Constructing oxygen-deficient V2O3@C nanospheres for high performance potassium ion batteries
-
* Corresponding author.
E-mail address: esyangc@scut.edu.cn (C. Yang).
Citation:
Qiang Deng, Luolan Wang, Jing Li, Qian Cheng, Xiaozhao Liu, Changdong Chen, Qimeng Zhang, Wentao Zhong, Hua Wang, Lijue Wu, Chenghao Yang. Constructing oxygen-deficient V2O3@C nanospheres for high performance potassium ion batteries[J]. Chinese Chemical Letters,
;2023, 34(3): 107372.
doi:
10.1016/j.cclet.2022.03.095

Transition metal-catalyzed C-H bond functionalization reactions are among the most straightforward and atom-economic synthetic methodologies for the construction of complex molecules [1-4]. Typically, a directing group is need to facilitate a regioselective C-H activation event. And the use of oxidizing directing group has received tremendous attentions by offering enhanced reactivity and eliminating the employment of external oxidant [5-12]. In this respect, various five- and six-numbered rings are mildly and effectively constructed through the oxidizing directing group strategy. For example, an elegant seminal work from Fagnou reported a Rh(Ⅲ)-catalyzed redox-neutral annulation of benzhydroxamic acids with alkynes towards the synthesis of isoquinolone derivatives by using N-O bond as a built-in oxidant (Scheme 1a) [13]. However, since the eight- membered rhodacycle intermediates are energetically unstable, utilizing this tactics to construct seven-membered rings is elusive [14, 15].
Fluorinated organic molecules have attracted significant attention in drug discovery and agricultural chemistry due to their unique physicochemical and bioactivity properties [16-18]. Traditional methods for the incorporation of fluorine into the molecules often suffer from the need of substrate pre-activation, the use of non-readily available starting materials, low regio- or stereo-selectivity and/or poor functional group tolerance due to the employment of sensitive reagents [19-21]. Compared with the above-mentioned protocols, transition metal-catalyzed C-H/C-F bond activation assisted by a directing group provides a concise and reliable alternative in an atom- and step-economic pattern. In this context, the group of Loh reported a RhⅢ-catalysed tandem C-H/C-F activation for the synthesis of (hetero)arylated monofluoroalkenes using gem-difluoroalkenes as electrophiles (Scheme 1b-1) [22-24]. The group of Wang disclosed a solvent-dependent enantioselective synthesis of alkynyl and monofluoroalkenyl isoindolinones by asymmetric CpRhⅢ-catalyzed C-H activation with α, α-difluoromethylene alkyne as the substrate (Scheme 1b-2) [25-28]. In these two cases, metal-mediated β-fluorine elimination was observed as key step. Previously, we discovered that different directing groups (N-OMe and N-OPiv amides) enabled dictate the selectivity of C-N formation versus β-F elimination with 2, 2-difluorovinyl tosylate as a substrate (Scheme 1c) [29-33]. With N-OMe benzamide being a directing group (DG), the reaction delivered a monofluorinated alkene with the retention of the tosylate functionality. When N-OPiv benzamides were used, however, [4 + 2] cyclization occurred to provide gem-difluorinated dihydroisoquinolin-1(2H)-ones.
Herein, we report a rhodium-catalyzed formal [4 + 3] cycloaddition reaction of N-methoxybenzamides with easily accessible gem-difluorocyclopropenes [34-49]. The reaction allows the formation of highly functionalized fluorinated 2H-azepin-2-one frameworks with excellent regioselectivity and functional group tolerance (Scheme 1d). Some interesting features of the transformation include: i) Both C-N bond formation and fluorine elimination occur in the reaction with N-OMe as an internal oxidant; ii) The combination of [4 + 2] cycloaddition and retro-[2 + 1] strategy eliminates the formation of eight-membered rhodacycle, thereby providing a feasible and reliable route for the construction of seven-membered aromatic heterocycles; iii) This reaction proceeds under rather mild conditions, and a series of bioactive fluorinated 2H-azepin-2-one derivatives (Fig. 1) [50-52] are obtained in moderate to good yields.
