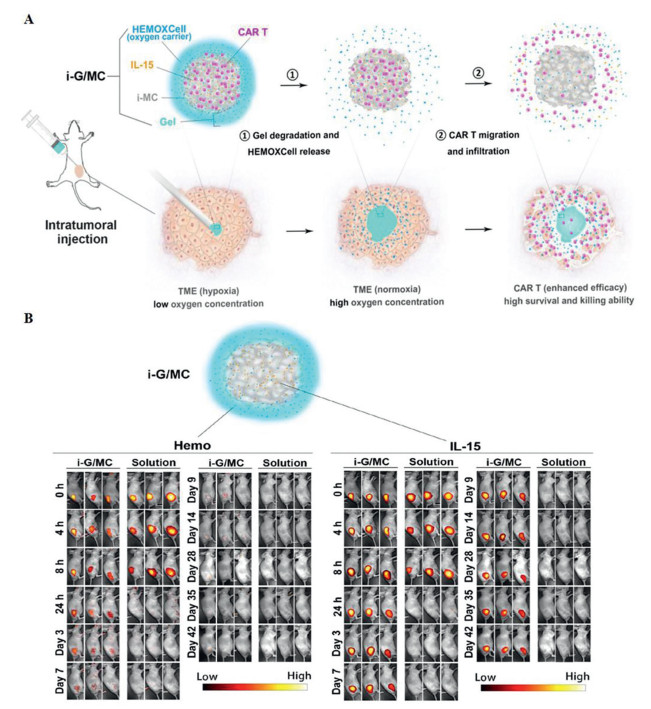
Precise tumor targeting and on-demand drug release show great potentials in cancer therapy, which have been achieved by the continuously emerging smart prodrugs activated by the specific stimuli [1]. Most of the reported activatable prodrugs (>80%) are sensitive to a single trigger, including the tumor microenvironments (TMEs) like pH [2], glutathione (GSH) [3,4], reactive oxygen species (ROS) [5,6] and enzyme [7], or the external triggers like light, ultrasound, heat, and magnetic field [8,9]. However, in most of the cases, the nonspecific drug accumulation or activation in normal tissues is inevitable because some TMEs are not “specific” enough to sensitively differentiate tumor and normal tissues [10]. Applying “logic gate” involving multiple triggers into the molecular structures can remarkably magnify the specific tumor targeting and attenuate the undesired toxicity via the introduction of multistage “locks” and sophisticating the “unlock” conditions [11]. More than two stimuli co-govern the “on and off” of the drug, exhibiting “OR gate” or “AND gate” features [12]. Most of the reported prodrugs with logic gates are “OR gates”, that is, drugs can be partially activated by either stimulus [13,14]. Even though sometimes the tumor targeting and anti-cancer efficacy can be magnified upon the combination of multiple stimuli, the partial activation feature of the “OR gate” determines the toxicity induced by single stimulus cannot be thoroughly avoided [15]. Compared to “OR gate”, “AND gate” endows the prodrug with the “only” activation upon the co-existence of all the stimuli [16,17], which was first reported in the design of a boron dipyrromethene responsive to both Na+ and H+ ions by Akkaya [18]. However, due to the molecule synthesis and design challenging, the “AND gate” is infrequent among numerous prodrugs.
Among the stimuli, a combination of external stimulus and internal stimulus owns advanced strengths due to the tumor-specific and spatio-temporal control features. Ultrasound (US) as a mechanical force has been applied into the cancer treatment as an external stimulus, called sonodynamic therapy (SDT) [19]. Sonosensitizers bearing macrocyclic structures like porphyrin and phthalocyanine, can be activated under low-intensity US irradiation to produce highly toxic ROS through the sonoluminescence mechanism [20,21]. Compared to other treatments, SDT owns the advantages of spatio-temporal control, noninvasiveness, minor energy attenuation, and deep tissue-penetration [22,23]. However, the US-triggered “AND gate” prodrug has been rarely reported due to the following challenges. First, unlike the pro-chemodrug which the drug activation is based on the transformation or exposure of specific functional groups upon the triggers, it is hard to inhibit the sonosensitizers’ toxicity upon US trigger via simple change of functional groups [24,25]. As the toxicity of sonosensitizers is mainly originated from the US's energy and sonoluminescence, Förster resonance energy transfer (FRET) effect which has been widely applied in the design of dyes, is a promising strategy to suppress the toxicity from the “only” US irradiation [26]. Second, killing of the tumor cells for SDT greatly relies on the ROS (singlet oxygen (1O2) and oxygen-based radicals (•OH)) produced by the US-irradiated sonosensitizers. Limited by the tolerate dosage and intracellular uptake level of the sonosensitizers, as well as the oxidative ability and life time of the ROS, the anticancer efficacy for SDT cannot reach as good as that for chemotherapy or radiotherapy [27,28]. Therefore, stronger ROS agents or toxic agents are extremely needed to boost the cytotoxicity.
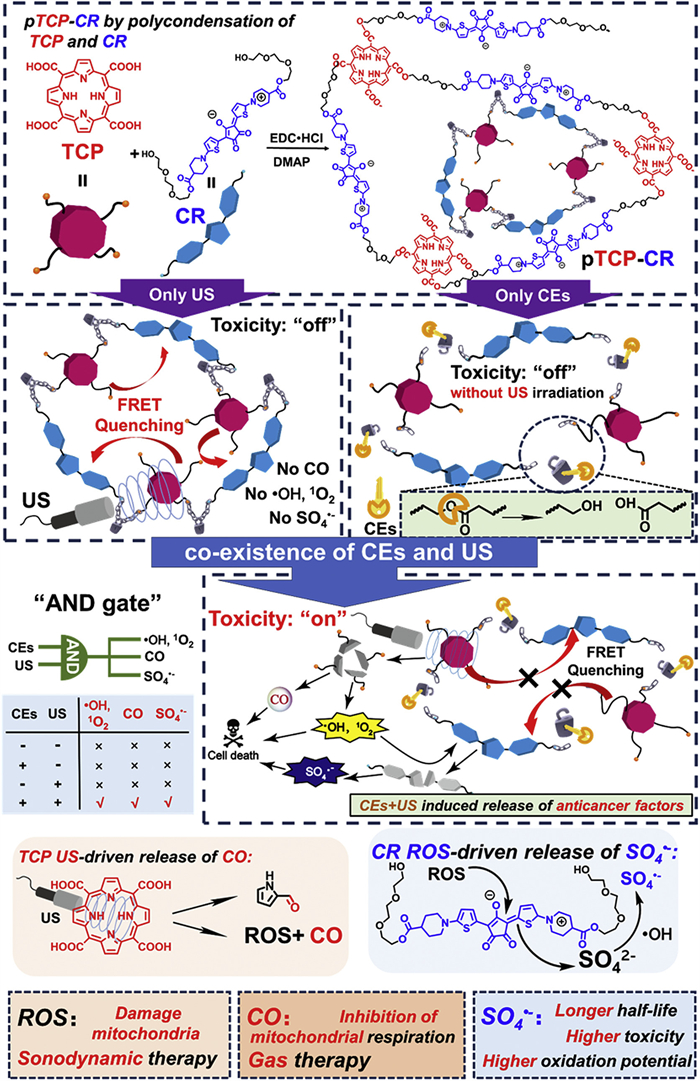
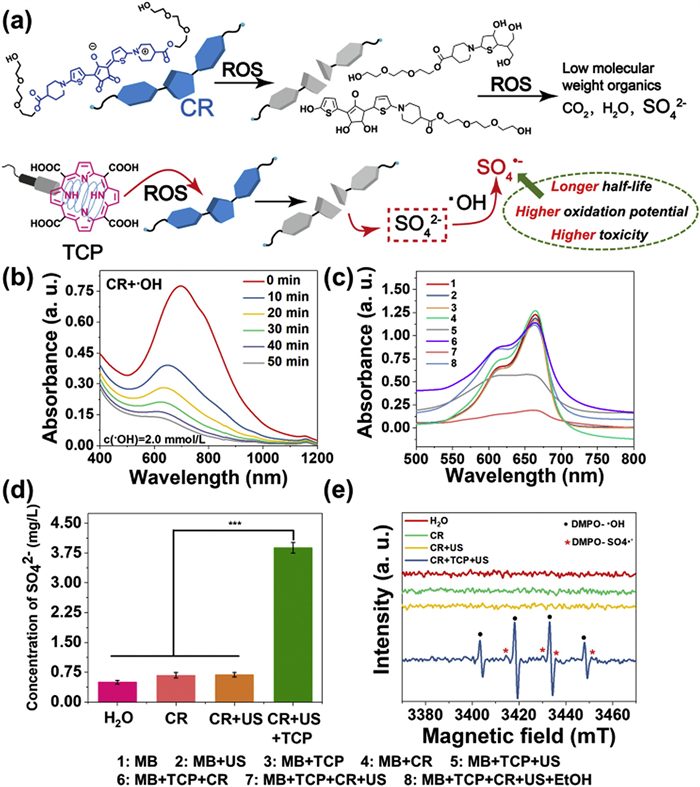
Considering the above-mentioned two issues in the design of “AND gate” SDT prodrugs, we specially design two monomers including a meso‑carboxyl-porphyrin-based sonosensitizer (5,10,15,20-tetrakis(carboxyl)porphyrin, TCP) and a croconium (thiophenyl-croconium (2,5-bis[(2-(2-(2-hydroxyethoxy)ethoxy)ethyl-4-carboxylate-piperidylamino)thiophenyl]-croconium, CR), as well as their polymers (pTCP-CR) to solve all the listed obstacles in a US and enzyme co-activated system. 5,10,15,20-Tetrakis(carboxyl)porphyrin (TCP) was selected since it can release ROS and carbon monoxide (CO) with US irradiation [29]. To further improve the toxicity of the ROS generated by SDT, sulfate radicals (SO4•−) with stronger oxidation capability are introduced into the molecule design. SO4•− has an oxidation potential of 2.5–3.1 eV, which is similar to that of •OH (2.80 eV), but the half-life (SO4•−, 30 µs) is 30 times versus •OH, which means that it has stronger cytotoxicity due to longer diffusion distance and leads to more effective oxidation reactions [30]. Recently, it has been revealed that SO4•− can be produced by sulfate ions (SO42−) and •OH, which is a mild method appliable in biomedical field [31]. Therefore, a specific CR dye bearing thiophene group is designed as a SO42− precursor since sulfur-containing dye owns the potential to release SO42− upon ROS treatment [32]. Third, the absorbance of the CR usually covers the emission of most porphyrin-based sonosensitizers upon US irradiation. Thus, CR can act as a quencher to lock the undesired US-induced ROS generated due to the FRET effect once it is connected with porphyrin via suitable linkers [26].
