A TICS-fluorophore based probe for dual-color GSH imaging
-
* Corresponding author.
E-mail address: zcxu@dicp.ac.cn (Z. Xu).
Citation:
Wenjuan Liu, Jie Chen, Qinglong Qiao, Xiaogang Liu, Zhaochao Xu. A TICS-fluorophore based probe for dual-color GSH imaging[J]. Chinese Chemical Letters,
;2022, 33(11): 4943-4947.
doi:
10.1016/j.cclet.2022.03.121

Light-induced charge transfer and separation are fundamental processes in numerous chemical reactions and are widely used in the design of fluorescent probes. Photoexcitation induced twisted intramolecular charge shuttle (TICS) is a newly discovered photo-induced charge transfer phenomenon, which refers to the role swap of electron donor and electron acceptor after intramolecular rotation when the dye molecule absorbs photons and transitions to the excited state [1]. TICS are ubiquitous in a variety of fluorophores including BODIPY, rhodamine and coumarin. The TICS fluorophore has the following typical structural characteristics. Taking rhodamine as the representative fluorophore which is substituted with a tertiary amine at the meso-position, in the ground state, the carbon atom at the 10-position of xanthene is in a state of lack of electricity, which is prone to nucleophilic substitution reaction. After being excited by light, electrons flow to the 10-carbon, and the consequent twist of the meso C-N bond leads to fluorescence quenching. TICS fluorophores are excellent candidates for designing reactive fluorescent probes based on the nucleophilic substitution reaction at the meso site and the concomitant significant fluorescence enhancement [2-5].
Biothiols are the most widely distributed nucleophilic molecules in living systems, among which cysteine (Cys), homocysteine (Hcy) and glutathione (GSH) mainly exist in cells [6]. The concentration of the first two in cells is about 30–200 µmol/L, respectively, while the highest content of GSH is concentrated in the mitochondria, and the concentration can reach 1–10 mmol/L [7]. GSH plays a key role in mitochondrial respiration and is used to maintain mitochondrial redox balance. GSH also plays an important role in intracellular protein synthesis and metabolism [7, 8]. Detecting and tracking the distribution and content dynamics of intracellular GSH is essential for understanding the redox balance and other physiological states of cells, which has stimulated the research upsurge of fluorescent probes for intracellular imaging of GSH [9-13].
GSH probes are mainly based on the electrophilicity of thiol or amino groups, and are designed by reactions such as nucleophilic addition, nucleophilic cleavage, or ligand exchange [9, 14, 15]. Since Cys and Hcy also contain thiol and amino groups, probes that can distinguish GSH from Cys/Hcy have always been a challenge. Niu et al. found that the thiol group of Cys or Hcy would first undergo nucleophilic substitution reaction with BODIPY derivative, and then the amino group would undergo intramolecular substitution of thiol group. However, due to the long distance between the thiol group and the amino group in GSH, this continuous intramolecular nucleophilic substitution is difficult to occur (Scheme 1a) [16]. Thiol substituted and amino substituted fluorophores show two colors of fluorescence with very different wavelengths, thus effectively distinguishing GSH from Cys/Hcy. The mechanism by which the difference in the distance between the thiol and amino groups in the molecule leads to different final thiol or amino substituted products has been widely used in the design of fluorescent probes to distinguish GSH from Cys/Hcy [17-24]. In addition to this single-site cascade reaction, Liu et al. proposed a strategy to simultaneously react with thiol and amino groups at multiple reactive sites within the probe (Scheme 1a), respectively [25]. Depending on the position difference of the reaction site, the conjugated system after the reaction is different, and different fluorescence channels are formed, so as to achieve the effect of distinguishing GSH and Cys/Hcy. This strategy was subsequently used to design more functionally enhanced fluorescent probes [26-31].
So far, only Cys and Hcy have been found to be able to undergo a cascade of thiol/amino substitution reactions, sometimes accompanied by the transition from long-wavelength fluorescence to short-wavelength fluorescence [17], while GSH does not have this reaction characteristic, so the reported GSH probes show for single wavelength fluorescence enhancement.
In this paper, we report the first probe capable of cascading nucleophilic substitution reactions with the thiol and amino groups of GSH successively at a single reaction site, and demonstrated dual-color recognition of GSH (Scheme 1b). The probe Rho-DEA is based on the TICS fluorophore, and the continuous intramolecular nucleophilic substitution reaction occurs with Cys/Hcy, while the intermolecular nucleophilic substitution reaction occurs with GSH. Since the intersystem crossing to triplet state of the thiol substitution in the first step leads to fluorescence quenching for Cys/Hcy, GSH can be selectively recognized from the fluorescence signals. Due to the excellent membrane permeability and the accurate mitochondrial localization properties of Rho-DEA itself, also profit from the excellent brightness and photostability of the reaction product with GSH, Rho-DEA can be used for GSH dynamic tracking in live cell mitochondria. Other four compounds were synthesized and their fluorescence response with biothiols was studied for reference (Scheme S1 in Supporting information).