The reaction was initially investigated by using N-methoxybenzamide 1a and gem-difluorocyclopropene 2a as model substrates, [Cp*RhCl2]2 as catalyst and K3PO4 as base in CH2Cl2 at 50 ℃ under argon atmosphere. To our delight, the desired product 3a was obtained in 41% yield (Table 1, entry 1). Solvent screening revealed that only chlorinated alkanes promoted the transformation, with 1, 1, 2, 2-tetrachloroethane being the solvent of choice to give a high yield of to 82% (Table 1, entries 2–7). Replacing [Cp*RhCl2]2 with Cp*Rh(OAc)2 led to a reduced yield of 61% (Table 1, entry 8). The reaction did not proceed with other transition metal catalysts such as [Cp*IrCl2]2, [RuCl2(p-cymene)]2 and [CoCp*(CO)I2] (Table 1, entries 9–11). Other inorganic bases were also subsequently used in the reaction, however, the yield of 3a was not further improved (Table 1, entries 12–16).
With optimized conditions in hand, we set to examine the scope. As shown in Scheme 2, the aromatic amide substrates bearing electron-donating (such as Me, t-Bu, OMe and N(CH3)2) and electron-withdrawing substituents (such as Ac, CN, NO2, CF3, CO2Me) at para position underwent reaction smoothly, delivering the cyclized products 3a-3f and 3k-3o in good yields. Substrates bearing halogen substituents were also compatible well (3g-3j), thus providing valuable handles for follow-up transformations. When meta-substituted N-methoxyamide 1p was used, the rhodation occurred at the less hindered site to provide exclusively the C-6 substituted regioisomer (3p). Specially, the 2-methyl substituted substrate did not retard the process (3q), although the yield was slightly reduced. To further highlight the synthetic versatility of our method, several substrates derived from complex natural products and drugs were also subjected to the reaction and the corresponding fluorination products were obtained without difficulty (3s-3v). Furthermore, various alkenyl amides with 2-alkyl substitution were also feasible substrates, producing the products 3w-3aa in moderate yields. Out of our expectation, N-methoxy-2-phenylacrylamide showed no reactivity in the protocol. To demonstrate the scalability of this methodology, the reaction of 1a with 2a was performed on 10 mmol scale, affording 76% yield of the product 3a.
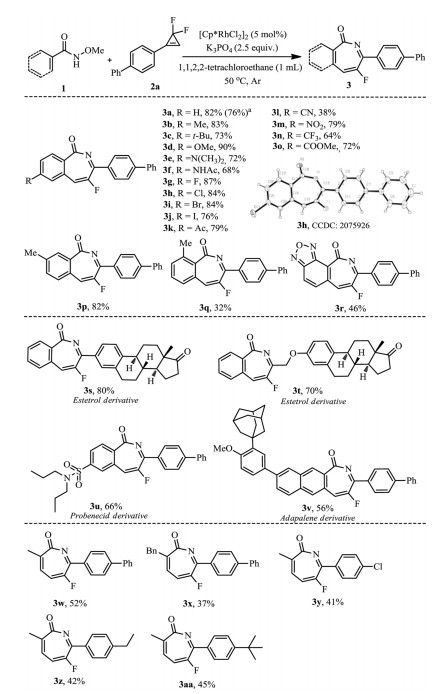
The substrate scope for gem-difluorocyclopropenes was also explored (Scheme 3). It was found the reaction was not sensitive to the electron nature of the substituents on the aryl ring, as a diverse of substituents such as Me, n-Pr, F, Cl, Br, CF3, NO2 and CO2Me well survived in the reaction (3ab-3al). Interestingly, higher yields were obtained for meta-substituted aryl gem-difluorocyclopropenes (3ai-3al). The observed higher bench stability of meta-substituted aryl gem-difluorocyclopropenes could be a reason for this result. Not unexpectedly, substrates with other aromatic heterocycles, for example, thiochroman, pyridine, thiophene and benzoxazole were also tolerant, obtaining the products 3an-3aq in 44%−65% yields. It was intriguing that alkyl-substituted gem-difluorocyclopropenes were also compatible (3ar-3av), greatly expanding the diversity of the title reaction.
Also interesting was the applicability of N-methoxybenzothioamide 4 in the reaction. The cyclization reaction proceeded smoothly to give the desired product 5 in 73% yield (Scheme 4).
When N-methoxy-2-naphthamide 6 was used in the reaction, the 3-position C-H bond with less steric hindrance was exclusively functionalized to give the product 7 in 72% yield (Scheme 5a). Interestingly, treatment of N-methoxybenzo[d][1, 3]dioxole-5-carboxamide 8 with 2a provided the 4-position annulation product 9 in 78% yield (Scheme 5b). The later could be explained by a coordination effect between the oxygen atom at the 3-position and the transition metal.
Intermolecular competitive reactions were performed to understand the reactivity of N-methoxybenzamides and gem-difluorocyclopropenes (Scheme 6). Treatment of N-methoxybenzamide 1a and N-methoxybenzothioamide 4 with 2a under the standard conditions gave only 3a in 80% yield (Scheme 6a). Competition reaction of 4-methoxy-N-methoxybenzamide 1d and 4-acetyl-N-methoxybenzamide 1k with 2a gave exclusively 3k in 76% yield (Scheme 6b). This result demonstrated that the benzamide substrates with electron-donating substituents are less reactive. Furthermore, when 1a was treated with 2ac and 2ah, the corresponding products 3ac and 3ah were isolated in 13% and 68% yields, respectively, suggesting that electron-poorer 2ah is good for the reaction (Scheme 6c).
To further probe the mechanism, several control experiments were conducted (Scheme 7). When D2O was added to the reaction in the absence of 2a, a 47% deuterium incorporation at ortho position of 1a was observed without N-O bond cleavage (Scheme 7a). And a kinetic isotope effect (KIE) value of kH/kD = 1.3 was observed (Scheme 7b). These results suggested that the C-H bond cleavage is reversible and not be involved in the turnover-limiting step. A hydroamination product 10 was unexpectedly obtained in the absence of rhodium catalyst (Scheme 7c). However, this compound was demonstrated not to be an effective intermediate for the title reaction. A rhodacycle Rh-1 was prepared and its intermediacy in the reaction was confirmed by stoichiometric and catalytic reactions, suggesting a C-H activation took place.
To further cast light on the mechanism, theoretical calculations were performed at the density functional theory level (B3LYP). For the convenience of calculation, the active catalyst Cp*Rh(OAc)2 was chosen as the starting point (zero value of energy). Using N-methoxy benzamide 1a as a substrate, N-H deprotonation followed by C-H activation were performed via a concerted metalation-deprotonation (CMD) mechanism with acetate acting as intramolecular base, through transition states TS-1 (ΔG‡ = 13.0 kcal/mol) and TS-2 (ΔG‡ = 19.8 kcal/mol), respectively (Fig. 2). The intermediate verification experiments in Scheme 7c echoed the calculation results. Thereafter, the insertion of gem-difluorocyclopropene 2ai into the rhodacycle INT-5 presented two characteristic spatial arrangements, TS-3 (ΔG‡ = 27.5 kcal/mol) and TS-3′ (ΔG‡ = 28.9 kcal/mol), both of which had a higher activation barrier than the first two steps (Fig. 3). The computational results indicated that C-H activation was not the turnover limiting step in the reaction, consistent with the observed small experimental KIE values (Scheme 7b). Taking into account the higher energy barrier of TS-3′, especially TS-4′, therefore subsequent calculations revolved around TS-3. From INT-7, the priority of either β-fluorine elimination or C-N bond formation was discussed. The results revealed that the direct β-fluorine elimination with or without the assistance of acetic acid, followed by C-N bond formation step via TS-4a and TS-4b, featured a high energy barrier of 33.2 and 35.1 kcal/mol, reaspectively. Two possible pathways for C-N bond formation prior to the defluorination were then calculated. Considering the high energy barrier of TS-4c (ΔG‡ = 68.6 kcal/mol), the direct migration of the methoxy group from the amide to the trivalent rhodium to form INT-9c was tough. The migration process was more reasonable in the assistance of acetic acid, because the energy barrier of TS-4 was reduced to 28.8 kcal/mol and a Rh(Ⅴ) intermediate INT-10 was produced with the free-energy of −54.3 kcal/mol. the synergistic effect of rhodium and acetate accelerated the ring-opening defluorination of INT-10 to release the final product 3ai (ΔG‡ = −71.0 kcal/mol). Overall, the computed Gibbs free-energy changes of the reaction pathway demonstrated a redox-neutral Rh(Ⅲ)-Rh(Ⅴ)-Rh(Ⅲ) catalytic cycle for the developed protocol involving HOAc-prompted oxidative addition and unprecedented C-F bond cleavage/ring expansion processes.
On the basis of the above studies and previous reports [53-57], a plausible mechanism is proposed in Scheme 8. A ligand exchange between [Cp*RhCl2]2 and K3PO4 forms a reactive catalyst. Rhodacycle A is then formed via consecutive N-H and ortho C-H bonds activation. These processes may occur via a CMD (concerted metalation deprotonation)-like mechanism in the aid of internal PO43− base [29]. Afterwards, a migratory insertion of the rhodacycle A into gem-difluorocyclopropene 2 delivers the intermediate B. Rh(Ⅲ) in intermediate B is oxidized to Rh(Ⅴ) nitrenoid intermediate C in the aid of K2HPO4. Rh(Ⅴ) intermediate C returns to Rh(Ⅲ) through a C-N migratory insertion into the nitrenoid. Finally, the synergistic effect of rhodium and K2HPO4 accelerates the ring-opening defluorination to release the product 3.
In summary, we developed a novel [4 + 3] cycloaddition reaction of N-methoxyamides with gem-difluorocyclopropenes, enabling a modular, concise and efficient approach for accessing highly functionalized fluorine-substituted 2H-azepin-2-ones in moderate to good yields. Other appealing features include simple and readily available substrates, mild conditions and broad substrate scope. DFT studies revealed a consecutive C-N bond formation and fluorine elimination events in the annulation reaction. Given the importance of 7-membered heterocycles as well as fluorine atom in medicinal chemistry, we anticipate this protocol will find applications. During the preparation of this work, Yi and Zhou reported a similar work [58].
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
This work was financially supported by National Natural Science Foundation of China (Nos. 21861007, 21702034), Natural Science Foundation of Guangxi Province (No. 2021GXNSFAA075024), "BAGUI Scholar" Program of Guangxi Province of China, High-Level Innovation Team and Distinguished Scholar Program in Guangxi Colleges and Universities.
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.cclet.2022.01.068.
S.M. Mousavi, M. Zarei, S.A. Hashemi, et al., Potassium Ion Batter. Mater. Appl. (2020) 43–98.
Y. Li, Y. Lu, C. Zhao, et al., Energy Storage Mater. 7 (2017) 130–151.
doi: 10.1016/j.ensm.2017.01.002
B. Scrosati, J. Hassoun, Y. -. K. Sun, Energy Environ. Sci. 4 (2011) 3287–3295.
doi: 10.1039/c1ee01388b
S.W. Kim, D.H. Seo, X. Ma, et al., Adv. Energy Mater. 2 (2012) 710–721.
doi: 10.1002/aenm.201200026
Q. Zhang, Z. Wang, X. Li, et al., Chem. Eng. J. 431 (2021) 133456.
X. Wu, D.P. Leonard, X. Ji, Chem. Mater. 29 (2017) 5031–5042.
doi: 10.1021/acs.chemmater.7b01764
Q. Deng, F.H. Zheng, W.T. Zhong, et al., Chem. Eng. J. 392 (2020) 12375.
Y. Liu, Q. Deng, Y. Li, et al., ACS Nano 15 (2021) 1121–1132.
doi: 10.1021/acsnano.0c08094
Y. Xia, W. Jin, Y. Qi, et al., Chin. Chem. Lett. 32 (2021) 2433–2437.
doi: 10.1016/j.cclet.2021.01.025
K. Lei, F. Li, C. Mu, et al., Energy Environ. Sci. 10 (2017) 552–557.
doi: 10.1039/C6EE03185D
S. Komaba, T. Hasegawa, M. Dahbi, et al., Electrochem. Commun. 60 (2015) 172–175.
doi: 10.1016/j.elecom.2015.09.002
W. Zhang, Y. Liu, Z. Guo, Sci. Adv. 5 (2019) eaav7412.
doi: 10.1126/sciadv.aav7412
X. Zou, P. Xiong, J. Zhao, et al., Phys. Chem. Chem. Phys. 19 (2017) 26495–26506.
doi: 10.1039/C7CP03852F
X. Min, J. Xiao, M. Fang, et al., Energy Environ. Sci. 14 (2021) 2186–2243.
doi: 10.1039/D0EE02917C
W. Zhu, A. Li, Z. Wang, et al., Small 17 (2021) 2006424.
doi: 10.1002/smll.202006424
Y. Li, W. Zhong, C. Yang, et al., Chem. Eng. J. 358 (2019) 1147–1154.
doi: 10.1016/j.cej.2018.10.135
A.P. Cohn, N. Muralidharan, R. Carter, et al., J. Mater. Chem. A 4 (2016) 14954–14959.
doi: 10.1039/C6TA06797B
B. Xu, S. Qi, F. Li, et al., Chin. Chem. Lett. 31 (2020) 217–222.
doi: 10.1016/j.cclet.2019.10.009
V. Gabaudan, R. Berthelot, L. Stievano, et al., J. Phys. Chem. C 122 (2018) 18266–18273.
doi: 10.1021/acs.jpcc.8b04575
J. Huang, X. Lin, H. Tan, et al., Adv. Energy Mater. 8 (2018) 1703496.
doi: 10.1002/aenm.201703496
W. Zhang, W. Huang, Q. Zhang, Chem. Eur. J. 27 (2021) 6131–6144.
doi: 10.1002/chem.202005259
Y. Liang, C. Luo, F. Wang, et al., Adv. Energy Mater. 9 (2019) 1802986.
doi: 10.1002/aenm.201802986
J. Zhou, H. Zhao, Q. Zhang, et al., Chem. Commun. 55 (2019) 1406–1409.
doi: 10.1039/C8CC09205B
Y. Li, Q. Zhang, Y. Yuan, et al., Adv. Energy Mater. 10 (2020) 2000717.
doi: 10.1002/aenm.202000717
D. Xu, L. Chen, X. Su, et al., Adv. Funct. Mater. 32 (2022) 2110223.
doi: 10.1002/adfm.202110223
C. Hu, K. Ma, Y. Hu, et al., Green Energy Environ. 6 (2021) 75–82.
doi: 10.1016/j.gee.2020.02.001
Y. Li, C. Yang, F. Zheng, et al., Nano Energy 59 (2019) 582–590.
doi: 10.1016/j.nanoen.2019.03.002
T. Jin, H. Li, Y. Li, et al., Nano Energy 50 (2018) 462–467.
doi: 10.1016/j.nanoen.2018.05.056
F. Chen, S. Wang, X.D. He, et al., J. Mater. Chem. A 8 (2020) 13261–13266.
doi: 10.1039/D0TA01057J
J. Hu, Y. Xie, J. Zheng, et al., ACS Appl. Mater. Interfaces 13 (2021) 12149–12158.
doi: 10.1021/acsami.1c01303
J. Lu, C. Wang, G. Xia, et al., J. Mater. Chem. A 8 (2020) 23939–23946.
doi: 10.1039/D0TA08845E
J. Cao, D. Zhang, Y. Yue, et al., Nano Energy 84 (2021) 105876.
doi: 10.1016/j.nanoen.2021.105876
X. Deng, K. Zou, R. Momen, et al., Sci. Bull. 66 (2021) 1858–1868.
doi: 10.1016/j.scib.2021.04.042
Q. Gan, H. He, Y. Zhu, et al., ACS Nano 13 (2019) 9247–9258.
doi: 10.1021/acsnano.9b03766
Y. Ding, Y. Peng, S. Chen, et al., ACS Appl. Mater. Interfaces 11 (2019) 44109–44117.
doi: 10.1021/acsami.9b13729
Y. Li, K. Xiao, C. Huang, et al., Nano Micro Lett. 13 (2021) 1.
doi: 10.1007/s40820-020-00525-y
S. Petnikota, J.J. Toh, J.Y. Li, et al., ChemElectroChem 6 (2019) 493–503.
doi: 10.1002/celc.201801244
H. Luo, B. Wang, C. Wang, et al., Energy Storage Mater. 33 (2020) 390–398.
doi: 10.1016/j.ensm.2020.08.011
N. Liu, X. Wu, L. Fan, et al., Adv. