Label-free photoelectric sensor for lactic acid determination in human sweat
-
* Corresponding authors.
E-mail addresses: dxhan@gzhu.edu.cn (D. Han), lniu@gzhu.edu.cn (L. Niu).
Citation:
Dongfang Han, Xiaolei Li, Zhishan Liang, Bolin Zhao, Zhifang Wu, Fangjie Han, Dongxue Han, Li Niu. Label-free photoelectric sensor for lactic acid determination in human sweat[J]. Chinese Chemical Letters,
;2023, 34(4): 107722.
doi:
10.1016/j.cclet.2022.08.002

Bacterial infectious diseases have posed a serious challenge to the health of human beings and increasingly raised public and medical concerns over the past few decades [1]. Antibiotics were the most effective treatments for bacteria when they were first invented. However, some bacteria have developed severe antibiotic resistance after a long-term abuse or misuse, which is expected to cause 10 million deaths per year by 2050 and a significant economic burden [2]. Despite such a foreseeable crisis for human health nowadays, very few new antibiotics have been developed and marketed, which is mainly due to rapid acquisition of resistance, the long drug development cycle, and poor return on investment [3]. Accordingly, recent efforts have been made to develop alternative new antimicrobial strategies.
Photodynamic therapy (PDT) as a new non-invasive treatment modality for infection, tumor and other diseases has attracted widespread attention due to high spatiotemporal selectivity, minimal side effects and low systemic toxicity over the last few decades [4–6]. During the process of PDT, photosensitizers (PSs) and light were employed to sensitize adjacent normal oxygen (3O2) to generate singlet oxygen (1O2) or other types of reactive oxygen species (ROS) via energy transfer or electron transfer of the excited triplet PSs, respectively [7]. The highly reactive ROS can cause irreversible damages to bacteria by chemical oxidation, so that it is scarcely possible for bacteria to develop resistance to PDT [8]. As far as we know, the treatment outcome of PDT is closely related to the PS employed, i.e., effective PSs with high ROS sensitizing efficiency and target specificity greatly favor the PDT applications [9]. Traditional PSs (e.g., porphyrins, BODIPY, phenothiazinium salts, cyanines) have been widely applied in the field of photodynamic antibacterial and anticancer agents [10–12]. However, these PSs are usually featured with giant hydrophobic coplanar structures, and inclined to experience strong π-π interactions at high concentrations or in the aggregated state, which will result in distinct fluorescence quenching and reduced ROS sensitizing efficiency owing to the decay of the excited state energy via non-radiative pathways [13], thus severely limiting their applications in both imaging and PDT therapy. In comparison, PSs with aggregation-induced emission (AIE) properties, which exhibit both enhanced emission and elevated photosensitization efficiency in the aggregated state or even in aqueous media as a result of restricted intramolecular motions, have recently emerged as a class of promising candidates [14]. To date, a large number of AIE PSs have been successfully developed to kill bacteria [15–19]. Detailed studies have revealed that the bactericidal efficiency of these AIE PSs can be remarkably enhanced when a positively charged AIE PS binds to the bacteria via electrostatic interactions [20], which is attributed to the short lifetimes and small effective working radii of ROS [21]. According to the structural features of the phospholipid bilayer of the bacterial membrane, i.e., the negatively charged "polar head" and the "non-polar tail" of phospholipid, exploring an amphiphilic AIE PS with membrane-targeting capabilities through electrostatic and hydrophobic interactions is highly desirable for higher antibacterial efficiency.
In this work, we have proposed an amphiphilic membrane-targeting AIE PS (TPA-Py, as shown in Fig. 1), in which two d-A type photosensitizers were covalently linked through a flexible butyl chain enabling both the hydrophilic and hydrophobic parts of TPA-Py more easy to insert into the bacterial membranes. More importantly, the rational design has endowed TPA-Py with four main features: (Ⅰ) the triphenylamine (TPA) acting as both hydrophobic donor (D) and AIE active moiety; (Ⅱ) the pyridinium salt acting as hydrophilic acceptor (A); (Ⅲ) the amphiphilic structure favoring bacterial membrane-targeting abilities; (Ⅳ) 2Br– as counter anions facilitating the intersystem crossing (ISC) process due to heavy atomic effect [22]. Moreover, TPA-Py can specifically target bacterial membranes through electrostatic and hydrophobic interactions. As expected, TPA-Py was proved to show a broad-spectrum bacterial staining performance and high 1O2 generation efficiency under irradiation with 420 nm light, resulting in rapid and complete inactivation of S. aureus and E. coli.