The spectral properties of probe Rho-DEA were first investigated. The maximum absorption in water was about 470 nm while the maximum emission was about 550 nm (Fig. 1a). Other main spectral properties of Rho-DEA and ethylamine substituted non-TICS reference compound Rho-EA in various solvents were summarized in Table S1 (Supporting information). The TICS-based probe Rho-DEA has an anti-solvation effect in both the ground state (UV-vis absorption) and the excited state (emission), which is due to the absence of charge separation. In contrast, for Rho-EA, due to the presence of an obvious intramolecular dipole, with the increase of solvent polarity, the charge transfer is enhanced, and the charge separation is intensified, resulting in a red-shift in both the absorption and the emission. From the perspective of quantum yield, TICS molecule Rho-DEA exhibits a very low quantum yield (< 0.01) in most solvents, that is, it is in a dark state, while Rho-EA exhibited pretty good optical properties in most solvents with high quantum yields.
By testing the spectroscopic properties of Rho-DEA in aqueous solutions of different pH, we found that with the increase of basicity, the maximum absorption of the compound at 470 nm decreased sharply, and a new absorption peak appeared at 400 nm (Fig. S1a in Supporting information). Correspondingly, the maximum emission intensity at 550 nm decreased, a new emission peak appears at 475 nm, and the intensity gradually increased (Fig. S1b in Supporting information). This change was completely irreversible, that was, once absorption at 400 nm and emission peak at 475 nm appeared upon increase of alkalinity of the solution, the original absorption and emission cannot be restored by lowering the pH of the solution. This phenomenon indicated that the compound undergoes irreversible structural changes at this time. Meanwhile, we noted that the spectra profile in this condition was very close to that of the starting material DMBPN (Fig. S1c in Supporting information). Combined with spectral properties and HRMS analysis, we proposed that DMBPN was generated when Rho-DEA was treated with alkaline conditions. As shown in Fig. S1d (Supporting information), the meso-C of Rho-DEA was attacked by hydroxyl groups which was abundant herein. Then the diethylamine group as a large sterically hindered leaving group quickly left, resulting in the stable resonance form DMBPN. This property of the probe Rho-DEA in alkaline solution demonstrated its typical TICS properties: The meso site is easily attacked by nucleophiles. Then we nest tested its reaction with biothiols in detail.
Considering the concentration of GSH in physiological environment, we first tested the reaction of the probe Rho-DEA with 1 mmol/L GSH in 200 mmol/L PB buffer. Referring to previous work, we also initially speculated that the probe Rho-DEA would generate Rho-S (GSH) accompanied by significant long-wavelength fluorescence. Spectral test showed that when the probe Rho-DEA (10 µmol/L) was mixed with 100 equiv. GSH (1 mmol/L) for 10 min, the UV-vis absorption peak was obviously narrowed, the maximum absorption around 470 nm was slightly reduced, and a new absorption peak around 590 nm appeared (Fig. S2 in Supporting information), associating with the appearance of an emission at 613 nm (Fig. 1b). The above spectra profiles were quite similar to that of reference compound Rho-SEA (Fig. S3 in Supporting information). The new emission band at 613 nm and absorption band at 590 nm were ascribed to the thiol-substituted product of Rho-DEA with GSH. After 3 h, the emission at 613 nm decreased, while a short-wavelength emission at 534 nm appeared and increased quickly (Fig. 1c), associated with the increase of a new absorption band at 430 nm (Fig. S4 in Supporting information). The solution color changed from bright purple to slightly green. Based on the optical properties of reference compound Rho-EA, the emission band at 534 nm and absorption band at 430 nm belong to the subsequent substitution reaction of amino group in GSH with the thiol-substituted product (Table S1, Scheme 1b). From the kinetics of the cascade reactions (Fig. 1d), the reaction of thiol substitution was rapid and reached the maximum fluorescence intensity quickly. The subsequent amino substitution reaction was slower and was affected by the concentration of GSH, which indicated that the second step was an intermolecular amino substitution reaction.