Although the instability and bleachability of squarylium dyes and CR dyes in the presence of metal ions or light have been reported [34], the phenomenon that CR dye can be degraded by ROS and transformed into active agents has not been reported. As sulfur-containing dyes owned the potentials to be converted to SO42− after being treated by ROS [32], CR bearing thiophenol group was selected as the special monomer for the releasing of surfur-based radicals. CR was synthesized by the nucleophilic substitution, alkali saponification/acid hydrolysis, condensation processes with croconic acid as shown in Scheme S2. All the intermediates and finally compounds were fully characterized (Figs. S1–S8 in Supporting information). It has been demonstrated before that US could trigger the porphyrin derivatives to generate •OH, similar with the porphyrin-based photodynamic therapy mechanism [35]. We then evaluated whether the ROS generated by the US and TCP would lead to the decomposition of CR into SO42− as well as more active SO4•− radicals (Fig. 1a). Adding •OH to a phosphate buffer saline (PBS) solution of CR led to a significant reduction in CR absorption in the near-infrared (NIR) region (around 700 nm) (Fig. 1b). 1H nuclear magnetic resonance spectroscopy (1H NMR) and high resolution mass spectrometry (HRMS) analyses confirmed CR decomposition after •OH treatment for 20 and 50 min, with CR molecules breaking down into low molecular weight compounds (Figs. S9 and S10 in Supporting information). Additionally, we assessed if •OH generated by TCP could decompose CR. As anticipated, CR absorption in the NIR region decreased after US treatment of a mixed solution of TCP and CR in PBS (Fig. S11 in Supporting information), while minimal change occurred in the CR + US group and the CR+TCP group (Fig. S11). This suggested CR decomposition relied on ROS from TCP and US combination. Furthermore, high performance high performance (HPIC) was utilized to determine SO42− concentration in various CR solutions (only CR group, CR + US group, CR + TCP + US group, and deionized water group). Results showed a significantly higher SO42− concentration in the CR + TCP + US group (3.88 mg/L) compared to control groups (0.5–0.7 mg/L) (Fig. 1d, Fig. S12 and Table S1 in Supporting information).
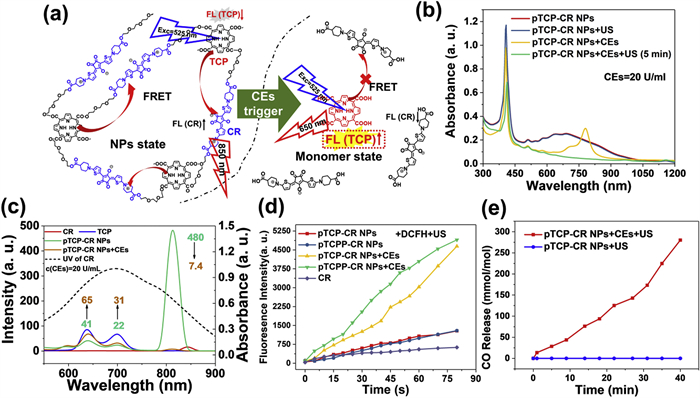
As a result, all the active agents were double-locked into pTCP-CR NPs, which could be only unlocked by the simultaneous existence of US and CEs (Scheme S4 in Supporting information). As shown in Fig. 2b, pTCP-CR NPs is processed by both US and CEs, both the TCP absorbance at 300–450 nm and CR absorbance at 600–1000 nm dramatically decreased. This indicates that both TCP and CR are decomposed after CEs/US dual processing. As a control, pTCP-CR NPs showed strong stability under only US or CEs irradiation (Fig. S25 in Supporting information). Subsequently, the ROS generation ability of CR, TCP + CR, pTCP-CR NPs and pTCPP-CR NPs in water upon different treatment conditions was evaluated. 1,3-Diphenylisobenzofuran (DPBF) and 2′,7′-dichlorodihyrofluorescein (DCFH) were used as 1O2 and ROS analytical reagent [43]. For CEs/US-co-treated pTCP-CR NPs and pTCPP-CR NPs, the absorption of DPBF (420 nm) decreased significantly with the prolongation of US treatment time, indicating the 1O2 generation upon US irradiation. As controls, if pTCP-CR NPs and pTCPP-CR NPs were only irradiation by US without CEs treatment, the absorption of DPBF did not change and no generation of 1O2 (Fig. S26 in Supporting information). Such results were in consistent with the fluorescence results that FRET effect inhibited the generation of 1O2 from TCP unit. Apart from DPBF, DCFH as a fluorescent probe, was also selected to demonstrate it. Compared with the only US triggered group, CEs/US co-triggered pTCP-CR NPs or pTCPP-CR NPs showed notable stronger fluorescence intensity, revealing the “on and off” of ROS was fully determined by the co-existence of CEs and US (Fig. 2d and Fig. S27 in Supporting information).
The CEs/US co-triggered CO release ability of pTCP-CR NPs was quantified by hemoglobin method, 9-(diethylamino)−5H-benzo[a]phenoxazin-5-one palladium complex (1-Ac) CO fluorescence probe method and CO gas sensor method. UV spectra indicated conversion of Hb-Fe(Ⅱ) to Hb-Fe(CO) after incubating CEs-treated pTCP-CR NPs with hemoglobin under US treatment. Fluorescence intensity of CEs-treated pTCP-CR NPs with 1-Ac increased significantly under US treatment, confirming CO release (Fig. S28 in Supporting information). CO gas sensor method quantified CO release at 280 mmol CO/mol TCP after 40 min of US treatment. No CO release was observed in pTCP-CR NPs treated only with US irradiation (Fig. 2e). Similar results were observed with pTCP-HG NPs (Fig. S28). Additionally, SO42− release from pTCP-CR NPs under CEs/US dual activation was significantly higher compared to other groups (Fig. S29 in Supporting information). In summary, the co-existence of CEs and US triggered the generation of CO, 1O2, and SO4•−.
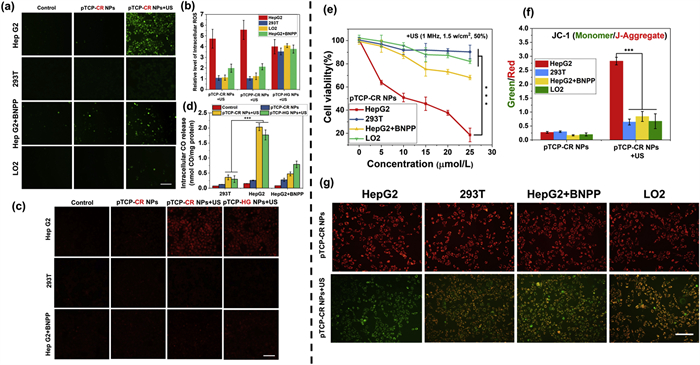
DCFH-diacetate (DA) and 1-Ac were furthermore used to detect the intracellular ROS and CO. In HepG2 cells, both pTCP-CR NPs and pTCPP-CR NPs showed green fluorescence upon US irradiation, while fluorescence dropped significantly in 293T and LO2 with or without US (Fig. 3a, Figs. S31 and S32 in Supporting information). Sole US treatment did not induce green fluorescence. The US-triggered ROS generation of pTCP-HG NPs was not influenced by cell type, blurring the tumor-normal cell distinction (Fig. S32). Adding CEs inhibitor bis-p-nitrophenyl phosphate (BNPP) to HepG2 cells reduced green fluorescence in pTCP-CR NPs + US and pTCPP-CR NPs + US groups, but not in pTCP-HG NPs + US group (Fig. 3b and Fig. S32). This aligns with ROS generation tuned by CEs and US co-existence, targeting toxicity to tumor cells under US. Similarly, intracellular CO release upon US showed CEs/US dependency. HepG2 cells incubated with pTCP-CR NPs and pTCP-HG NPs displayed intracellular CO production after US, evidenced by strong red fluorescence using 1-Ac (Figs. 3c and d, Fig. S33 in Supporting information). No CO was detected in 293T cells or HepG2 cells treated with BNPP. Thus, pTCP-CR NPs specifically generate ROS and CO only with CEs and US co-existence, targeting high CEs tumor cells under US.
The MTT assay evaluated the toxicity of pTCP-CR NPs, pTCPP-CR NPs, and pTCP-HG NPs on cancer cells. All NPs showed low cytotoxicity without US irradiation, indicating their safety (Fig. 3e and Fig. S34 Supporting information). Upon US irradiation, HepG2 cells treated with pTCP-CR NPs and pTCPP-CR NPs exhibited significantly higher cytotoxicity (Fig. 3f and Fig. S35 in Supporting information). Pre-treatment of HepG2 cells with BNPP decreased therapeutic efficiency, consistent with results in cells with limited CEs expression. At 25 µmol/L concentration (porphyrin equivalent), cell mortality was significantly higher in the pTCP-CR NPs + US group (81.37% ± 3.27%) compared to pTCPP-CR NPs + US (59.77% ± 1.35%) due to CO gas therapy from the TCP moiety (Fig. 3f). Additionally, mortality was higher in the pTCP-CR NPs + US group than pTCP-HG NPs + US (64.36% ± 2.25%) due to more efficient oxidative toxicity of SO4•− from CR (Fig. S36 in Supporting information). These results demonstrate the contribution of both SDT and GT therapy to cell death, activated only by CEs and US. CO and ROS can induce mitochondrial dysfunction and lead to cell apoptosis by decreasing mitochondrial membrane potential (MMP). To assess the therapeutic effect, MMP reduction was measured using 5,5′,6,6′-tetrachloro-1,1′,3,3′-tetraethylbenzimidazolo carbocyanine iodide (JC-1) dye. Following US-triggered treatment, HepG2 cells showed a significant decrease in MMP, shifting JC-1 dye fluorescence from red to green, indicating mitochondrial dysfunction (Fig. 3g). The green/red fluorescence intensity ratio, indicative of mitochondrial damage, was highest in HepG2 cells treated with pTCP-CR NPs and US, demonstrating the most effective mitochondrial damage compared to other cell lines (Figs. S36–S38 in Supporting information).
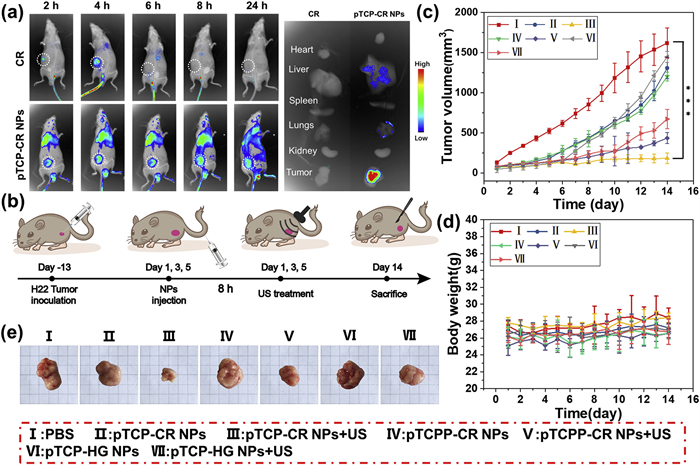
In summary, a CEs and US co-triggered polysonosensitizer pTCP-CR NPs with “AND gate” logic was developed, in which TCP with ROS and CO generation ability was polymerized with CR with FRET quenching effect and SO4•− generation capability via ester linkers. Either CEs or US itself could not “unlock” pTCP-CR NPs with the releasing of active agents while the anti-cancer efficacy could only be activated upon the co-existence of CEs and US. Such design could avoid the nonspecific targeting and drug activation in the normal tissues via the combination of CEs as the internal trigger and US as an external trigger. A spatio-temporal control of the toxicity activation could also be achieved. Upon activation by two triggers, advanced anti-cancer agents beyond traditional ROS from SDT were simultaneously generated due to the smart design of TCP and CR monomers. TCP monomer can not only generate traditional ROS upon US irradiation, but also be decomposed into CO as therapeutic gas. CR monomer can not only quench the toxicity of TCP at polymer state due to FRET effect, but also be decomposed into SO4•− as more toxic ROS for toxicity boosting. Post SDT treatment, pTCP-CR NPs can be thoroughly decomposed into low-toxic molecules for the fast clearance, avoiding phototoxic side-ffect. Such “AND gate” logic design provided a promising strategy for the precise tumor treatment with limited side-effect.
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
This work was supported by grants from the National Natural Science Foundation of China (No. 22375027), the Natural Science Foundation of Jiangsu Province (Nos. BK20221265, BK20211100), the Fundamental Research Funds for the Central Universities (No. DUT23YG133), and the Research Funds from Liaoning Cancer Hospital (No. 2024ZLKF-35). The authors acknowledge the assistance of DUT Instrumental Analysis Center.
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.cclet.2024.110032.
-
[1]
P. Vyas, S.E. Jacobsen, Cell Stem Cell 8 (2011) 242–244.
doi: 10.1016/j.stem.2011.02.015
-
[2]
C. Sordo-Bahamonde, M. Vitale, S. Lorenzo-Herrero, A. López-Soto, S. Gonzalez, Cancers 12 (2020) 893.
doi: 10.3390/cancers12040893
-
[3]
J.N. Kochenderfer, S.A. Rosenberg, Nat. Rev. Clin. Oncol. 10 (2013) 267–276.
doi: 10.1038/nrclinonc.2013.46
-
[4]
C.H. June, M. Sadelain, New Engl. J. Med. 379 (2018) 64–73.
doi: 10.1056/nejmra1706169
-
[5]
M.V. Maus, Nature 543 (2017) 48–49.
doi: 10.1038/nature21506
-
[6]
M.V. Maus, C.H. June, Clin. Cancer Res. 22 (2016) 1875–1884.
doi: 10.1158/1078-0432.CCR-15-1433
-
[7]
S. Stoiber, B.L. Cadilha, M.R. Benmebarek, et al., Cells 8 (2019) 472.
doi: 10.3390/cells8050472
-
[8]
Z. Walsh, Y. Yang, M.E. Kohler, Immunol. Rev. 290 (2019) 100–113.
doi: 10.1111/imr.12794
-
[9]
M.C. Burger, C. Zhang, P.N. Harter, et al., Front. Immunol. 10 (2019) 2683.
doi: 10.3389/fimmu.2019.02683
-
[10]
Y. Hu, Z.G. Tian, C. Zhang, Acta Pharmacol. Sin. 39 (2018) 167–176.
doi: 10.1038/aps.2017.125
-
[11]
Z. Eshhar, T. Waks, G. Gross, D.G. Schindler, Proc. Natl. Acad. Sci. U. S. A. 90 (1993) 720–724.
doi: 10.1073/pnas.90.2.720
-
[12]
D. Moritz, W. Wels, J. Mattern, B. Groner, Proc. Natl. Acad. Sci. U. S. A. 91 (1994) 4318–4322.
doi: 10.1073/pnas.91.10.4318
-
[13]
I. Stancovski, D.G. Schindler, T. Waks, et al., J. Immunol. 151 (1993) 6577–6582.
doi: 10.4049/jimmunol.151.11.6577
-
[14]
C. Imai, K. Mihara, M. Andreansky, et al., Leukemia 18 (2004) 676–684.
doi: 10.1038/sj.leu.2403302
-
[15]
A.A. Hombach, J. Heiders, M. Foppe, M. Chmielewski, H. Abken, Oncoimmunology 1 (2012) 458–466.
doi: 10.4161/onci.19855
-
[16]
M. Chmielewski, A.A. Hombach, H. Abken, Immunol. Rev. 257 (2014) 83–90.
doi: 10.1111/imr.12125
-
[17]
V. Hoyos, B. Savoldo, C. Quintarelli, et al., Leukemia 24 (2010) 1160–1170.
doi: 10.1038/leu.2010.75
-
[18]
B. Hu, J. Ren, Y. Luo, et al., Cell Rep. 20 (2017) 3025–3033.
doi: 10.1016/j.celrep.2017.09.002
-
[19]
M.C. O'Leary, X. Lu, Y. Huang, et al., Clin. Cancer Res. 25 (2019) 1142–1146.
doi: 10.1158/1078-0432.ccr-18-2035
-
[20]
N. Bouchkouj, Y.L. Kasamon, R.A. de Claro, et al., Clin. Cancer Res. 25 (2019) 1702–1708.
doi: 10.1158/1078-0432.ccr-18-2743
-
[21]
C.H. June, R.S. O'Connor, O.U. Kawalekar, S. Ghassemi, M.C. Milone, Science 359 (2018) 1361–1365.
doi: 10.1126/science.aar6711
-
[22]
A. Schmidts, M.V. Maus, Front. Immunol. 9 (2018) 2593.
doi: 10.3389/fimmu.2018.02593
-
[23]
K. Newick, S. O'Brien, E. Moon, S.M. Albelda, Annu. Rev. Med. 68 (2017) 139–152.
doi: 10.1146/annurev-med-062315-120245
-
[24]
S. Rafiq, C.S. Hackett, R.J. Brentjens, Nat. Rev. Clin. Oncol. 17 (2020) 147–167.
doi: 10.1038/s41571-019-0297-y
-
[25]
T. Schioppa, B. Uranchimeg, A. Saccani, et al., J. Exp. Med. 198 (2003) 1391–1402.
doi: 10.1084/jem.20030267
-
[26]
G.W. Tormoen, M.R. Crittenden, M.J. Gough, Adv. Radiat. Oncol. 3 (2018) 520–526.
doi: 10.1016/j.adro.2018.08.018
-
[27]
S. Wilkie, S.E. Burbridge, L. Chiapero-Stanke, et al., J. Biol. Chem. 285 (2010) 25538–25544.
doi: 10.1074/jbc.M110.127951
-
[28]
E.I. Buchbinder, A. Desai, Am. J. Clin. Oncol. 39 (2016) 98–106.
doi: 10.1097/COC.0000000000000239
-
[29]
L. Labanieh, R.G. Majzner, C.L. Mackall, Nat. Biomed. Eng. 2 (2018) 377–391.
doi: 10.1038/s41551-018-0235-9
-
[30]
S.L. Buchan, L. Dou, M. Remer, et al., Immunity 49 (2018) 958–970 e957.
doi: 10.1016/j.immuni.2018.09.014
-
[31]
R. Parihar, C. Rivas, M. Huynh, et al., Cancer Immunol. Res. 7 (2019) 363–375.
doi: 10.1158/2326-6066.cir-18-0572
-
[32]
M. Ruella, M. Klichinsky, S.S. Kenderian, et al., Cancer Discov. 7 (2017) 1154–1167.
doi: 10.1158/2159-8290.CD-16-0850
-
[33]
S.A. Grupp, M. Kalos, D. Barrett, et al., New Engl. J. Med. 368 (2013) 1509–1518.
doi: 10.1056/NEJMoa1215134
-
[34]
J.C. Fitzgerald, S.L. Weiss, S.L. Maude, et al., Crit. Care Med. 45 (2017) e124–e131.
doi: 10.1097/CCM.0000000000002053
-
[35]
S.J. Schuster, J. Svoboda, E.A. Chong, et al., New Engl. J. Med. 377 (2017) 2545–2554.
doi: 10.1056/NEJMoa1708566
-
[36]
D.W. Lee, B.D. Santomasso, F.L. Locke, et al., Biol. Blood Marrow Transplant. 25 (2019) 625–638.
doi: 10.1016/j.bbmt.2018.12.758
-
[37]
H. Klingemann, Oncoimmunology 3 (2014) e28147.
doi: 10.4161/onci.28147
-
[38]
L.B. Kennedy, A.K.S. Salama, CA Cancer J. Clin. 70 (2020) 86–104.
doi: 10.3322/caac.21596
-
[39]
J. Gust, K.A. Hay, L.A. Hanafi, et al., Cancer Discov. 7 (2017) 1404–1419.
doi: 10.1158/2159-8290.CD-17-0698
-
[40]
B.D. Santomasso, J.H. Park, D. Salloum, et al., Cancer Discov. 8 (2018) 958–971.
doi: 10.1158/2159-8290.cd-17-1319
-
[41]
K.R. Parker, D. Migliorini, E. Perkey, et al., Cell 183 (2020) 126–142 e117.
doi: 10.1016/j.cell.2020.08.022
-
[42]
J.N. Brudno, J.N. Kochenderfer, Blood Rev. 34 (2019) 45–55.
doi: 10.1016/j.blre.2018.11.002
-
[43]
M. Namuduri, R.J. Brentjens, Expert Rev. Hematol. 9 (2016) 511–513.
doi: 10.1080/17474086.2016.1183479
-
[44]
S.L. Maude, N. Frey, P.A. Shaw, et al., New Engl. J. Med. 371 (2014) 1507–1517.
doi: 10.1056/NEJMoa1407222
-
[45]
D. Kalyane, N. Raval, R. Maheshwari, et al., Mater. Sci. Eng. C Mater. Biol. Appl. 98 (2019) 1252–1276.
doi: 10.1016/j.msec.2019.01.066
-
[46]
J. Wu, J. Pers. Med. 11 (2021) 771.
doi: 10.3390/jpm11080771
-
[47]
M. Zhao, D. van Straten, M.L.D. Broekman, V. Préat, R.M. Schiffelers, Theranostics 10 (2020) 1355–1372.
doi: 10.7150/thno.38147
-
[48]
C. Hamers-Casterman, T. Atarhouch, S. Muyldermans, et al., Nature 363 (1993) 446–448.
doi: 10.1038/363446a0
-
[49]
P. Safarzadeh Kozani, A. Naseri, S.M.J. Mirarefin, et al., Biomark. Res. 10 (2022) 24.
doi: 10.1186/s40364-022-00371-7
-
[50]
J.R. Ingram, F.I. Schmidt, H.L. Ploegh, Annu. Rev. Immunol. 36 (2018) 695–715.
doi: 10.1146/annurev-immunol-042617-053327
-
[51]
H. Revets, P. De Baetselier, S. Muyldermans, Expert Opin. Biol. Ther. 5 (2005) 111–124.
doi: 10.1517/14712598.5.1.111
-
[52]
S. Muyldermans, Annu. Rev. Biochem. 82 (2013) 775–797.
doi: 10.1146/annurev-biochem-063011-092449
-
[53]
J. Jayaraman, M.P. Mellody, A.J. Hou, et al., EBioMedicine 8 (2020) 102931.
doi: 10.1016/j.ebiom.2020.102931
-
[54]
W. Sun, J. Xie, H. Lin, et al., Protein Expr. Purif. 83 (2012) 21–29.
doi: 10.1016/j.pep.2012.02.006
-
[55]
S. Muyldermans, J. Biotechnol. 74 (2001) 277–302.
-
[56]
B.M. Tijink, T. Laeremans, M. Budde, et al., Mol. Cancer Ther. 7 (2008) 2288–2297.
doi: 10.1158/1535-7163.MCT-07-2384
-
[57]
C. Bao, Q. Gao, L.L. Li, et al., Biomolecules 11 (2021) 238.
doi: 10.3390/biom11020238
-
[58]
M.M. Harmsen, H.J. De Haard, Appl. Microbiol. Biotechnol. 77 (2007) 13–22.
doi: 10.1007/s00253-007-1142-2
-
[59]
F.J. Iri-Sofla, F. Rahbarizadeh, D. Ahmadvand, M.J. Rasaee, Exp. Cell Res. 317 (2011) 2630–2641.
doi: 10.1016/j.yexcr.2011.08.015
-
[60]
S. Khaleghi, F. Rahbarizadeh, D. Ahmadvand, M.J. Rasaee, P. Pognonec, Int. J. Hematol. 95 (2012) 434–444.
doi: 10.1007/s12185-012-1037-6
-
[61]
Z. Sharifzadeh, F. Rahbarizadeh, M.A. Shokrgozar, et al., Cancer Lett. 334 (2013) 237–244.
doi: 10.1016/j.canlet.2012.08.010
-
[62]
S.H. Bakhtiari, F. Rahbarizadeh, S. Hasannia, et al., Hybridoma (2005) 28 (2009) 85–92.
doi: 10.1089/hyb.2008.0079
-
[63]
N. Li, H. Fu, S.M. Hewitt, D.S. Dimitrov, M. Ho, Proc. Natl. Acad. Sci. U. S. A. 114 (2017) E6623–e6631.
doi: 10.1073/pnas.1700536114
-
[64]
M. Hassani, F. Hajari Taheri, Z. Sharifzadeh, et al., J. Cell. Biochem. 120 (2019) 10787–10795.
doi: 10.1002/jcb.28370
-
[65]
Y.J. Xie, M. Dougan, N. Jailkhani, et al., Proc. Natl. Acad. Sci. U. S. A. 116 (2019) 7624–7631.
doi: 10.1073/pnas.1817147116
-
[66]
F. Hajari Taheri, M. Hassani, Z. Sharifzadeh, et al., IUBMB Life 71 (2019) 1259–1267.
doi: 10.1002/iub.2019
-
[67]
F.R. Jamnani, F. Rahbarizadeh, M.A. Shokrgozar, et al., Biochim. Biophys. Acta 1840 (2014) 378–386.
doi: 10.1016/j.bbagen.2013.09.029
-
[68]
N. An, Y.N. Hou, Q.X. Zhang, et al., Mol. Pharm. 15 (2018) 4577–4588.
doi: 10.1021/acs.molpharmaceut.8b00584
-
[69]
J. Xu, L.J. Chen, S.S. Yang, et al., Proc. Natl. Acad. Sci. U. S. A. 116 (2019) 9543–9551.
doi: 10.1073/pnas.1819745116
-
[70]
S. De Munter, A. Van Parys, L. Bral, et al., Int. J. Mol. Sci. 21 (2020) 833.
doi: 10.3390/ijms21030833
-
[71]
T. Zhang, T. Wang, F. You, et al., Transpl. Immunol. 71 (2022) 101538.
doi: 10.1016/j.trim.2022.101538
-
[72]
M. Zhang, D. Chen, X. Fu, et al., Clin. Cancer Res. (2022) 2830–2843.
doi: 10.1158/1078-0432.ccr-21-4097
-
[73]
D. Chen, F. You, S. Xiang, et al., Am. J. Cancer Res. 11 (2021) 5263–5281.
-
[74]
M.A. Nix, K. Mandal, H. Geng, et al., Cancer Discov. 11 (2021) 2032–2049.
doi: 10.1158/2159-8290.cd-20-0242
-
[75]
Z. Feng, X. He, X. Zhang, et al., Nat. Cancer (2022) 581–594.
doi: 10.1038/s43018-022-00344-7
-
[76]
F. Mo, S. Duan, X. Jiang, et al., Signal Transduct. Target. Ther. 6 (2021) 80.
doi: 10.1038/s41392-021-00462-1
-
[77]
L. Han, J. Zhou, K. Zhou, et al., J. Immunother. Cancer 8 (2020) e000927.
doi: 10.1136/jitc-2020-000927
-
[78]
S. De Munter, J. Ingels, G. Goetgeluk, et al., Int. J. Mol. Sci. 19 (2018) 403.
doi: 10.3390/ijms19020403
-
[79]
H. Wang, L. Wang, Y. Li, et al., Cancer Cell Int. 21 (2021) 450.
doi: 10.1186/s12935-021-02151-z
-
[80]
R.C. Larson, M.V. Maus, Nat. Rev. Cancer 21 (2021) 145–161.
doi: 10.1038/s41568-020-00323-z
-
[81]
S. Albert, C. Arndt, A. Feldmann, et al., Oncoimmunology 6 (2017) e1287246.
doi: 10.1080/2162402X.2017.1287246
-
[82]
S. Albert, C. Arndt, S. Koristka, et al., Oncotarget 9 (2018) 25597–25616.
doi: 10.18632/oncotarget.25390
-
[83]
K. L A.R.K. Kumar, Y. Shou, B. Chan, A. Tay, Adv. Mater. 33 (2021) e2007421.
doi: 10.1002/adma.202007421
-
[84]
B.L. Levine, J. Miskin, K. Wonnacott, C. Keir, Mol. Ther. Methods Clin. Dev. 4 (2017) 92–101.
doi: 10.1016/j.omtm.2016.12.006
-
[85]
V. Poletti, F. Mavilio, Mol. Ther. Methods Clin. Dev. 8 (2018) 31–41.
doi: 10.1016/j.omtm.2017.10.001
-
[86]
C.H. June, B.R. Blazar, J.L. Riley, Nat. Rev. Immunol. 9 (2009) 704–716.
doi: 10.1038/nri2635
-
[87]
P. Vormittag, R. Gunn, S. Ghorashian, F.S. Veraitch, Curr. Opin. Biotechnol. 53 (2018) 164–181.
doi: 10.1016/j.copbio.2018.01.025
-
[88]
H. Nakai, E. Montini, S. Fuess, et al., Nat. Genet. 34 (2003) 297–302.
doi: 10.1038/ng1179
-
[89]
A. Donsante, D.G. Miller, Y. Li, et al., Science 317 (2007) 477.
doi: 10.1126/science.1142658
-
[90]
A. Tay, N. Melosh, Adv. Ther. 2 (2019) 1900133.
doi: 10.1002/adtp.201900133
-
[91]
Y.C. Wu, T.H. Wu, D.L. Clemens, et al., Nat. Methods 12 (2015) 439–444.
doi: 10.1038/nmeth.3357
-
[92]
M.P. Stewart, A. Sharei, X. Ding, et al., Nature 538 (2016) 183–192.
doi: 10.1038/nature19764
-
[93]
S. Sun, V.B. Rao, M.G. Rossmann, Curr. Opin. Struct. Biol. 20 (2010) 114–120.
doi: 10.1016/j.sbi.2009.12.006
-
[94]
E. Neumann, M. Schaefer-Ridder, Y. Wang, P.H. Hofschneider, EMBO J. 1 (1982) 841–845.
doi: 10.1002/j.1460-2075.1982.tb01257.x
-
[95]
T.Y. Tsong, Biophys. J. 60 (1991) 297–306.
doi: 10.1016/S0006-3495(91)82054-9
-
[96]
X. Xu, D. Gao, P. Wang, et al., Sci. Rep. 8 (2018) 11649.
doi: 10.1038/s41598-018-30227-w
-
[97]
J.T. Sengel, M.I. Wallace, Proc. Natl. Acad. Sci. U. S. A. 113 (2016) 5281–5286.
doi: 10.1073/pnas.1517437113
-
[98]
J.C. Weaver, Y.A. Chizmadzhev, Bioelectrochem. Bioenerg. 41 (1996) 135–160.
doi: 10.1016/S0302-4598(96)05062-3
-
[99]
D. Morita, N. Nishio, S. Saito, et al., Mol. Ther. Methods Clin. Dev. 8 (2018) 131–140.
doi: 10.1016/j.omtm.2017.12.003
-
[100]
S. Saito, Y. Nakazawa, A. Sueki, et al., Cytotherapy 16 (2014) 1257–1269.
doi: 10.1016/j.jcyt.2014.05.022
-
[101]
I. Querques, A. Mades, C. Zuliani, et al., Nat. Biotechnol. 37 (2019) 1502–1512.
doi: 10.1038/s41587-019-0291-z
-
[102]
Q. Gao, X. Dong, Q. Xu, et al., Cancer Med. 8 (2019) 4254–4264.
doi: 10.1002/cam4.2257
-
[103]
L. Mátés, M.K. Chuah, E. Belay, et al., Nat. Genet. 41 (2009) 753–761.
doi: 10.1038/ng.343
-
[104]
X. Xue, X. Huang, S.E. Nodland, et al., Blood 114 (2009) 1319–1330.
doi: 10.1182/blood-2009-03-210005
-
[105]
T.T. Smith, S.B. Stephan, H.F. Moffett, et al., Nat. Nanotechnol. 12 (2017) 813–820.
doi: 10.1038/nnano.2017.57
-
[106]
C.J. McKinlay, N.L. Benner, O.A. Haabeth, R.M. Waymouth, P.A. Wender, Proc. Natl. Acad. Sci. U. S. A. 115 (2018) E5859–E5866.
-
[107]
B. Mostaghaci, J. Susewind, G. Kickelbick, C.M. Lehr, B. Loretz, ACS Appl. Mater. Interfaces 7 (2015) 5124–5133.
doi: 10.1021/am507193a
-
[108]
L. Raes, S.C. De Smedt, K. Raemdonck, K. Braeckmans, Biotechnol. Adv. 49 (2021) 107760.
doi: 10.1016/j.biotechadv.2021.107760
-
[109]
B.R. Olden, Y. Cheng, J.L. Yu, S.H. Pun, J. Control. Release 282 (2018) 140–147.
doi: 10.1016/j.jconrel.2018.02.043
-
[110]
W.P. Verdurmen, R. Wallbrecher, S. Schmidt, et al., J. Control. Release 170 (2013) 83–91.
doi: 10.1016/j.jconrel.2013.05.001
-
[111]
S.A.B. Riedl, P. Kaiser, A. Raup, et al., Processes 6 (2018) 188.
doi: 10.3390/pr6100188
-
[112]
W. Nawaz, S. Xu, Y. Li, et al., Acta Biomater. 109 (2020) 21–36.
doi: 10.1016/j.actbio.2020.04.015
-
[113]
H. Yin, R.L. Kanasty, A.A. Eltoukhy, et al., Nat. Rev. Genet. 15 (2014) 541–555.
doi: 10.1038/nrg3763
-
[114]
Y. Song, J. Hormes, C.S. Kumar, Small 4 (2008) 698–711.
doi: 10.1002/smll.200701029
-
[115]
H. Dong, M. Zhu, J.A. Yoon, et al., J. Am. Chem. Soc. 130 (2008) 12852–12853.
doi: 10.1021/ja8038097
-
[116]
J. Xie, S. Peng, N. Brower, et al., Pure Appl. Chem. 78 (2006) 1003–1014.
doi: 10.1351/pac200678051003
-
[117]
O. Boussif, F. Lezoualc'h, M.A. Zanta, et al., Proc. Natl. Acad. Sci. U. S. A. 92 (1995) 7297–7301.
doi: 10.1073/pnas.92.16.7297
-
[118]
J.J. Virgen-Ortíz, J.C.S. Dos Santos, Á. Berenguer-Murcia, et al., J. Mater. Chem. B 5 (2017) 7461–7490.
doi: 10.1039/C7TB01639E
-
[119]
J. Fan, Q. He, Z. Jin, W. Chen, W. Huang, RSC Adv. 8 (2018) 14975–14982.
doi: 10.1039/c8ra02133c
-
[120]
Q. Yu, M. Zhang, Y. Chen, et al., Int. J. Nanomed. 15 (2020) 483–495.
doi: 10.2147/ijn.s229858
-
[121]
I.A. Khalil, K. Kogure, H. Akita, H. Harashima, Pharmacol. Rev. 58 (2006) 32–45.
doi: 10.1124/pr.58.1.8
-
[122]
K.A. Mislick, J.D. Baldeschwieler, Proc. Natl. Acad. Sci. U. S. A. 93 (1996) 12349–12354.
doi: 10.1073/pnas.93.22.12349
-
[123]
A. Harizaj, M. Wels, L. Raes, et al., Adv. Funct. Mater. 31 (2021) 2102472.
doi: 10.1002/adfm.202102472
-
[124]
Y. Zhao, L. Huang, Adv. Genet. 88 (2014) 13–36.
-
[125]
D. Luo, W.M. Saltzman, Nat. Biotechnol. 18 (2000) 33–37.
doi: 10.1038/71889
-
[126]
A. Yen, Y. Cheng, M. Sylvestre, et al., Mol. Pharm. 15 (2018) 2268–2276.
doi: 10.1021/acs.molpharmaceut.8b00134
-
[127]
S. Warashina, T. Nakamura, Y. Sato, et al., J. Control. Release 225 (2016) 183–191.
doi: 10.1016/j.jconrel.2016.01.042
-
[128]
T. Nakamura, M. Kuroi, Y. Fujiwara, et al., Sci. Rep. 6 (2016) 37849.
doi: 10.1038/srep37849
-
[129]
Y. Sawalha, A.S. Advani, Int. J. Hematol. Oncol. 7 (2018) Ijh02.
doi: 10.2217/ijh-2017-0023
-
[130]
E. Samaridou, J. Heyes, P. Lutwyche, Adv. Drug. Deliv. Rev. 154-155 (2020) 37–63.
doi: 10.1016/j.addr.2020.06.002
-
[131]
X. Zhao, J. Chen, M. Qiu, et al., Angew Chem. Int. Ed. 59 (2020) 20083–20089.
doi: 10.1002/anie.202008082
-
[132]
M.M. Billingsley, N. Singh, P. Ravikumar, et al., Nano Lett. 20 (2020) 1578–1589.
doi: 10.1021/acs.nanolett.9b04246
-
[133]
M.M. Billingsley, A.G. Hamilton, D. Mai, et al., Nano Lett. (2021) 533–542.
-
[134]
H.F. Moffett, M.E. Coon, S. Radtke, et al., Nat. Commun. 8 (2017) 389.
doi: 10.1038/s41467-017-00505-8
-
[135]
K. Garber, Nat. Biotechnol. 36 (2018) 777–778.
doi: 10.1038/nbt0918-777
-
[136]
H. Pan, P. Zhang, D. Gao, et al., ACS Nano 8 (2014) 5468–5477.
doi: 10.1021/nn501028b
-
[137]
H. Pan, P. Li, G. Li, et al., Adv. Funct. Mater. 29 (2019) 1807528.
doi: 10.1002/adfm.201807528
-
[138]
A. Tay, N. Melosh, Small 17 (2021) e2103198.
doi: 10.1002/smll.202103198
-
[139]
L.H. Wang, D.C. Wu, H.X. Xu, Y.Z. You, Angew. Chem. Int. Ed. 55 (2016) 755–759.
doi: 10.1002/anie.201508695
-
[140]
Y. Zheng, Z.R. Li, R. Yue, et al., Am. J. Transl. Res. 11 (2019) 7126–7136.
-
[141]
R.P. Somerville, M.E. Dudley, Oncoimmunology 1 (2012) 1435–1437.
doi: 10.4161/onci.21206
-
[142]
R.P. Somerville, L. Devillier, M.R. Parkhurst, S.A. Rosenberg, M.E. Dudley, J. Transl. Med. 10 (2012) 69.
doi: 10.1186/1479-5876-10-69
-
[143]
B.L. Levine, Cancer Gene Ther. 22 (2015) 79–84.
doi: 10.1038/cgt.2015.5
-
[144]
D. Hollyman, J. Stefanski, M. Przybylowski, et al., J. Immunother. 32 (2009) 169–180.
doi: 10.1097/CJI.0b013e318194a6e8
-
[145]
A.D. Kaiser, M. Assenmacher, B. Schröder, et al., Cancer Gene Ther. 22 (2015) 72–78.
doi: 10.1038/cgt.2014.78
-
[146]
P. Bajgain, R. Mucharla, J. Wilson, et al., Mol. Ther. Methods Clin. Dev. 1 (2014) 14015.
doi: 10.1038/mtm.2014.15
-
[147]
J. Jin, M. Sabatino, R. Somerville, et al., J. Immunother. 35 (2012) 283–292.
doi: 10.1097/CJI.0b013e31824e801f
-
[148]
U. Mock, L. Nickolay, B. Philip, et al., Cytotherapy 18 (2016) 1002–1011.
doi: 10.1016/j.jcyt.2016.05.009
-
[149]
M. Apel, M. Bruening, M. Granzin, et al., Chem. Ing. Tech. 85 (2013) 103–110.
doi: 10.1002/cite.201200175
-
[150]
H. Lin, Q. Li, O. Wang, et al., Adv. Healthc. Mater. 7 (2018) e1701297.
doi: 10.1002/adhm.201701297
-
[151]
M.E. Coon, S.B. Stephan, V. Gupta, C.P. Kealey, M.T. Stephan, Nat. Biomed. Eng. 4 (2020) 195–206.