The design principle of amphiphilic AIE photosensitizer TPA-Py with membrane-targeting capacity is illustrated in Fig. 1. The triphenylamine (TPA) fragment is elegantly selected in consideration of its superior AIE performance and natural hydrophobicity which will be embedded into the "non-polar tail" of phospholipid by hydrophobic interactions [23]. Besides, the positively charged pyridinium units can anchor on the surface of phospholipid bilayer and bind to "polar head" of phospholipid with negative charge by intense electrostatic interactions [24]. So, considering the amphiphilic feature of the bacterial membranes [25], the conjugation of two amphiphilic chromophores through a flexible butyl chain favors the insertion into the bacterial membranes by the dual hydrophobic and electrostatic interactions, leading to a highly efficient antimicrobial performance. Moreover, once bound to the bacterial membrane structures, the intramolecular motions of TPA-Py are restricted and generate intense fluorescence, implying that AIE phenomenon occurs inside bacteria, which will facilitate bacterial imaging. As depicted in Scheme S1 (Supporting information), the amphiphilic AIE photosensitizer TPA-Py was prepared by two steps in a yield of 63%. And its chemical structure was characterized by 1H NMR, 13C NMR and HRMS spectroscopy (Figs. S2-S4 in Supporting information).
With this photosensitizer in hand, we first examined the photophysical properties of TPA-Py in different solvents. As illustrated in Fig. 2a, TPA-Py exhibited a maximal absorption band ranging from 345 nm to 550 nm in toluene, CHCl3 and DMSO, which is resulted from intramolecular charge transfer (ICT) from TPA moiety to pyridinium unit [26,27]. In contrast to that in DMSO, the maximal absorption wavelength of TPA-Py exhibited a distinct bathochromic-shift in the less polar solvents (toluene and CHCl3), implying a negative solvent-dependent photophysical behavior, which may be attributed to the poor solubility in the small polar solvents causing the formation of the aggregates. Subsequently, its AIE properties were further evaluated by using a mixed solvents (toluene/DMSO, toluene is a poor solvent for TPA-Py). As depicted in Figs. 2b and c, almost no fluorescence was detected for TPA-Py in pure DMSO. When toluene fraction (fT) was increased from 0% to 80%, TPA-Py showed negligible fluorescence change. With continuously raising the fraction of toluene to 90%, the fluorescence intensity of TPA-Py at 545 nm dramatically increased by ca. 275-fold and reached to its maximum along with an intense yellow fluorescence appearance (insert in Fig. 2c), which definitely indicates a typical AIE feature. This fluorescence enhancement phenomenon can be explained by the restriction of rotational motions owing to the formation of the aggregates (Fig. S1 in Supporting information). The average particle diameter of the aggregates was determined to be ca. 69 nm by dynamic light scattering (DLS) analysis (Fig. 2d). In addition, TPA-Py exhibited a relatively high quantum yields (ΦF = 9.6%) in the mixtures of DMSO/toluene with fT = 90% compared to that in pure DMSO (ΦF = 0.23%), as well as a fluorescence lifetime of 3.08 ns (Fig. 2e). As anticipated, a strong yellow fluorescence at 545 nm was observed for TPA-Py in the solid state (Fig. 2f), accompanied with a high quantum yield (ΦF = 8.2%) and shorter lifetime (τ = 1.66 ns) (Fig. 2e).
To further understand the relationships between the electronic features and photophysical properties of TPA-Py, density functional theory (DFT) calculations were conducted to explore its electron densities and optimized molecular geometries in Gaussian 09 B3LYP/6–31G* level [28]. As illustrated in Fig. 2g, the HOMO orbital energy of TPA-Py was mainly delocalized around the TPA fragments, while its LUMO was distributed over the pyridinium units due to electron-deficient effect of pyridinium group, indicating the separation of HOMO-LUMO and a typical D-A type structural feature for either side of TPA-Py, which would favor the ROS generation. In addition, a lower HOMO-LUMO energy gap (Eg = 2.30 eV) was obtained. Based on DFT calculations, the optimized ground-state geometry of TPA-Py presented a "Z-type" configuration between two TPA-pyridinium dyads owing to the presence of the middle flexible C4 chain (Fig. 2 h), which helps to easily insert into the bacterial membranes for efficient antimicrobial performance.
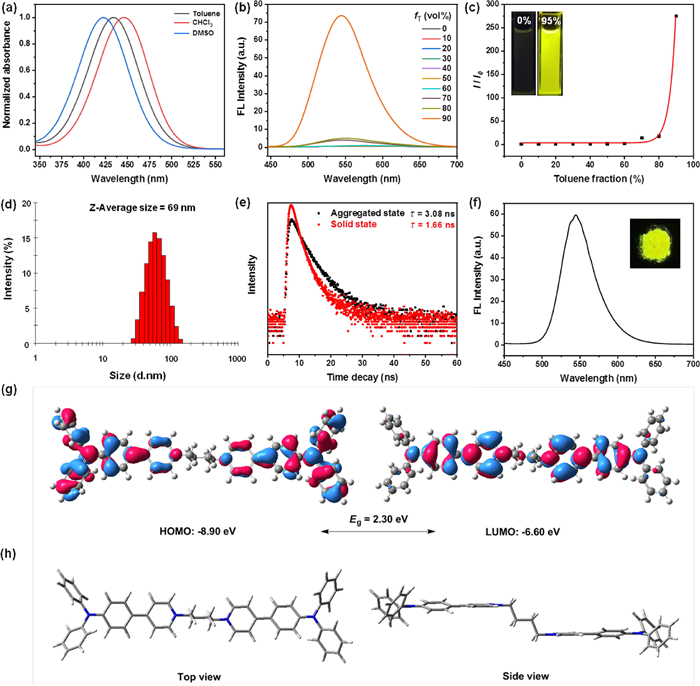
Although some progress has been made in AIEgens-based photosensitizers in recent years, the poor imaging capability for Gram negative (G−) bacteria limits their further research and applications [29,30]. In consideration of the amphiphilic nature of the outer phospholipid membrane of the G− bacteria, the amphiphilic TPA-Py could enhance the binding affinity to the G− bacteria through the dual hydrophobic and electrostatic interactions, thus it is expected to achieve the broad-spectrum imaging of G+ and G− bacteria. We selected S. aureus and E. coli as representatives of G+ and G− bacteria to preliminarily assess the bacterial staining ability of TPA-Py. As depicted in Fig. 3a, when S. aureus was incubated with 10 µmol/L TPA-Py, the bright yellow fluorescence signal was visualized within 15 min, showing excellent bacteria imaging for G+ bacteria. For the previously reported AIE PSs, most showed a very weak staining capability for the G− bacteria. This is mainly attributed to the multilayer outer membranes in G− bacteria composed of phospholipid membranes [31–35], which provides a natural barrier preventing the invasion of the interbedded peptidoglycan network by the extraneous PSs. However, after 15 min of incubating E. coli with TPA-Py, some intense yellow fluorescence was clearly detected in sharp contrast to the background, implying that this amphiphilic AIE PS has a high binding affinity to the outer membrane in G− bacteria. Therefore, these results revealed that TPA-Py could achieve the broad-spectrum bacteria imaging for G+ and G− bacteria.
To further demonstrate targeting capability of TPA-Py toward the bacterial membrane, a phospholipid, 1,2-dihexadecanoyl-sn-glycero-3-phosphocholine (DOPC), was utilized to explore the interactions between TPA-Py and phospholipid. As illustrated in Fig. 3b, TPA-Py exhibited almost no fluorescence in phosphate buffered saline (PBS) with 1% DMSO fraction, while its emission intensity at 546 nm was significantly enhanced by ca. 400-fold with the addition of DOPC (5 mg/mL), accompanied by the emergence of a strong yellow fluorescence under irradiation with a hand-held UV lamp at 365 nm (insert in Fig. 3b). Compared to the maximum emission in the aggregated state (λem = 545 nm) and solid state (λem = 545 nm), TPA-Py showed an analogical emission peak (λem = 546 nm) in DOPC-containing PBS solution, which may be ascribed to essentially the same aggregation form in the bacterial membranes as solution and solid states.
Encouraged by the structural features and broad-spectrum bacterial staining of TPA-Py, its singlet oxygen (1O2) generation capability was further evaluated in PBS with 1% DMSO fraction, which was monitored by time-dependent absorption degradation (378 nm) of commercial 9,10-anthracenediyl-bis-(methylene)-dimalonic acid (ABDA) as an 1O2 indicator upon irradiation with 420 nm light (8.9 mW/cm2) (Scheme S2 in Supporting information). As shown in Fig. 4a, in sharp contrast to ABDA alone, the absorption intensity of ABDA gradually decreased under 420 nm light in the presence of TPA-Py, which comes of the decomposition by the increasing generation of 1O2. To validate its 1O2 generation efficiency, a commercial PS Rose Bengal (RB) was used to perform the same operation in the presence of ABDA. Furthermore, the decomposition rate of ABDA treated with RB was lower than that of TPA-Py by monitoring the attenuation of absorption at 378 nm, indicating the superior 1O2 generation efficiency for TPA-Py. What is more, the absorbance of ABDA in the presence of TPA-Py was decreased by 63.6% upon irradiation for 180 s, revealing that 10.6 µmol of ABDA was consumed per minute when 5 µmol/L of TPA-Py was exposed to 420 nm light. In comparison, degradation with 55.9% of the absorbance at 378 nm of ABDA was obtained for 5 µmol/L of RB, and 9.3 µmol of ABDA was consumed under the same irradiation conditions, which implied a relatively inferior photosensitizing performance compared with the presented amphiphilic TPA-Py. Besides, the 1O2 quantum yield of TPA-Py was determined as 28% with RB as the reference photosensitizer.
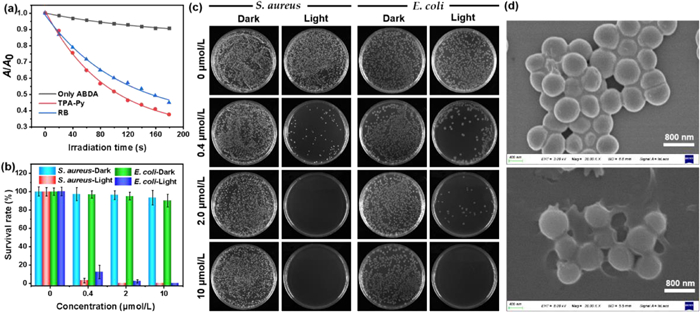
The superior singlet oxygen generation efficiency of TPA-Py inspired us to ultimately investigate its photodynamic antibacterial activity against Gram-positive S. aureus and Gram-negative E. coli under 420 nm light irradiation through a standard plate colony-counting method [36]. As depicted in Figs. 4b and c, in the absence of TPA-Py, almost no obvious changes in the survival rates of S. aureus and E. coli were detected in the dark or under light irradiation, indicating both S. aureus and E. coli can grow and multiply healthily without TPA-Py treatment. For both bacterial strains, although the dark toxicity of TPA-Py was slightly raised with increasing concentration, the survival rates of S. aureus and E. coli in the dark were still as high as ca. 93% and 90% even at a concentration of 10 µmol/L, respectively, which implied a low dark toxicity for TPA-Py. Under the treatment of light irradiation, more than 92% of S. aureus and 87% of E. coli were killed at a low concentration of 0.4 µmol/L. The survival rates of S. aureus and E. coli incubated with 2 µmol/L TPA-Py descended to nearly 0% and 2%, respectively, suggesting a high photodynamic efficiency of TPA-Py toward S. aureus and E. coli. Almost 100% of E. coli was eradicated when the concentration of TPA-Py was increased to 10 µmol/L. Therefore, TPA-Py showed broad-spectrum photodynamic antibacterial activity, which can be reasonably ascribed to a high binding affinity of amphiphilic TPA-Py to the bacterial membranes and the efficient generation of singlet oxygen. Subsequently, scanning electron microscopy (SEM) was utilized to obtain in-depth insights into the morphological changes of S. aureus and E. coli upon treatment with TPA-Py without or with light irradiation. As illustrated in Fig. 4d, when treated with only TPA-Py, the morphology of S. aureus still remained intact with smooth bodies and well-defined borders. Upon treatment with both TPA-Py and light, the bacterial shape was significantly changed along with the shrinkage and fusion of cell walls. Thus, the SEM results definitely provide a direct evidence of the photodynamic antibacterial for TPA-Py toward S. aureus. Unfortunately, treating E. coli by the same method did not obtain a change in bacterial morphology through SEM.
In summary, we rationally designed and successfully developed an amphiphilic AIE photosensitizer (TPA-Py), in which TPA acted as both hydrophobic donor and AIE active moiety and pyridinium salt with positive charge acted as hydrophilic acceptor, thus allowing for easily inserting into the bacterial membranes due to carrying both the hydrophobic and hydrophilic entities. TPA-Py showed excellent AIE properties in the mixtures of DMSO/toluene. As expected, it presented a broad-spectrum bacterial staining ability for S. aureus and E. coli due to electrostatic and hydrophobic interactions. In addition, TPA-Py showed a relatively superior 1O2 generation efficiency compared with the commercial RB. Moreover, TPA-Py exhibited broad-spectrum photodynamic antibacterial activity toward S. aureus and E. coli. This study will provide a promising therapeutic platform for membrane-targeting AIE photosensitizer in the field of antimicrobial PDT.
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
The authors acknowledge financial support from Natural Science Foundation of Henan Province (No. 222300420501), the Key Scientific and Technological Project of Henan province (No. 212102210549), the Key Scientific Research Project of Higher Education of Henan Province (No. 22A430007), and National College Students Innovation and Entrepreneurship Training Program (No. 202210482028).
Supplementary material associated with this article can be found, in the online version, at doi:
S.P. Mathupala, Y.H. Ko, P.L. Pedersen, Semin. Cancer Biol. 19 (2008) 17–24.
D. Baba, A.S. Nugraha, M. Iqbal, RSC Adv. 8 (2018) 10446–10449.
doi: 10.1039/C7RA13026K
J.S. Weiner, R. Van Heyningen, Nature 164 (1949) 351.
doi: 10.1038/164351b0
L. Klous, C.J. de Ruiter, S. Scherrer, N. Gerrett, H.A.M. Daanen, Eur. J. Appl. Physiol. 121 (2021) 803–816.
doi: 10.1007/s00421-020-04562-8
Y. Mao, W. Yue, T. Zhao, Biosensors 10 (2020) 75.
doi: 10.3390/bios10070075
S. Garcia-Rey, E. Ojeda, U.B. Gunatilake, L. Basabe-Desmonts, F. Benito-Lopez, Biosensors 11 (2021) 3–12.
O. Smutok, M. Karkovska, R. Serkiz, Sens Actuators B: Chem 250 (2017) 469–475.
doi: 10.1016/j.snb.2017.04.192
S. Biagi, S. Ghimenti, M. Onor, E. Bramanti, Biomed. Chromatogr. 26 (2012) 1408–1415.
doi: 10.1002/bmc.2713
S. Sugase, T. Tsuda, Jpn. Soc. Anal. Chem. 6 (2002) 429–435.
doi: 10.2116/bunsekikagaku.51.429
Y. Jiang, J. Sun, X. Huang, Analyst 144 (2019) 7017–7023.
doi: 10.1039/C9AN01385G
A. Roda, M. Guardigli, D. Calabria, Analyst 139 (2014) 6494–6501.
doi: 10.1039/C4AN01612B
J. Li, Y. Zhai, B. Zhang, Polym. Int. 57 (2008) 268–274.
doi: 10.1002/pi.2339
W. Dungchai, O. Chailapakul, C.S. Henry, Anal. Chem. 81 (2009) 5821–5826.
doi: 10.1021/ac9007573
S. Baek, J. Kwon, T. Mano, S. Tokito, S. Jung, Macromol. Biosci. 20 (2020) e2000144.
doi: 10.1002/mabi.202000144
W. Gao, S. Emaminejad, H.Y.Y. Nyein, Nature 529 (2016) 509–514.
doi: 10.1038/nature16521
X. Cai, J. Yan, H. Chu, M. Wu, Y. Tu, Sens. Actuators B: Chem. 143 (2010) 655–659.
doi: 10.1016/j.snb.2009.10.002
F. Alam, A.H. Jalal, S. Forouzanfar, IEEE Sens. J. 20 (2020) 5102–5109.
doi: 10.1109/JSEN.2020.2968278
H. Lee, C. Song, Y.S. Hong, Sci. Adv. 3 (2017) e1601314.
doi: 10.1126/sciadv.1601314
G. Baysal, S. Önder, I. Göcek, Textile Res. J. 84 (2014) 1729–1741. ·
doi: 10.1177/0040517514528565
K. Enomoto, R. Shimizu, H. Kudo, Electron. Commun. Jpn. 101 (2018) 41–46.
doi: 10.1002/ecj.12061
F. Han, Z. Song, M. Nawaz, Anal. Chem. 91 (2019) 10657–10662.
doi: 10.1021/acs.analchem.9b01889
B. Gao, X. Zhao, Z. Liang, Anal. Chem. 93 (2021) 820–827.
doi: 10.1021/acs.analchem.0c03315
L. Liang, N. Shuang, M. Dai, Chem. J. Chin. Univ. Chin. 40 (2019) 2081–2089.
M. Dai, W. Ma, F. Han, Chem. Res. Chin. Univ. 37 (2020) 763–771.
F. Han, M. Dai, Z. Liang, Chem. J. Chin. Univ. Chin. 41 (2020) 591–603.
X. Li, H. Li, D. Chen, Cheng, Dalton Trans. 44 (2015) 20316–20320.
doi: 10.1039/C5DT03931B
B. Paul, S. Demuru, C. Lafaye, M. Saubade, D. Briand, Adv. Mater. Technol. 6 (2021) 2000910.
doi: 10.1002/admt.202000910
G. Liu, M. Alomari, B. Sahin, Appl. Phys. Lett. 106 (2015) 133702.
doi: 10.1063/1.4916831
Y.M. Choi, H. Lim, H.N. Lee, Biosensors 10 (2020) 111.
doi: 10.3390/bios10090111
J.Y. Han, M. Li, H. Li, Biosens. Bioelectron. 170 (2020) 112675.
doi: 10.1016/j.bios.2020.112675
S.P. Mathupala, Y.H. Ko, P.L. Pedersen, Semin. Cancer Biol. 19 (2008) 17–24.
D. Baba, A.S. Nugraha, M. Iqbal, RSC Adv. 8 (2018) 10446–10449.
doi: 10.1039/C7RA13026K
J.S. Weiner, R. Van Heyningen, Nature 164 (1949) 351.
doi: 10.1038/164351b0
L. Klous, C.J. de Ruiter, S. Scherrer, N. Gerrett, H.A.M. Daanen, Eur. J. Appl. Physiol. 121 (2021) 803–816.
doi: 10.1007/s00421-020-04562-8
Y. Mao, W. Yue, T. Zhao, Biosensors 10 (2020) 75.
doi: 10.3390/bios10070075
S. Garcia-Rey, E. Ojeda, U.B. Gunatilake, L. Basabe-Desmonts, F. Benito-Lopez, Biosensors 11 (2021) 3–12.
O. Smutok, M. Karkovska, R. Serkiz, Sens Actuators B: Chem 250 (2017) 469–475.
doi: 10.1016/j.snb.2017.04.192
S. Biagi, S. Ghimenti, M. Onor, E. Bramanti, Biomed. Chromatogr. 26 (2012) 1408–1415.
doi: 10.1002/bmc.2713
S. Sugase, T. Tsuda, Jpn. Soc. Anal. Chem. 6 (2002) 429–435.
doi: 10.2116/bunsekikagaku.51.429
Y. Jiang, J. Sun, X. Huang, Analyst 144 (2019) 7017–7023.
doi: 10.1039/C9AN01385G
A. Roda, M. Guardigli, D. Calabria, Analyst 139 (2014) 6494–6501.
doi: 10.1039/C4AN01612B
J. Li, Y. Zhai, B. Zhang, Polym. Int. 57 (2008) 268–274.
doi: 10.1002/pi.2339
W. Dungchai, O. Chailapakul, C.S. Henry, Anal. Chem. 81 (2009) 5821–5826.
doi: 10.1021/ac9007573
S. Baek, J. Kwon, T. Mano, S. Tokito, S. Jung, Macromol. Biosci. 20 (2020) e2000144.
doi: 10.1002/mabi.202000144
W. Gao, S. Emaminejad, H.Y.Y. Nyein, Nature 529 (2016) 509–514.
doi: 10.1038/nature16521
X. Cai, J. Yan, H. Chu, M. Wu, Y. Tu, Sens. Actuators B: Chem. 143 (2010) 655–659.
doi: 10.1016/j.snb.2009.10.002
F. Alam, A.H. Jalal, S. Forouzanfar, IEEE Sens. J. 20 (2020) 5102–5109.
doi: 10.1109/JSEN.2020.2968278
H. Lee, C. Song, Y.S. Hong, Sci. Adv. 3 (2017) e1601314.
doi: 10.1126/sciadv.1601314
G. Baysal, S. Önder, I. Göcek, Textile Res. J. 84 (2014) 1729–1741. ·
doi: 10.1177/0040517514528565
K. Enomoto, R. Shimizu, H. Kudo, Electron. Commun. Jpn. 101 (2018) 41–46.
doi: 10.1002/ecj.12061
F. Han, Z. Song, M. Nawaz, Anal. Chem. 91 (2019) 10657–10662.
doi: 10.1021/acs.analchem.9b01889
B. Gao, X. Zhao, Z. Liang, Anal. Chem. 93 (2021) 820–827.
doi: 10.1021/acs.analchem.0c03315
L. Liang, N. Shuang, M. Dai, Chem. J. Chin. Univ. Chin. 40 (2019) 2081–2089.
M. Dai, W. Ma, F. Han, Chem. Res. Chin. Univ. 37 (2020) 763–771.
F. Han, M. Dai, Z. Liang, Chem. J. Chin. Univ. Chin. 41 (2020) 591–603.
X. Li, H. Li, D. Chen, Cheng, Dalton Trans. 44 (2015) 20316–20320.
doi: 10.1039/C5DT03931B
B. Paul, S. Demuru, C. Lafaye, M. Saubade, D. Briand, Adv. Mater. Technol. 6 (2021) 2000910.
doi: 10.1002/admt.202000910
G. Liu, M. Alomari, B. Sahin, Appl. Phys. Lett. 106 (2015) 133702.
doi: 10.1063/1.4916831
Y.M. Choi, H. Lim, H.N. Lee, Biosensors 10 (2020) 111.
doi: 10.3390/bios10090111
J.Y. Han, M. Li, H. Li, Biosens. Bioelectron. 170 (2020) 112675.
doi: 10.1016/j.bios.2020.112675

Ying Wang , Hong Yang , Caixia Zhu , Qing Hong , Xuwen Cao , Kaiyuan Wang , Yuan Xu , Yanfei Shen , Songqin Liu , Yuanjian Zhang . Cascading oxidoreductases-like nanozymes for high selective and sensitive fluorescent detection of ascorbic acid. Chinese Chemical Letters, 2025, 36(4): 110153-. doi: 10.1016/j.cclet.2024.110153
Caixia Zhu , Qing Hong , Kaiyuan Wang , Yanfei Shen , Songqin Liu , Yuanjian Zhang . Single nanozyme-based colorimetric biosensor for dopamine with enhanced selectivity via reactivity of oxidation intermediates. Chinese Chemical Letters, 2024, 35(10): 109560-. doi: 10.1016/j.cclet.2024.109560
Congyan Liu , Xueyao Zhou , Fei Ye , Bin Jiang , Bo Liu . Confined electric field in nano-sized channels of ionic porous framework towards unique adsorption selectivity. Chinese Chemical Letters, 2025, 36(2): 109969-. doi: 10.1016/j.cclet.2024.109969
Zimo Yang , Yan Tong , Yongbo Liu , Qianlong Liu , Zhihao Ni , Yuna He , Yu Rao . Developing selective PI3K degraders to modulate both kinase and non-kinase functions. Chinese Chemical Letters, 2024, 35(11): 109577-. doi: 10.1016/j.cclet.2024.109577
Conghui Wang , Lei Xu , Zhenhua Jia , Teck-Peng Loh . Recent applications of macrocycles in supramolecular catalysis. Chinese Chemical Letters, 2024, 35(4): 109075-. doi: 10.1016/j.cclet.2023.109075
Junyi Yu , Yin Cheng , Anhong Cai , Xianfeng Huang , Qingrui Zhang . Synthetic Cu(Ⅲ) from copper plating wastewater for onsite decomplexation of Cu(Ⅱ)- and Ni(Ⅱ)-organic complexes. Chinese Chemical Letters, 2025, 36(2): 110549-. doi: 10.1016/j.cclet.2024.110549
Weidan Meng , Yanbo Zhou , Yi Zhou . Green innovation unleashed: Harnessing tungsten-based nanomaterials for catalyzing solar-driven carbon dioxide conversion. Chinese Chemical Letters, 2025, 36(2): 109961-. doi: 10.1016/j.cclet.2024.109961
Wei-Bin Li , Xiao-Chao Huang , Pei Liu , Jie Kong , Guo-Ping Yang . Recent advances in directing group assisted transition metal catalyzed para-selective C-H functionalization. Chinese Chemical Letters, 2025, 36(6): 110543-. doi: 10.1016/j.cclet.2024.110543
Weihan Zhang , Menglu Wang , Ankang Jia , Wei Deng , Shuxing Bai . 表面硫物种对钯-硫纳米片加氢性能的影响. Acta Physico-Chimica Sinica, 2024, 40(11): 2309043-. doi: 10.3866/PKU.WHXB202309043
Qijun Tang , Wenguang Tu , Yong Zhou , Zhigang Zou . High efficiency and selectivity catalyst for photocatalytic oxidative coupling of methane. Chinese Journal of Structural Chemistry, 2023, 42(12): 100170-100170. doi: 10.1016/j.cjsc.2023.100170
Rui HUANG , Shengjie LIU , Qingyuan WU , Nanfeng ZHENG . Enhanced selectivity of catalytic hydrogenation of halogenated nitroaromatics by interfacial effects. Chinese Journal of Inorganic Chemistry, 2025, 41(1): 201-212. doi: 10.11862/CJIC.20240356
Shiqi Xu , Zi Ye , Shuang Shang , Fengge Wang , Huan Zhang , Lianguo Chen , Hao Lin , Chen Chen , Fang Hua , Chong-Jing Zhang . Pairs of thiol-substituted 1,2,4-triazole-based isomeric covalent inhibitors with tunable reactivity and selectivity. Chinese Chemical Letters, 2024, 35(7): 109034-. doi: 10.1016/j.cclet.2023.109034
Shaoming Dong , Yiming Niu , Yinghui Pu , Yongzhao Wang , Bingsen Zhang . Subsurface carbon modification of Ni-Ga for improved selectivity in acetylene hydrogenation reaction. Chinese Chemical Letters, 2024, 35(12): 109525-. doi: 10.1016/j.cclet.2024.109525
Sanmei Wang , Dengxin Yan , Wenhua Zhang , Liangbing Wang . Graphene-supported isolated platinum atoms and platinum dimers for CO2 hydrogenation: Catalytic activity and selectivity variations. Chinese Chemical Letters, 2025, 36(4): 110611-. doi: 10.1016/j.cclet.2024.110611
Xinyue Lan , Junguang Liang , Churan Wen , Xiaolong Quan , Huimin Lin , Qinqin Xu , Peixian Chen , Guangyu Yao , Dan Zhou , Meng Yu . Photo-manipulated polyunsaturated fatty acid-doped liposomal hydrogel for flexible photoimmunotherapy. Chinese Chemical Letters, 2024, 35(4): 108616-. doi: 10.1016/j.cclet.2023.108616
Yulin Mao , Jingyu Ma , Jiecheng Ji , Yuliang Wang , Wanhua Wu , Cheng Yang . Crown aldoxime ethers: Their synthesis, structure, acid-catalyzed/photo-induced isomerization and adjustable guest binding. Chinese Chemical Letters, 2024, 35(11): 109927-. doi: 10.1016/j.cclet.2024.109927
Lijun Yan , Shiqi Chen , Penglu Wang , Xiangyu Liu , Lupeng Han , Tingting Yan , Yuejin Li , Dengsong Zhang . Hydrothermally stable metal oxide-zeolite composite catalysts for low-temperature NOx reduction with improved N2 selectivity. Chinese Chemical Letters, 2024, 35(6): 109132-. doi: 10.1016/j.cclet.2023.109132
Hui Li , Yanxing Qi , Jia Chen , Juanjuan Wang , Min Yang , Hongdeng Qiu . Synthesis of amine-pillar[5]arene porous adsorbent for adsorption of CO2 and selectivity over N2 and CH4. Chinese Chemical Letters, 2024, 35(11): 109659-. doi: 10.1016/j.cclet.2024.109659
Shanyuan Bi , Jin Zhang , Dengchao Peng , Danhong Cheng , Jianping Zhang , Lupeng Han , Dengsong Zhang . Improved N2 selectivity for low-temperature NOx reduction over etched ZSM-5 supported MnCe oxide catalysts. Chinese Chemical Letters, 2025, 36(5): 110295-. doi: 10.1016/j.cclet.2024.110295
Hua Liu , Jian Zhao , Qi Li , Xiang-Yu Zhang , Zhi-Wei Zheng , Kun Huang , Da-Bin Qin , Bin Zhao . Indium-captured zirconium-porphyrin frameworks displaying rare multi-selectivity for catalytic transfer hydrogenation of aldehydes and ketones. Chinese Chemical Letters, 2025, 36(6): 110593-. doi: 10.1016/j.cclet.2024.110593