However, the previously reported meso-S-substituted pyronine derivative Rho-SPhR did not find a nucleophilic substitution reaction with the amino group of GSH (Scheme 1a) [18, 19]. We speculate that the reactivity of such molecules is affected by the amino substituent on xanthene. For a TICS fluorophore, the greater the electron-donating ability of the amino substituent on xanthene, the lower the charge deficiency of the meso-C, and the lower reactivity. Conversely, reducing the reactivity of the amino substituent enhanced the reactivity of the meso-C (Fig. 2a). Based on such a mechanism, we performed calculations on the Gibbs free energy difference between S-substituted and N-substituted pyronine derivatives with different amino substituents. We found that as the electron-donating ability of the amino substituent on xanthene increases, (represented by the S-substituted Rho-TDEA), the Gibbs free energy difference decreased, associated with the decreased reaction rate. When the concentration of GSH was increased in the solution of Rho-TDEA, the products of GSH amino-substitution were indeed found, but the reaction rate was much slower than that of Rho-DEA (Fig. 2b). Accordingly, when the concentration of GSH was reduced in the solution of Rho-DEA, the green fluorescent specie of the amino substituted product was not found in a short period of time (Fig. 2c and Fig. S5 in Supporting information). The above results were consistent with the calculations of Gibbs free energy. Finally, the process of nucleophilic substitution reaction of Rho-DEA with GSH thiol group and then a cascade reaction with another GSH amino group was confirmed (Fig. 2d).
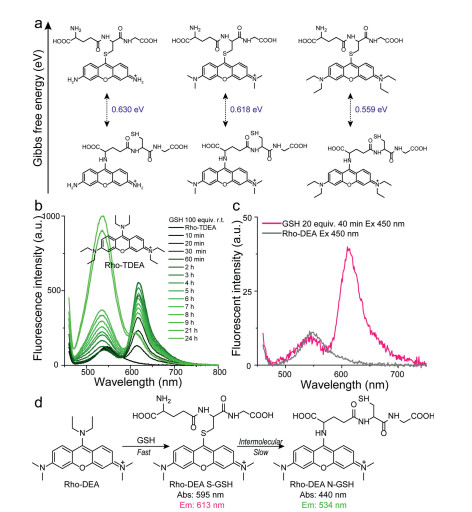
Subsequently, we explored the reaction between Rho-DEA and Cys. With the presence of a high concentration of Cys, it was observed that the absorption peak was significantly narrowed with a large blue shift from ~470 nm to ~430 nm, and a strong new absorption peak at ~590 nm appeared in 5 min (Fig. 3a), while the fluorescence intensity at 534 nm was significantly enhanced (48 fold) (Fig. 3b). Based on the above results and the spectra of reference compounds Rho-EA and Rho-SEA, the absorption at 430 nm and the fluorescence emission at 534 nm were ascribed to the product Rho-N(Cys), and the absorption at 590 nm to the product Rho-S(Cys) with none long wavelength emission. After 30 min, the absorption and emission spectra of the solution did not change significantly, and even the maximum emission intensity at 534 nm was slightly reduced (Fig. S6 in Supporting information). During this process, the S-substituted specie of Rho-DEA with Cys was difficult to be observed. This may be due to the extremely fast reaction rate of Rho-DEA with Cys thiol group, which can be completed within tens of minutes (refer to the dynamic study in Fig. 3c). When we reduced the concentration of Cys to avoid the rapid consumption of the stage product Rho-S(Cys), the absorption band of S-substituted specie of Rho-DEA with Cys thiol group could not be observed (Fig. 3d). The UV-vis absorption at 590 nm showed a trend of increasing first and then decreasing (Fig. 3d), while the fluorescence intensity at 534 nm continued to increase (Fig. S7 in Supporting information). It means that Rho-S (Cys) has a process of generation, accumulation and further consumption. Therefore, the reaction of Rho-DEA with Cys also undergoes the cascade reaction process with thiol and then amino groups (Fig. 3e).
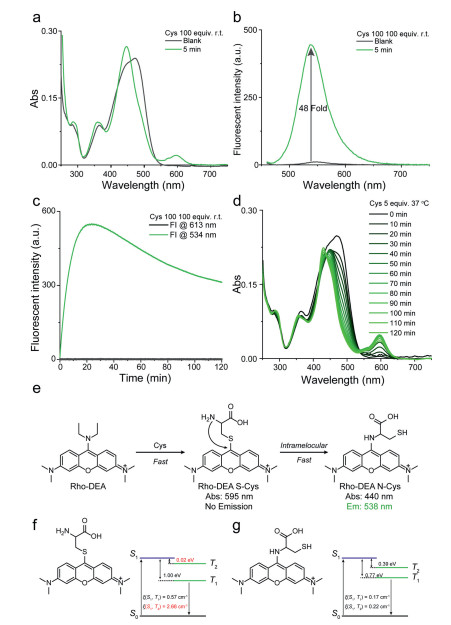
Unlike the reaction between GSH and Rho-DEA, the thiol substitution product between Cys and Rho-DEA is fluorescence quenched. Our calculations showed that Rho-S(Cys) exhibited smaller energy gap (ΔEST) and larger spin-orbit coupling (SOC) between the S1 and the second triplet state (T2) at the ground state geometry, which leads to high intersystem crossing probability and generate triplet state species [32] (Fig. 3f). As for Rho-N(Cys), although it also has a small energy gap (ΔEST), the spin-orbit coupling (SOC) between S1 and T1/T2 is too low to generate triplet species (Fig. 3g). For the same reason, Rho-S (GSH) is also unable to produce triplet products and thus has strong long-wavelength fluorescence (Fig. S8 in Supporting information). We conclude that the main reason for the fluorescence quenching of the Rho-S (Cys) is due to intersystem crossing to triplet state, not a photo-induced electron transfer (PET) process from Cys amino to the fluorophore pyronin [18].
To further investigate the selectivity of Rho-DEA, we measured the fluorescent spectra of Rho-DEA with GSH and 20 various amino acids including Cys and Hcy (1 mmol/L). As shown in Figs. 4a and b, dual color fluorescence was only observed at 613 nm and 534 nm simultaneously when GSH was added. The high selectivity of Rho-DEA to GSH is attributed to the unique cascade reaction with thiol and then amino groups, with the formation of reactive intermediates Rho-S and then stable products Rho-N.
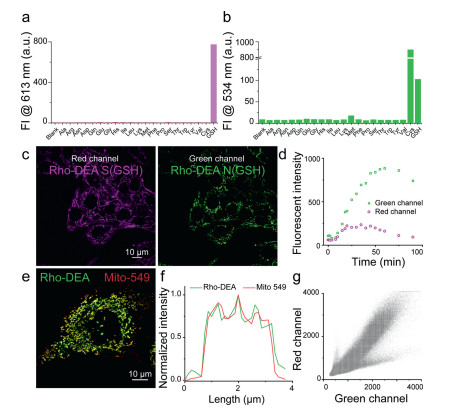
The Rho-DEA can target and enrich in mitochondria selectively because of the cationic rhodamine scaffold. It has been shown that the accumulation of cationic compounds in mitochondria can reach the millimole level concentration [33, 34]. In our case, Rho-DEA can fast target to mitochondria and accumulate to a high concentration, triggering the localized reaction with GSH in mitochondria both at millimolar level. At such high concentration, the conversion from Rho-S(GSH) to Rho-N(GSH) by the attack of free GSH would occur rapidly, meanwhile, the effect of other biothiols can be negligible due to their low concentration.
We incubated HeLa cells with Rho-DEA and tracked the fluorescence changes immediately. As shown in Figs. 4c and d, the fluorescent signals in red channel and green channel appeared rapidly after adding Rho-DEA to cell culture medium. The fluorescence intensity in red channel exhibited increasing first and then decreased, finally quenched completely after 100 min, while the fluorescence intensity of green channel continued to increase until achieving a maximum, which was completely consistent with the phenomenon of the probe reacted with GSH in vitro. It can be seen that the product of the reaction between Rho-DEA and GSH was prominent in mitochondria. By colocalization experiments with the commercial mitochondria probe Mito-549, it is observed that Rho-N (GSH) was mainly distributed in mitochondria, the fluorescence signals of two probes were well overlapped with a Pearson's coeff. of 0.93 (Figs. 4e–g). In addition, the changes of fluorescent signal during the incubation of Rho-DEA also reflected the redox states in mitochondria.
In conclusion, we report a TICS probe, Rho-DEA, which selectively recognizes GSH with dual-color fluorogenicity, which can be used in the tracking of glutathione dynamics and redox balance dynamics in cellular mitochondria.
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
This work is supported by the National Natural Science Foundation of China (Nos. 22078314, 21878286, 21908216).
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.cclet.2022.03.121.