-
[152]
T.R. Fadel, F.A. Sharp, N. Vudattu, et al., Nat. Nanotechnol. 9 (2014) 639–647.
doi: 10.1038/nnano.2014.154
-
[153]
A.S. Cheung, D.K.Y. Zhang, S.T. Koshy, D.J. Mooney, Nat. Biotechnol. 36 (2018) 160–169.
doi: 10.1038/nbt.4047
-
[154]
Y. Wang, F. Luo, J. Yang, C. Zhao, Y. Chu, Front. Immunol. 8 (2017) 1934.
doi: 10.3389/fimmu.2017.01934
-
[155]
M. Binnewies, E.W. Roberts, K. Kersten, et al., Nat. Med. 24 (2018) 541–550.
doi: 10.1038/s41591-018-0014-x
-
[156]
W. Zou, Nat. Rev. Cancer 5 (2005) 263–274.
doi: 10.1038/nrc1586
-
[157]
J.R. Groom, A.D. Luster, Immunol. Cell Biol. 89 (2011) 207–215.
doi: 10.1038/icb.2010.158
-
[158]
E.M. Hanson, V.K. Clements, P. Sinha, D. Ilkovitch, S. Ostrand-Rosenberg, J. Immunol. 183 (2009) 937–944.
doi: 10.4049/jimmunol.0804253
-
[159]
C.Y. Slaney, M.H. Kershaw, P.K. Darcy, Cancer Res. 74 (2014) 7168–7174.
doi: 10.1158/0008-5472.CAN-14-2458
-
[160]
A. Ohta, E. Gorelik, S.J. Prasad, et al., Proc. Natl. Acad. Sci. U. S. A. 103 (2006) 13132–13137.
doi: 10.1073/pnas.0605251103
-
[161]
C. Cekic, J. Linden, Cancer Res. 74 (2014) 7239–7249.
doi: 10.1158/0008-5472.CAN-13-3581
-
[162]
P.A. Beavis, M.A. Henderson, L. Giuffrida, et al., J. Clin. Invest. 127 (2017) 929–941.
doi: 10.1172/JCI89455
-
[163]
J. Li, W. Li, K. Huang, et al., J. Hematol. Oncol. 11 (2018) 22.
doi: 10.1504/ijhpcn.2018.10025202
-
[164]
R.G. Majzner, S. Heitzeneder, C.L. Mackall, Cancer Cell 31 (2017) 476–485.
doi: 10.1016/j.ccell.2017.03.002
-
[165]
C.J. Turtle, L.A. Hanafi, C. Berger, et al., J. Clin. Invest. 126 (2016) 2123–2138.
doi: 10.1172/JCI85309
-
[166]
R. Gardner, D. Wu, S. Cherian, et al., Blood 127 (2016) 2406–2410.
doi: 10.1182/blood-2015-08-665547
-
[167]
E. Jacoby, S.M. Nguyen, T.J. Fountaine, et al., Nat. Commun. 7 (2016) 12320.
doi: 10.1038/ncomms12320
-
[168]
R.G. Majzner, C.L. Mackall, Cancer Discov. 8 (2018) 1219–1226.
doi: 10.1158/2159-8290.cd-18-0442
-
[169]
S.L. Maude, D.T. Teachey, S.R. Rheingold, et al., J. Clin. Oncol. 34 (2016) 3011.
doi: 10.1200/JCO.2016.34.15_suppl.3011
-
[170]
S.L. Maude, T.W. Laetsch, J. Buechner, et al., New Engl. J. Med. 378 (2018) 439–448.
doi: 10.1056/nejmoa1709866
-
[171]
R. Gardner, O. Finney, H. Smithers, et al., Blood 128 (2016) 219.
doi: 10.1182/blood.v128.22.219.219
-
[172]
D.W. Lee, M. Stetler-Stevenson, C.M. Yuan, et al., Blood 128 (2016) 218.
doi: 10.1182/blood.V128.22.218.218
-
[173]
T.J. Fry, N.N. Shah, R.J. Orentas, et al., Nat. Med. 24 (2018) 20–28.
doi: 10.1038/nm.4441
-
[174]
A.R. Pyzer, D. Stroopinsky, H. Rajabi, et al., Blood 129 (2017) 1791–1801.
doi: 10.1182/blood-2016-07-730614
-
[175]
R. Jitschin, D. Saul, M. Braun, et al., J. Immunother. Cancer 6 (2018) 116.
doi: 10.1186/s40425-018-0432-9
-
[176]
A.K. Park, Y. Fong, S.I. Kim, et al., Sci. Transl. Med. 12 (2020) eaaz1863.
doi: 10.1126/scitranslmed.aaz1863
-
[177]
N. Nishio, G. Dotti, Oncoimmunology 4 (2015) e988098.
doi: 10.4161/21505594.2014.988098
-
[178]
E.K. Moon, L.S. Wang, K. Bekdache, et al., Oncoimmunology 7 (2018) e1395997.
doi: 10.1080/2162402X.2017.1395997
-
[179]
A. Aalipour, F. Le Boeuf, M. Tang, et al., Mol. Ther. Oncolytics 17 (2020) 232–240.
doi: 10.1016/j.omto.2020.03.018
-
[180]
J. Ren, X. Liu, C. Fang, et al., Clin. Cancer Res. 23 (2017) 2255–2266.
doi: 10.1158/1078-0432.CCR-16-1300
-
[181]
J.R. Hamilton, C.A. Tsuchida, D.N. Nguyen, et al., Cell Rep. 35 (2021) 109207.
doi: 10.1016/j.celrep.2021.109207
-
[182]
M. Hale, B. Lee, Y. Honaker, et al., Mol. Ther. Methods Clin. Dev. 4 (2017) 192–203.
doi: 10.1016/j.omtm.2016.12.008
-
[183]
J. Eyquem, J. Mansilla-Soto, T. Giavridis, et al., Nature 543 (2017) 113–117.
doi: 10.1038/nature21405
-
[184]
L.J. Rupp, K. Schumann, K.T. Roybal, et al., Sci. Rep. 7 (2017) 737.
doi: 10.1038/s41598-017-00462-8
-
[185]
T. Nakazawa, A. Natsume, F. Nishimura, et al., Cells 9 (2020) 998.
doi: 10.3390/cells9040998
-
[186]
K. Reinhard, B. Rengstl, P. Oehm, et al., Science 367 (2020) 446–453.
doi: 10.1126/science.aay5967
-
[187]
M.N. Garas, S.V. Tillib, O.V. Zubkova, et al., Acta Nat. 6 (2014) 95–105.
doi: 10.32607/20758251-2014-6-2-95-105
-
[188]
Y. Zhai, W. Ran, J. Su, et al., Adv. Mater. (2018) e1802378.
-
[189]
G.B. Kim, V. Aragon-Sanabria, L. Randolph, et al., Bioact. Mater. 5 (2020) 624–635.
doi: 10.1016/j.bioactmat.2020.04.011
-
[190]
V. de Turris, M. Cardoso Trabuco, G. Peruzzi, et al., Nanoscale 9 (2017) 647–655.
doi: 10.1039/C6NR07129E
-
[191]
C.T. Jiang, K.G. Chen, A. Liu, et al., Nat. Commun. 12 (2021) 1359.
doi: 10.1038/s41467-021-21497-6
-
[192]
X. Huang, J.Z. Williams, R. Chang, et al., Nat. Nanotechnol. 16 (2021) 214–223.
doi: 10.1038/s41565-020-00813-z
-
[193]
L. Ma, T. Dichwalkar, J.Y.H. Chang, et al., Science 365 (2019) 162–168.
doi: 10.1126/science.aav8692
-
[194]
D. Schmid, C.G. Park, C.A. Hartl, et al., Nat. Commun. 8 (2017) 1747.
doi: 10.1038/s41467-017-01830-8
-
[195]
F. Zhang, S.B. Stephan, C.I. Ene, et al., Cancer Res. 78 (2018) 3718–3730.
doi: 10.1158/0008-5472.can-18-0306
-
[196]
N. Siriwon, Y.J. Kim, E. Siegler, et al., Cancer Immunol. Res. 6 (2018) 812–824.
doi: 10.1158/2326-6066.cir-17-0502
-
[197]
C. Wang, Y. Ye, G.M. Hochu, H. Sadeghifar, Z. Gu, Nano Lett. 16 (2016) 2334–2340.
doi: 10.1021/acs.nanolett.5b05030
-
[198]
C. Wang, W. Sun, Y. Ye, et al., Nat. Biomed. Eng. 1 (2017) 0011.
doi: 10.1038/s41551-016-0011
-
[199]
S. Han, W. Wang, S. Wang, et al., Theranostics 11 (2021) 2892–2916.
doi: 10.7150/thno.50928
-
[200]
W. Ou, R.K. Thapa, L. Jiang, et al., J. Control. Release 281 (2018) 84–96.
doi: 10.1016/j.jconrel.2018.05.018
-
[201]
Q. Liu, D. Zhang, H. Qian, et al., Int. J. Nanomed. 15 (2020) 3669–3680.
doi: 10.2147/ijn.s249174
-
[202]
J. Li, D.J. Mooney, Nat. Rev. Mater. 1 (2016) 16071.
doi: 10.1038/natrevmats.2016.71
-
[203]
S.W. Jung, S.H. Oh, I.S. Lee, J.H. Byun, J.H. Lee, Tissue Eng. Regen. Med. 16 (2019) 479–490.
doi: 10.1007/s13770-019-00206-x
-
[204]
Y. Ando, E.L. Siegler, H.P. Ta, et al., Adv. Healthc. Mater. 8 (2019) e1900001.
doi: 10.1002/adhm.201900001
-
[205]
Y. Hori, A.M. Winans, C.C. Huang, E.M. Horrigan, D.J. Irvine, Biomaterials 29 (2008) 3671–3682.
doi: 10.1016/j.biomaterials.2008.05.033
-
[206]
K. Wang, Y. Chen, S. Ahn, et al., Nat. Cancer 1 (2020) 990–997.
doi: 10.1038/s43018-020-00119-y
-
[207]
Y. Dong, S. Li, X. Li, X. Wang, Int. J. Biol. Macromol. 190 (2021) 693–699.
doi: 10.1016/j.ijbiomac.2021.09.037
-
[208]
Y. Cao, Y. Zhou, Z. Chen, et al., Adv. Healthc. Mater. 10 (2021) e2100814.
doi: 10.1002/adhm.202100814
-
[209]
C. Liu, P. Leon-Plata, M. Zaroudi, et al., ACS Appl. Bio. Mater. 3 (2020) 7357–7362.
doi: 10.1021/acsabm.0c00992
-
[210]
E.A. Ogunnaike, A. Valdivia, M. Yazdimamaghani, et al., Sci. Adv. 7 (2021) eabg5841.
doi: 10.1126/sciadv.abg5841
-
[211]
X. Dong, A. Yang, Y. Bai, D. Kong, F. Lv, Biomaterials 230 (2020) 119659.
doi: 10.1016/j.biomaterials.2019.119659
-
[212]
A.F. Atik, C.M. Suryadevara, R.M. Schweller, et al., J. Clin. Neurosci. 56 (2018) 163–168.
doi: 10.1016/j.jocn.2018.06.005
-
[213]
Q. Hu, H. Li, E. Archibong, et al., Nat. Biomed. Eng. 5 (2021) 1038–1047.
doi: 10.1038/s41551-021-00712-1
-
[214]
Z. Luo, Z. Liu, Z. Liang, et al., ACS Appl. Mater. Interfaces 12 (2020) 56712–56722.
doi: 10.1021/acsami.0c15239
-
[215]
T. Shang, X. Yu, S. Han, B. Yang, Biomater. Sci. 8 (2020) 5241–5259.
doi: 10.1039/d0bm01158d
-
[216]
X. Xu, T. Li, S. Shen, et al., Theranostics 9 (2019) 7889–7905.
doi: 10.7150/thno.38583
-
[217]
R. Ahmad, J. Fu, N. He, S. Li, J. Nanosci. Nanotechnol. 16 (2016) 67–80.
doi: 10.1166/jnn.2016.10770
-
[218]
Y. Huang, G. Zeng, Q. Xin, et al., MedComm 1 (2020) 202–210.
doi: 10.1002/mco2.28
-
[219]
T. Yata, Y. Takahashi, M. Tan, et al., Biomaterials 146 (2017) 136–145.
doi: 10.1016/j.biomaterials.2017.09.014
-
[220]
J. Nam, S. Son, L.J. Ochyl, et al., Nat. Commun. 9 (2018) 1074.
doi: 10.1038/s41467-018-03473-9
-
[221]
A.L. Oei, L.E. Vriend, J. Crezee, N.A. Franken, P.M. Krawczyk, Radiat. Oncol. 10 (2015) 165.
doi: 10.1186/s13014-015-0462-0
-
[222]
S. Wang, Q. Zhang, X.F. Luo, et al., Biomaterials 35 (2014) 9473–9483.
doi: 10.1016/j.biomaterials.2014.07.064
-
[223]
H. Yang, H. He, Z. Tong, et al., J. Colloid Interface Sci. 565 (2020) 186–196.
doi: 10.1016/j.jcis.2020.01.026
-
[224]
Z. Liao, W. Zhang, Z. Qiao, et al., J. Colloid Interface Sci. 562 (2020) 81–90.
doi: 10.1016/j.jcis.2019.11.055
-
[225]
X. Zhang, J. Tang, C. Li, et al., Bioact. Mater. 6 (2021) 472–489.
doi: 10.1016/j.bioactmat.2020.08.024
-
[226]
F. Zhang, G. Lu, X. Wen, et al., J. Control. Release 326 (2020) 131–139.
doi: 10.1016/j.jconrel.2020.06.015
-
[227]
Q. Yang, J. Peng, K. Shi, et al., J. Control. Release 308 (2019) 29–43.
doi: 10.1016/j.jconrel.2019.06.031
-
[228]
X. Yi, Q.Y. Duan, F.G. Wu, Research 2021 (2021) 9816594.
-
[229]
I.C. Miller, A. Zamat, L.K. Sun, et al., Nat. Biomed. Eng. (2021) 1348–1359.
doi: 10.1038/s41551-021-00781-2
-
[230]
W. Ma, D. Zhu, J. Li, et al., Theranostics 10 (2020) 1281–1295.
doi: 10.7150/thno.40291
-
[231]
Q. Chen, Q. Hu, E. Dukhovlinova, et al., Adv. Mater. 31 (2019) e1900192.
doi: 10.1002/adma.201900192
-
[232]
L. Zhu, J. Liu, G. Zhou, et al., Small (2021) e2102624.
-
[233]
Z. Chen, H. Pan, Y. Luo, et al., Small 17 (2021) e2007494.
doi: 10.1002/smll.202007494
-
[234]
H.J. Jackson, S. Rafiq, R.J. Brentjens, Nat. Rev. Clin. Oncol. 13 (2016) 370–383.
doi: 10.1038/nrclinonc.2016.36
-
[235]
S.S. Neelapu, S. Tummala, P. Kebriaei, et al., Nat. Rev. Clin. Oncol. 15 (2018) 47–62.
doi: 10.1038/nrclinonc.2017.148
-
[236]
B. Wolf, S. Zimmermann, C. Arber, et al., Drug Saf. 42 (2019) 315–334.
doi: 10.1007/s40264-018-0779-3
-
[237]
M. Norelli, B. Camisa, G. Barbiera, et al., Nat. Med. 24 (2018) 739–748.
doi: 10.1038/s41591-018-0036-4
-
[238]
T. Giavridis, S.J.C. van der Stegen, J. Eyquem, et al., Nat. Med. 24 (2018) 731–738.
doi: 10.1038/s41591-018-0041-7
-
[239]
Y. Liu, Y. Fang, X. Chen, et al., Sci. Immunol. 5 (2020) eaax7969.
doi: 10.1126/sciimmunol.aax7969
-
[240]
A. Taraseviciute, V. Tkachev, R. Ponce, et al., Cancer Discov. 8 (2018) 750–763.
doi: 10.1158/2159-8290.cd-17-1368
-
[241]
S.J. Schuster, J. Svoboda, E.A. Chong, et al., New Engl. J. Med. 377 (2017) 2545–2554.
doi: 10.1056/NEJMoa1708566
-
[242]
S.L. Maude, N. Frey, P.A. Shaw, et al., New Engl. J. Med. 371 (2014) 1507–1517.
doi: 10.1056/NEJMoa1407222
-
[243]
M.L. Davila, I. Riviere, X. Wang, et al., Sci. Transl. Med. 6 (2014) 224 ra25.
-
[244]
C.J. Turtle, K.A. Hay, J. Gust, et al., J. Clin. Oncol. 35 (2017) 3020.
doi: 10.1200/JCO.2017.35.15_suppl.3020
-
[245]
D.W. Lee, R. Gardner, D.L. Porter, et al., Blood 124 (2014) 188–195.
doi: 10.1182/blood-2014-05-552729
-
[246]
C.J. Turtle, L.A. Hanafi, C. Berger, et al., J. Clin. Invest. 126 (2016) 2123–2138.
doi: 10.1172/JCI85309
-
[247]
J.N. Brudno, J.N. Kochenderfer, Blood Rev. 34 (2019) 45–55.
doi: 10.1016/j.blre.2018.11.002
-
[248]
T. Giavridis, S.J.C. van der Stegen, J. Eyquem, et al., Nat. Med. 24 (2018) 731–738.
doi: 10.1038/s41591-018-0041-7
-
[249]
R.M. Sterner, R. Sakemura, M.J. Cox, et al., Blood 133 (2019) 697–709.
doi: 10.1182/blood-2018-10-881722
-
[250]
A. Shetty, R. Hanson, P. Korsten, et al., Drug Des. Devel. Ther. 8 (2014) 349–364.
-
[251]
M. Hong, J.D. Clubb, Y.Y. Chen, Cancer Cell 38 (2020) 473–488.
doi: 10.1016/j.ccell.2020.07.005
-
[252]
E.W. Weber, R.C. Lynn, K.R. Parker, et al., bioRxiv (2020), https://doi.org/10.1101/2020.01.26.920496.
doi: 10.1101/2020.01.26.920496
-
[253]
A. Juillerat, A. Marechal, J.M. Filhol, et al., Sci. Rep. 7 (2017) 39833.
doi: 10.1038/srep39833
-
[254]
M. Casucci, L. Falcone, B. Camisa, et al., Front. Immunol. 9 (2018) 507.
doi: 10.3389/fimmu.2018.00507
-
[255]
A. Di Stasi, S.K. Tey, G. Dotti, et al., New Engl. J. Med. 365 (2011) 1673–1683.
doi: 10.1056/NEJMoa1106152
-
[256]
K.C. Straathof, M.A. Pulè, P. Yotnda, et al., Blood 105 (2005) 4247–4254.
doi: 10.1182/blood-2004-11-4564
-
[257]
B. Kwong, S.A. Gai, J. Elkhader, K.D. Wittrup, D.J. Irvine, Cancer Res. 73 (2013) 1547–1558.
-
[258]
Y. Zheng, L. Tang, L. Mabardi, S. Kumari, D.J. Irvine, ACS Nano 11 (2017) 3089–3100.
doi: 10.1021/acsnano.7b00078
-
[259]
Y.Q. Xie, H. Arik, L. Wei, et al., Biomater. Sci. 7 (2019) 1345–1357.
doi: 10.1039/c8bm01556b
-
[260]
L. Tang, Y. Zheng, M.B. Melo, et al., Nat. Biotechnol. 36 (2018) 707–716.
doi: 10.1038/nbt.4181
-
[261]
Y. Zhang, N. Li, H. Suh, D.J. Irvine, Nat. Commun. 9 (2018) 6.
doi: 10.1038/s41467-017-02251-3
-
[262]
T.T. Smith, H.F. Moffett, S.B. Stephan, et al., J. Clin. Invest. 127 (2017) 2176–2191.
doi: 10.1172/JCI87624
-
[263]
S.B. Stephan, A.M. Taber, I. Jileaeva, et al., Nat. Biotechnol. 33 (2015) 97–101.
doi: 10.1038/nbt.3104
-
[264]
S.K. Härmälä, R. Butcher, C.H. Roberts, Methods Mol. Biol. 1654 (2017) 135–149.
doi: 10.1007/978-1-4939-7231-9_9
-
[265]
L.B. Pinheiro, V.A. Coleman, C.M. Hindson, et al., Anal. Chem. 84 (2012) 1003–1011.
doi: 10.1021/ac202578x
-
[266]
E.D. Nair-Gill, C.J. Shu, C.G. Radu, O.N. Witte, Immunol. Rev. 221 (2008) 214–228.
doi: 10.1111/j.1600-065X.2008.00585.x
-
[267]
E.T. Ahrens, J.W. Bulte, Nat. Rev. Immunol. 13 (2013) 755–763.
doi: 10.1038/nri3531
-
[268]
H. Singh, A.M. Najjar, S. Olivares, et al., Leukemia 23 (2009) 620–622.
doi: 10.1038/leu.2008.256
-
[269]
G. Dotti, M. Tian, B. Savoldo, et al., Mol. Imaging 8 (2009) 230–237.
-
[270]
J. Grimm, M.F. Kircher, R. Weissleder, Radiologe 47 (2007) 25–33.
doi: 10.1007/s00117-006-1449-5
-
[271]
R. Zhou, D.H. Thomas, H. Qiao, et al., J. Nucl. Med. 46 (2005) 816–822.
-
[272]
R.G. Blasberg, J. Gelovani, Mol. Imaging 1 (2002) 280–300.
doi: 10.1162/153535002760235472
-
[273]
V. Ponomarev, M. Doubrovin, I. Serganova, et al., Eur. J. Nucl. Med. Mol. Imaging 31 (2004) 740–751.
doi: 10.1007/s00259-003-1441-5
-
[274]
B.A. Tannous, J. Grimm, K.F. Perry, et al., Nat. Methods 3 (2006) 391–396.
doi: 10.1038/nmeth875
-
[275]
R. Weissleder, A. Moore, U. Mahmood, et al., Nat. Med. 6 (2000) 351–355.
doi: 10.1038/73219
-
[276]
D.B. Kohn, M. Sadelain, J.C. Glorioso, Nat. Rev. Cancer 3 (2003) 477–488.
doi: 10.1038/nrc1122
-
[277]
P. Bhatnagar, Z. Li, Y. Choi, et al., Integr. Biol. (Camb) 5 (2013) 231–238.
doi: 10.1039/c2ib20093g
-
[278]
H. Li, L. Diaz, D. Lee, et al., Radiol. Med. 119 (2014) 269–276.
doi: 10.1007/s11547-013-0335-2
-
[279]
N. Emami-Shahri, J. Foster, R. Kashani, et al., Nat. Commun. 9 (2018) 1081.
doi: 10.1038/s41467-018-03524-1
-
[280]
F. Chapelin, S. Gao, H. Okada, et al., Sci. Rep. 7 (2017) 17748.
doi: 10.1038/s41598-017-17669-4
-
[281]
D.V. Hingorani, F. Chapelin, E. Stares, et al., Magn. Reson. Med. 83 (2020) 974–987.
doi: 10.1002/mrm.27988
-
[282]
L. Liu, Q. Ye, Y. Wu, et al., Nanomedicine 8 (2012) 1345–1354.
doi: 10.1016/j.nano.2012.02.017
-
[283]
A. Kulkarni, P. Rao, S. Natarajan, et al., Proc. Natl. Acad. Sci. U. S. A. 113 (2016) E2104–2113.
doi: 10.1073/pnas.1510959113
-
[284]
M. Horie, K. Nishio, K. Fujita, et al., Chem. Res. Toxicol. 22 (2009) 543–553.
doi: 10.1021/tx800289z
-
[285]
P. Chandran, J.E. Riviere, N.A. Monteiro-Riviere, Nanotoxicology 11 (2017) 507–519.
doi: 10.1080/17435390.2017.1314036
-
[286]
E. Preedia Babu, A. Subastri, A. Suyavaran, et al., Sci. Rep. 7 (2017) 4203.
doi: 10.1038/s41598-017-04440-y
-
[287]
H. Huang, W. Lai, M. Cui, et al., Sci. Rep. 6 (2016) 25518.
doi: 10.1038/srep25518
-
[288]
T. Wang, X. Jiang, ACS Appl. Mater. Interfaces 7 (2015) 129–136.
doi: 10.1021/am503865g
-
[289]
H.L. Karlsson, P. Cronholm, Y. Hedberg, et al., Toxicology 313 (2013) 59–69.
doi: 10.1016/j.tox.2013.07.012
-
[290]
X. He, W.G. Aker, H.M. Hwang, Nanotoxicology 8 (Suppl. 1) (2014) 185–195.
doi: 10.3109/17435390.2013.874050
-
[291]
A. Alalaiwe, G. Roberts, P. Carpinone, J. Munson, S. Roberts, Drug Deliv. 24 (2017) 591–598.
doi: 10.1080/10717544.2017.1282554
-
[292]
Y. Chen, C. Ren, S. Ouyang, X. Hu, Q. Zhou, Environ. Sci. Technol. 49 (2015) 10147–10154.
doi: 10.1021/acs.est.5b02220
-
[293]
J.R. Velicogna, E.E. Ritchie, R.P. Scroggins, J.I. Princz, Nanotoxicology 10 (2016) 1144–1151.
doi: 10.1080/17435390.2016.1181807
-
[294]
S.M. Moghimi, A.C. Hunter, T.L. Andresen, Annu. Rev. Pharmacol. Toxicol. 52 (2012) 481–503.
doi: 10.1146/annurev-pharmtox-010611-134623
-
[295]
D.E. Owens 3rd, N.A. Peppas, Int. J. Pharmacol. 307 (2006) 93–102.
doi: 10.1016/j.ijpharm.2005.10.010
-
[296]
S. Nie, Nanomedicine 5 (2010) 523–528.
doi: 10.2217/nnm.10.23
-
[297]
S.D. Li, L. Huang, Biochim. Biophys. Acta 1788 (2009) 2259–2266.
doi: 10.1016/j.bbamem.2009.06.022
-
[298]
L. Brannon-Peppas, J.O. Blanchette, Adv. Drug. Deliv. Rev. 56 (2004) 1649–1659.
doi: 10.1016/j.addr.2004.02.014
-
[299]
O. Veiseh, J.W. Gunn, M. Zhang, Adv. Drug. Deliv. Rev. 62 (2010) 284–304.
doi: 10.1016/j.addr.2009.11.002
-
[300]
H. Cao, Z. Dan, X. He, et al., ACS Nano 10 (2016) 7738–7748.
doi: 10.1021/acsnano.6b03148
-
[301]
H. Cao, B. Jiang, Y. Yang, et al., J. Colloid Interface Sci. 604 (2021) 596–603.
doi: 10.1016/j.jcis.2021.07.004
-
[302]
T. Kang, Q. Zhu, D. Wei, et al., ACS Nano 11 (2017) 1397–1411.
doi: 10.1021/acsnano.6b06477
-
[303]
Y. Guo, D. Wang, Q. Song, et al., ACS Nano 9 (2015) 6918–6933.
doi: 10.1021/acsnano.5b01042
-
[304]
D. Gao, X. Guo, X. Zhang, et al., Mater. Today Bio 5 (2020) 100035.
doi: 10.1016/j.mtbio.2019.100035
-
[305]
X. Yu, H. Zheng, M.T.V. Chan, W.K.K. Wu, Environ. Sci. Pollut. Res. Int. 25 (2018) 20569–20574.
doi: 10.1007/s11356-018-2426-z
-
[306]
H.S. Hwang, H. Shin, J. Han, K. Na, J. Pharm. Investig. 48 (2018) 143–151.
doi: 10.1007/s40005-017-0377-x
-
[307]
X. Xu, H. Lu, R. Lee, Front. Bioeng. Biotechnol. 8 (2020) 488.
doi: 10.3389/fbioe.2020.00488
-
[308]
G. Lan, K. Ni, Z. Xu, et al., J. Am. Chem. Soc. 140 (2018) 5670–5673.
doi: 10.1021/jacs.8b01072
-
[309]
D.W. Zheng, F. Gao, Q. Cheng, et al., Nat. Commun. 11 (2020) 1985.
doi: 10.1038/s41467-020-15927-0
-
[310]
Y. Wang, Y.X. Lin, S.L. Qiao, et al., Biomaterials 112 (2017) 153–163.
doi: 10.3901/JME.2017.05.153

 Login In
Login In





 DownLoad:
DownLoad:

 DownLoad:
DownLoad: