peacock, B.A. Couch, A.J. Koleske, Cytoskeleton 67 (2010) 666–675.
doi: 10.1002/cm.20479
Y. Li, L. Yao, Y. Mori, et al., Proc. Natl. Acad. Sci. U. S. A. 116 (2019) 23894–23900.
doi: 10.1073/pnas.1907625116
M. Tantama, J.R. Martínez-François, R. Mongeon, et al., Nat. Commun. 4 (2013) 2550.
J. Zhang, F. Goliwas Kayla, W. Wang, et al., Proc. Natl. Acad. Sci. U. S. A. 116 (2019) 7867–7872.
doi: 10.1073/pnas.1809964116
M.R. Zanotelli, A. Rahman-Zaman, J.A. VanderBurgh, et al., Nat. Commun. 10 (2019) 4185.
doi: 10.1038/s41467-019-12155-z
T. Isogai, J.S. Park, G. Danuser, Nat. Cell Bio. 19 (2017) 591–593.
D.E. Ingber, FASEB J. 20 (2006) 811–827.
doi: 10.1096/fj.05-5424rev
B. Ladoux, R.M. Mège, Nat. Rev. Mol. Cell Biol. 18 (2017) 743–757.
doi: 10.1038/nrm.2017.98
P.K. Viji Babu, C. Rianna, U. Mirastschijski, et al., Sci. Rep. 9 (2019) 12317.
doi: 10.1038/s41598-019-48566-7
M. Gómez-González, E. Latorre, M. Arroyo, et al., Nat. Rev. Phys. 2 (2020) 300–317.
doi: 10.1038/s42254-020-0184-6
X. Hu, F.M. Margadant, M. Yao, et al., Protein Sci. 26 (2017) 1337–1351.
doi: 10.1002/pro.3188
E.A.R. Morris, S. Bodin, B. Delaval, et al., Bio. Cell 109 (2017) 210–221.
doi: 10.1111/boc.201700001
S. Jain, V.M.L. Cachoux, G.H.N.S. Narayana, et al., Nat. Rev. Phys. 16 (2020) 802–809.
doi: 10.1038/s41567-020-0875-z
Y. Inoue, M. Suzuki, T. Watanabe, et al., Biomech. Model. Mech. 15 (2016) 1733–1746.
doi: 10.1007/s10237-016-0794-1
J.W. Song, L.L. Munn, Proc. Natl. Acad. Sci. U. S. A. 108 (2011) 15342–15347.
doi: 10.1073/pnas.1105316108
H. Zang, X. Li, Phys. Rev. E 101 (2020) 032406.
doi: 10.1103/PhysRevE.101.032406
G.M. Allen, K.C. Lee, E.L. Barnhart, et al., Cell Syst. 11 (2020) 286–299.
doi: 10.1016/j.cels.2020.08.008
S. Yokoyama, T.S. Matsui, S. Deguchi, Biochem. Bioph. Res. Co. 482 (2017) 975–979.
doi: 10.1016/j.bbrc.2016.11.142
S.R.K. Vedula, H. Hirata, M.H. Nai, et al., Nat. Mater. 13 (2014) 87–96.
doi: 10.1038/nmat3814
E. Bazellières, V. Conte, A. Elosegui-Artola, et al., Nat. Cell Biol. 17 (2015) 409–420.
doi: 10.1038/ncb3135
Z. Liu, J.L. Tan, D.M. Cohen, C.S. Chen, Proc. Natl. Acad. Sci. U. S. A. 107 (2010) 9944–9949.
doi: 10.1073/pnas.0914547107
G. Charras, A.S. Yap, Curr. Biol. 28 (2018) R445–R457.
doi: 10.1016/j.cub.2018.02.003
A. Ray, O. Lee, Z. Win, et al., Nat. Commun. 8 (2017) 14923.
doi: 10.1038/ncomms14923
C.M. Nelson, R.P. Jean, J.L. Tan, et al., Proc. Natl. Acad. Sci. U. S. A. 102 (2005) 11594–11599.
doi: 10.1073/pnas.0502575102
J.T. Parsons, A.R. Horwitz, M.A. Schwartz, Nat. Rev. Mol. Cell Biol. 11 (2010) 633–643.
doi: 10.1038/nrm2957
X.H. Wang, F. Yang, J.B. Pan, et al., Anal. Chem. 92 (2020) 16180–16187.
doi: 10.1021/acs.analchem.0c03935
B. Zhao, C. O'Brien, A.P.K.K.K. Mudiyanselage, et al., J. Am. Chem. Soc. 139 (2017) 18182–18185.
doi: 10.1021/jacs.7b11176
Y. Chang, Z. Liu, Y. Zhang, et al., J. Am. Chem. Soc. 138 (2016) 2901–2904.
doi: 10.1021/jacs.5b11602
V.P.Y. Ma, K. Salaita, Small 15 (2019) 1900961.
doi: 10.1002/smll.201900961
S.R.K. Vedula, M.C. Leong, T.L. Lai, et al., Proc. Natl. Acad. Sci. U. S. A. 109 (2012) 12974–12979.
doi: 10.1073/pnas.1119313109
W. Xi, S. Sonam, T. B. Saw, et al., Nat. Commun. 8 (2017) 1517.
doi: 10.1038/s41467-017-01390-x
R. Mayor, S. Etienne-Manneville, Nat. Rev. Mol. Cell Biol. 17 (2016) 97–109.
doi: 10.1038/nrm.2015.14
J.G. Peacock, B.A. Couch, A.J. Koleske, Cytoskeleton 67 (2010) 666–675.
doi: 10.1002/cm.20479
Y. Li, L. Yao, Y. Mori, et al., Proc. Natl. Acad. Sci. U. S. A. 116 (2019) 23894–23900.
doi: 10.1073/pnas.1907625116
M. Tantama, J.R. Martínez-François, R. Mongeon, et al., Nat. Commun. 4 (2013) 2550.
J. Zhang, F. Goliwas Kayla, W. Wang, et al., Proc. Natl. Acad. Sci. U. S. A. 116 (2019) 7867–7872.
doi: 10.1073/pnas.1809964116
M.R. Zanotelli, A. Rahman-Zaman, J.A. VanderBurgh, et al., Nat. Commun. 10 (2019) 4185.
doi: 10.1038/s41467-019-12155-z
T. Isogai, J.S. Park, G. Danuser, Nat. Cell Bio. 19 (2017) 591–593.

Haixian Ren , Yuting Du , Xiaojing Yang , Fangjun Huo , Le Zhang , Caixia Yin . Development of ESIPT-based specific fluorescent probes for bioactive species based on the protection-deprotection of the hydroxyl. Chinese Chemical Letters, 2025, 36(2): 109867-. doi: 10.1016/j.cclet.2024.109867
Kezuo Di , Jie Wei , Lijun Ding , Zhiying Shao , Junling Sha , Xilong Zhou , Huadong Heng , Xujing Feng , Kun Wang . A wearable sensor device based on screen-printed chip with biofuel cell-driven electrochromic display for noninvasive monitoring of glucose concentration. Chinese Chemical Letters, 2025, 36(2): 109911-. doi: 10.1016/j.cclet.2024.109911
Lixian Fu , Yiyun Tan , Yue Ding , Weixia Qing , Yong Wang . Water–soluble and polarity–sensitive near–infrared fluorescent probe for long–time specific cancer cell membranes imaging and C. Elegans label. Chinese Chemical Letters, 2024, 35(4): 108886-. doi: 10.1016/j.cclet.2023.108886
Yunan Yuan , Zhimin Luo , Jie Chen , Chaoliang He , Kai Hao , Huayu Tian . Constructing thermoresponsive PNIPAM-based microcarriers for cell culture and enzyme-free cell harvesting. Chinese Chemical Letters, 2024, 35(7): 109549-. doi: 10.1016/j.cclet.2024.109549
Gongcheng Ma , Qihang Ding , Yuding Zhang , Yue Wang , Jingjing Xiang , Mingle Li , Qi Zhao , Saipeng Huang , Ping Gong , Jong Seung Kim . Palladium-free chemoselective probe for in vivo fluorescence imaging of carbon monoxide. Chinese Chemical Letters, 2024, 35(9): 109293-. doi: 10.1016/j.cclet.2023.109293
Weiyu Chen , Zenghui Li , Chenguang Zhao , Lisha Zha , Junfeng Shi , Dan Yuan . Enzyme-modulate conformational changes in amphiphile peptide for selectively cell delivery. Chinese Chemical Letters, 2024, 35(12): 109628-. doi: 10.1016/j.cclet.2024.109628
Xu Qu , Pengzhao Wu , Kaixuan Duan , Guangwei Wang , Liang-Liang Gao , Yuan Guo , Jianjian Zhang , Donglei Shi . Self-calibrating probes constructed on a unique dual-emissive fluorescence platform for the precise tracking of cellular senescence. Chinese Chemical Letters, 2024, 35(12): 109681-. doi: 10.1016/j.cclet.2024.109681
Zhoupeng Zheng , Shengyi Gong , Qianhua Li , Shiya Zhang , Guoqiang Feng . Lipid droplets and gallbladder targeted fluorescence probe for ratiometric NO imaging in gallstones disease models. Chinese Chemical Letters, 2025, 36(5): 110191-. doi: 10.1016/j.cclet.2024.110191
Kun-Heng Li , Hong-Yang Zhao , Dan-Dan Wang , Ming-Hui Qi , Zi-Jian Xu , Jia-Mi Li , Zhi-Li Zhang , Shi-Wen Huang . Mitochondria-targeted nano-AIEgens as a powerful inducer for evoking immunogenic cell death. Chinese Chemical Letters, 2024, 35(5): 108882-. doi: 10.1016/j.cclet.2023.108882
Yang Liu , Yan Liu , Kaiyin Yang , Zhiruo Zhang , Wenbo Zhang , Bingyou Yang , Hua Li , Lixia Chen . A selective HK2 degrader suppresses SW480 cancer cell growth by degrading HK2. Chinese Chemical Letters, 2024, 35(8): 109264-. doi: 10.1016/j.cclet.2023.109264
Boran Cheng , Lei Cao , Chen Li , Fang-Yi Huo , Qian-Fang Meng , Ganglin Tong , Xuan Wu , Lin-Lin Bu , Lang Rao , Shubin Wang . Fluorine-doped carbon quantum dots with deep-red emission for hypochlorite determination and cancer cell imaging. Chinese Chemical Letters, 2024, 35(6): 108969-. doi: 10.1016/j.cclet.2023.108969
Jing Chen , Peisi Xie , Pengfei Wu , Yu He , Zian Lin , Zongwei Cai . MALDI coupled with laser-postionization and trapped ion mobility spectrometry contribute to the enhanced detection of lipids in cancer cell spheroids. Chinese Chemical Letters, 2024, 35(4): 108895-. doi: 10.1016/j.cclet.2023.108895
Yanjing Li , Jiayin Li , Yuqi Chang , Yunfeng Lin , Lei Sui . Tetrahedral framework nucleic acids promote the proliferation and differentiation potential of diabetic bone marrow mesenchymal stem cell. Chinese Chemical Letters, 2024, 35(9): 109414-. doi: 10.1016/j.cclet.2023.109414
Zhixue Liu , Haiqi Chen , Lijuan Guo , Xinyao Sun , Zhi-Yuan Zhang , Junyi Chen , Ming Dong , Chunju Li . Luminescent terphen[3]arene sulfate-activated FRET assemblies for cell imaging. Chinese Chemical Letters, 2024, 35(9): 109666-. doi: 10.1016/j.cclet.2024.109666
Ying Gao , Rong Zhou , Qiwen Wang , Shaolong Qi , Yuanyuan Lv , Shuang Liu , Jie Shen , Guocan Yu . Natural killer cell membrane doped supramolecular nanoplatform with immuno-modulatory functions for immuno-enhanced tumor phototherapy. Chinese Chemical Letters, 2024, 35(10): 109521-. doi: 10.1016/j.cclet.2024.109521
Yuanzheng Wang , Chen Zhang , Shuyan Han , Xiaoli Kong , Changyun Quan , Jun Wu , Wei Zhang . Cancer cell membrane camouflaged biomimetic gelatin-based nanogel for tumor inhibition. Chinese Chemical Letters, 2024, 35(11): 109578-. doi: 10.1016/j.cclet.2024.109578
Zheyi Li , Xiaoyang Liang , Zitong Qiu , Zimeng Liu , Siyu Wang , Yue Zhou , Nan Li . Ion-interferential cell cycle arrest for melanoma treatment based on magnetocaloric bimetallic-ion sustained release hydrogel. Chinese Chemical Letters, 2024, 35(11): 109592-. doi: 10.1016/j.cclet.2024.109592
Qian Ren , Xue Dai , Ran Cen , Yang Luo , Mingyang Li , Ziyun Zhang , Qinghong Bai , Zhu Tao , Xin Xiao . A cucurbit[8]uril-based supramolecular phosphorescent assembly: Cell imaging and sensing of amino acids in aqueous solution. Chinese Chemical Letters, 2024, 35(12): 110022-. doi: 10.1016/j.cclet.2024.110022
Zhi Li , Shuya Pan , Yuan Tian , Shaowei Liu , Weifeng Wei , Jinlin Wang , Tianfeng Chen , Ling Wang . Selenium nanoparticles enhance the chemotherapeutic efficacy of pemetrexed against non-small cell lung cancer. Chinese Chemical Letters, 2024, 35(12): 110018-. doi: 10.1016/j.cclet.2024.110018
Yanfei Liu , Yaqin Hu , Yifu Tan , Qiwen Chen , Zhenbao Liu . Tumor acidic microenvironment activatable DNA nanostructure for precise cancer cell targeting and inhibition. Chinese Chemical Letters, 2025, 36(1): 110289-. doi: 10.1016/j.cclet.2024.110289