W. Chi, Q. Qiao, R. Lee, et al., Angew. Chem. Int. Ed. 58 (2019) 7073–7077.
doi: 10.1002/anie.201902766
C. Wang, W. Chi, Q. Qiao, et al., Chem. Soc. Rev. 50 (2021) 12656–12678.
doi: 10.1039/D1CS00239B
Y. Song, H. Zhang, X. Wang, et al., Anal. Chem. 93 (2021) 1786–1791.
doi: 10.1021/acs.analchem.0c04644
W. Zhou, X. Fang, Q. Qiao, et al., Chin. Chem. Lett. 32 (2021) 943–946.
doi: 10.1016/j.cclet.2021.02.003
X. Li, J. Zheng, W. Liu, et al., Chin. Chem. Lett. 31 (2020) 2937–2940.
doi: 10.1016/j.cclet.2020.05.043
A. Meister, M.E. Anderson, Annu. Rev. Biochem. 52 (1983) 711–760.
doi: 10.1146/annurev.bi.52.070183.003431
R. Franco, O.J. Schoneveld, A. Pappa, et al., Arch. Physiol. Biochem. 113 (2007) 234–258.
doi: 10.1080/13813450701661198
K. Van Laer, C.J. Hamilton, J. Messens, Antioxid. Redox Signal. 18 (2013) 1642–1653.
doi: 10.1089/ars.2012.4964
W. Liu, J. Chen, Z. Xu, Coord. Chem. Rev. 429 (2021) 213638.
doi: 10.1016/j.ccr.2020.213638
W. Liu, R. Li, F. Deng, et al., ACS Appl. Mater. Interfaces 4 (2021) 2104–2112.
doi: 10.1021/acsabm.0c01269
X. Chen, Y. Zhou, X. Peng, et al., Chem. Soc. Rev. 39 (2010) 2120–2135.
doi: 10.1039/b925092a
T. Jin, M. Cui, D. Wu, et al., Chin. Chem. Lett. 32 (2021) 3899–3902.
doi: 10.1016/j.cclet.2021.06.033
X. Li, H. Wang, Y. Zhang, et al., Chin. Chem. Lett. 32 (2021) 1541–1544.
doi: 10.1016/j.cclet.2020.10.047
C.X. Yin, K.M. Xiong, F.J. Huo, et al., Angew. Chem. Int. Ed. 56 (2017) 13188–13198.
doi: 10.1002/anie.201704084
O. Rusin, N.N. St. Luce, R.A. Agbaria, et al., J. Am. Chem. Soc. 126 (2004) 438–439.
doi: 10.1021/ja036297t
L.Y. Niu, Y.S. Guan, Y.Z. Chen, et al., J. Am. Chem. Soc. 134 (2012) 18928–18931.
doi: 10.1021/ja309079f
F. Wang, Z. Guo, X. Li, et al., Chem. Eur. J. 20 (2014) 11471–11478.
doi: 10.1002/chem.201403450
J. Liu, Y.Q. Sun, H. Zhang, et al., Chem. Sci. 5 (2014) 3183–3188.
doi: 10.1039/c4sc00838c
M. Yang, J. Fan, W. Sun, et al., Anal. Chem. 91 (2019) 12531–12537.
doi: 10.1021/acs.analchem.9b03386
L. He, X. Yang, K. Xu, et al., Chem. Sci. 8 (2017) 6257–6265.
doi: 10.1039/C7SC00423K
X. Yang, L. He, K. Xu, et al., Anal. Chim. Acta 981 (2017) 86–93.
doi: 10.1016/j.aca.2017.05.016
L. Jia, L.Y. Niu, Q.Z. Yang, Anal. Chem. 92 (2020) 10800–10806.
doi: 10.1021/acs.analchem.0c02255
Y. Chen, Y. Wang, X.H. Wu, et al., Dyes Pigm. 186 (2021) 109015.
doi: 10.1016/j.dyepig.2020.109015
J. Yao, G. Yin, T. Yu, et al., Anal. Methods 13 (2021) 1358–1363.
doi: 10.1039/D0AY02206C
J. Liu, Y.Q. Sun, Y. Huo, et al., J. Am. Chem. Soc. 136 (2014) 574–577.
doi: 10.1021/ja409578w
X. Jiang, J. Chen, A. Bajic, et al., Nat. Commun. 8 (2017) 16087.
doi: 10.1038/ncomms16087
Y. Yue, F. Huo, P. Ning, et al., J. Am. Chem. Soc. 139 (2017) 3181–3185.
doi: 10.1021/jacs.6b12845
Y. Yue, F. Huo, P. Yue, et al., Anal. Chem. 90 (2018) 7018–7024.
doi: 10.1021/acs.analchem.8b01406
K. Xiong, F. Huo, J. Chao, et al., Anal. Chem. 91 (2019) 1472–1478.
doi: 10.1021/acs.analchem.8b04485
G.X. Yin, T.T. Niu, Y.B. Gan, et al., Angew. Chem. Int. Ed. 57 (2018) 4991–4994.
doi: 10.1002/anie.201800485
G. Yin, T. Niu, T. Yu, et al., Angew. Chem. Int. Ed. 58 (2019) 4557–4561.
doi: 10.1002/anie.201813935
D. Sasikumar, A.T. John, J. Sunny, et al., Chem. Soc. Rev. 49 (2020) 6122–6140.
doi: 10.1039/D0CS00484G
M.P. Murphy, R.A. Smith, Annu. Rev. Pharmacol. Toxicol. 47 (2007) 629–656.
doi: 10.1146/annurev.pharmtox.47.120505.105110
S. Jakobs, T. Stephan, P. Ilgen, et al., Annu. Rev. Biophys. 49 (2020) 289–308.
doi: 10.1146/annurev-biophys-121219-081550
W. Chi, Q. Qiao, R. Lee, et al., Angew. Chem. Int. Ed. 58 (2019) 7073–7077.
doi: 10.1002/anie.201902766
C. Wang, W. Chi, Q. Qiao, et al., Chem. Soc. Rev. 50 (2021) 12656–12678.
doi: 10.1039/D1CS00239B
Y. Song, H. Zhang, X. Wang, et al., Anal. Chem. 93 (2021) 1786–1791.
doi: 10.1021/acs.analchem.0c04644
W. Zhou, X. Fang, Q. Qiao, et al., Chin. Chem. Lett. 32 (2021) 943–946.
doi: 10.1016/j.cclet.2021.02.003
X. Li, J. Zheng, W. Liu, et al., Chin. Chem. Lett. 31 (2020) 2937–2940.
doi: 10.1016/j.cclet.2020.05.043
A. Meister, M.E. Anderson, Annu. Rev. Biochem. 52 (1983) 711–760.
doi: 10.1146/annurev.bi.52.070183.003431
R. Franco, O.J. Schoneveld, A. Pappa, et al., Arch. Physiol. Biochem. 113 (2007) 234–258.
doi: 10.1080/13813450701661198
K. Van Laer, C.J. Hamilton, J. Messens, Antioxid. Redox Signal. 18 (2013) 1642–1653.
doi: 10.1089/ars.2012.4964
W. Liu, J. Chen, Z. Xu, Coord. Chem. Rev. 429 (2021) 213638.
doi: 10.1016/j.ccr.2020.213638
W. Liu, R. Li, F. Deng, et al., ACS Appl. Mater. Interfaces 4 (2021) 2104–2112.
doi: 10.1021/acsabm.0c01269
X. Chen, Y. Zhou, X. Peng, et al., Chem. Soc. Rev. 39 (2010) 2120–2135.
doi: 10.1039/b925092a
T. Jin, M. Cui, D. Wu, et al., Chin. Chem. Lett. 32 (2021) 3899–3902.
doi: 10.1016/j.cclet.2021.06.033
X. Li, H. Wang, Y. Zhang, et al., Chin. Chem. Lett. 32 (2021) 1541–1544.
doi: 10.1016/j.cclet.2020.10.047
C.X. Yin, K.M. Xiong, F.J. Huo, et al., Angew. Chem. Int. Ed. 56 (2017) 13188–13198.
doi: 10.1002/anie.201704084
O. Rusin, N.N. St. Luce, R.A. Agbaria, et al., J. Am. Chem. Soc. 126 (2004) 438–439.
doi: 10.1021/ja036297t
L.Y. Niu, Y.S. Guan, Y.Z. Chen, et al., J. Am. Chem. Soc. 134 (2012) 18928–18931.
doi: 10.1021/ja309079f
F. Wang, Z. Guo, X. Li, et al., Chem. Eur. J. 20 (2014) 11471–11478.
doi: 10.1002/chem.201403450
J. Liu, Y.Q. Sun, H. Zhang, et al., Chem. Sci. 5 (2014) 3183–3188.
doi: 10.1039/c4sc00838c
M. Yang, J. Fan, W. Sun, et al., Anal. Chem. 91 (2019) 12531–12537.
doi: 10.1021/acs.analchem.9b03386
L. He, X. Yang, K. Xu, et al., Chem. Sci. 8 (2017) 6257–6265.
doi: 10.1039/C7SC00423K
X. Yang, L. He, K. Xu, et al., Anal. Chim. Acta 981 (2017) 86–93.
doi: 10.1016/j.aca.2017.05.016
L. Jia, L.Y. Niu, Q.Z. Yang, Anal. Chem. 92 (2020) 10800–10806.
doi: 10.1021/acs.analchem.0c02255
Y. Chen, Y. Wang, X.H. Wu, et al., Dyes Pigm. 186 (2021) 109015.
doi: 10.1016/j.dyepig.2020.109015
J. Yao, G. Yin, T. Yu, et al., Anal. Methods 13 (2021) 1358–1363.
doi: 10.1039/D0AY02206C
J. Liu, Y.Q. Sun, Y. Huo, et al., J. Am. Chem. Soc. 136 (2014) 574–577.
doi: 10.1021/ja409578w
X. Jiang, J. Chen, A. Bajic, et al., Nat. Commun. 8 (2017) 16087.
doi: 10.1038/ncomms16087
Y. Yue, F. Huo, P. Ning, et al., J. Am. Chem. Soc. 139 (2017) 3181–3185.
doi: 10.1021/jacs.6b12845
Y. Yue, F. Huo, P. Yue, et al., Anal. Chem. 90 (2018) 7018–7024.
doi: 10.1021/acs.analchem.8b01406
K. Xiong, F. Huo, J. Chao, et al., Anal. Chem. 91 (2019) 1472–1478.
doi: 10.1021/acs.analchem.8b04485
G.X. Yin, T.T. Niu, Y.B. Gan, et al., Angew. Chem. Int. Ed. 57 (2018) 4991–4994.
doi: 10.1002/anie.201800485
G. Yin, T. Niu, T. Yu, et al., Angew. Chem. Int. Ed. 58 (2019) 4557–4561.
doi: 10.1002/anie.201813935
D. Sasikumar, A.T. John, J. Sunny, et al., Chem. Soc. Rev. 49 (2020) 6122–6140.
doi: 10.1039/D0CS00484G
M.P. Murphy, R.A. Smith, Annu. Rev. Pharmacol. Toxicol. 47 (2007) 629–656.
doi: 10.1146/annurev.pharmtox.47.120505.105110
S. Jakobs, T. Stephan, P. Ilgen, et al., Annu. Rev. Biophys. 49 (2020) 289–308.
doi: 10.1146/annurev-biophys-121219-081550

Min Liu , Bin Feng , Feiyi Chu , Duoyang Fan , Fan Zheng , Fei Chen , Wenbin Zeng . An ESIPT-boosted NIR nanoprobe for ratiometric sensing of carbon monoxide via activatable aggregation-induced dual-color fluorescence. Chinese Chemical Letters, 2025, 36(5): 110043-. doi: 10.1016/j.cclet.2024.110043
Zhihui Zhang , Ru Sun , Chong Bian , Hongbo Wang , Zhen Zhao , Panpan Lv , Jianzhong Lu , Haixin Zhang , Hulie Zeng , Yuanyuan Chen , Zhijuan Cao . A dual-protease-triggered chemiluminescent probe for precise tumor imaging. Chinese Chemical Letters, 2025, 36(2): 109784-. doi: 10.1016/j.cclet.2024.109784
Huamei Zhang , Jingjing Liu , Mingyue Li , Shida Ma , Xucong Zhou , Aixia Meng , Weina Han , Jin Zhou . Imaging polarity changes in pneumonia and lung cancer using a lipid droplet-targeted near-infrared fluorescent probe. Chinese Chemical Letters, 2024, 35(12): 110020-. doi: 10.1016/j.cclet.2024.110020
Di ZHANG , Tianxiang XIE , Xu HE , Wanyu WEI , Qi FAN , Jie QIAO , Gang JIN , Ningbo LI . Construction and antitumor activity of pH/GSH dual-responsive magnetic nanodrug. Chinese Journal of Inorganic Chemistry, 2025, 41(4): 786-796. doi: 10.11862/CJIC.20240329
Jiayu Zeng , Minhui Liu , Ting Yang , Jia Huang , Songjiao Li , Wanting Zhang , Dan Cheng , Longwei He , Jia Zhou . Two-dimensional design strategy to construct smart dual-responsive fluorescent probe for the precise tracking of ischemic stroke. Chinese Chemical Letters, 2025, 36(5): 110166-. doi: 10.1016/j.cclet.2024.110166
Lixian Fu , Yiyun Tan , Yue Ding , Weixia Qing , Yong Wang . Water–soluble and polarity–sensitive near–infrared fluorescent probe for long–time specific cancer cell membranes imaging and C. Elegans label. Chinese Chemical Letters, 2024, 35(4): 108886-. doi: 10.1016/j.cclet.2023.108886
Han-Min Wang , Yan-Chen Li , Lu-Lu Sun , Ming-Ye Tang , Jia Liu , Jiahao Cai , Lei Dong , Jia Li , Yi Zang , Hai-Hao Han , Xiao-Peng He . Protein-encapsulated long-wavelength fluorescent probe hybrid for imaging lipid droplets in living cells and mice with non-alcoholic fatty liver. Chinese Chemical Letters, 2024, 35(11): 109603-. doi: 10.1016/j.cclet.2024.109603
Ying Wang , Hong Yang , Caixia Zhu , Qing Hong , Xuwen Cao , Kaiyuan Wang , Yuan Xu , Yanfei Shen , Songqin Liu , Yuanjian Zhang . Cascading oxidoreductases-like nanozymes for high selective and sensitive fluorescent detection of ascorbic acid. Chinese Chemical Letters, 2025, 36(4): 110153-. doi: 10.1016/j.cclet.2024.110153
Qiuye Wang , Yabing Sun , Liangxue Lai , Haijing Cui , Yonglong Ye , Ming Yang , Weihao Zhu , Bo Yuan , Quanliang Mao , Wenzhi Ren , Aiguo Wu . MMP-9-responsive probe for fluorescence-magnetic resonance dual-mode imaging of hepatocellular carcinoma models with different metastatic capacities. Chinese Chemical Letters, 2025, 36(4): 110212-. doi: 10.1016/j.cclet.2024.110212
Gongcheng Ma , Qihang Ding , Yuding Zhang , Yue Wang , Jingjing Xiang , Mingle Li , Qi Zhao , Saipeng Huang , Ping Gong , Jong Seung Kim . Palladium-free chemoselective probe for in vivo fluorescence imaging of carbon monoxide. Chinese Chemical Letters, 2024, 35(9): 109293-. doi: 10.1016/j.cclet.2023.109293
Chuan-Zhi Ni , Ruo-Ming Li , Fang-Qi Zhang , Qu-Ao-Wei Li , Yuan-Yuan Zhu , Jie Zeng , Shuang-Xi Gu . A chiral fluorescent probe for molecular recognition of basic amino acids in solutions and cells. Chinese Chemical Letters, 2024, 35(10): 109862-. doi: 10.1016/j.cclet.2024.109862
Tao Liu , Xuwei Han , Xueyi Sun , Weijie Zhang , Ke Gao , Runan Min , Yuting Tian , Caixia Yin . An activated fluorescent probe to monitor NO fluctuation in Parkinson’s disease. Chinese Chemical Letters, 2025, 36(3): 110170-. doi: 10.1016/j.cclet.2024.110170
Wei GAO , Meiqi SONG , Xuan REN , Jianliang BAI , Jing SU , Jianlong MA , Zhijun WANG . A self-calibrating fluorescent probe for the selective detection and bioimaging of HClO. Chinese Journal of Inorganic Chemistry, 2025, 41(6): 1173-1182. doi: 10.11862/CJIC.20250112
Lei ZHANG , Cheng HE , Yang JIAO . An azo-based fluorescent probe for the detection of hypoxic tumor cells. Chinese Journal of Inorganic Chemistry, 2025, 41(6): 1162-1172. doi: 10.11862/CJIC.20250081
Shuaige Bai , Shuai Huang , Ting Luo , Bin Feng , Yanpeng Fang , Feiyi Chu , Jie Dong , Wenbin Zeng . Debut of a responsive chemiluminescent probe for butyrylcholinesterase: Application in biological imaging and pesticide residue detection. Chinese Chemical Letters, 2025, 36(3): 110054-. doi: 10.1016/j.cclet.2024.110054
Dandan Tang , Ningge Xu , Yuyang Fu , Wei Peng , Jinsheng Wu , Heng Liu , Fabiao Yu . Rationally designed an innovative proximity labeling near-infrared fluorogenic probe for imaging of peroxynitrite in acute lung injury. Chinese Chemical Letters, 2025, 36(5): 110082-. doi: 10.1016/j.cclet.2024.110082
Zhoupeng Zheng , Shengyi Gong , Qianhua Li , Shiya Zhang , Guoqiang Feng . Lipid droplets and gallbladder targeted fluorescence probe for ratiometric NO imaging in gallstones disease models. Chinese Chemical Letters, 2025, 36(5): 110191-. doi: 10.1016/j.cclet.2024.110191
Baoli Yin , Xinlin Liu , Zhe Li , Zhifei Ye , Youjuan Wang , Xia Yin , Sulai Liu , Guosheng Song , Shuangyan Huan , Xiao-Bing Zhang . Ratiometric NIR-Ⅱ fluorescent organic nanoprobe for imaging and monitoring tumor-activated photodynamic therapy. Chinese Chemical Letters, 2025, 36(5): 110119-. doi: 10.1016/j.cclet.2024.110119
Meng Gao , Jiqiu Yin , XianChao Jia , Ye Gao , Yang Jiao . On-target site enriching fluorescent bioprobe for imaging of receptor tyrosine kinase in tumor. Chinese Chemical Letters, 2025, 36(6): 110297-. doi: 10.1016/j.cclet.2024.110297
Botao QU , Qian WANG , Xiaogang NING , Yuxin ZHOU , Ruiping ZHANG . Deeply penetrating photoacoustic imaging in tumor tissues based on dual-targeted melanin nanoparticle. Chinese Journal of Inorganic Chemistry, 2024, 40(5): 1025-1032. doi: 10.11862/CJIC.20230416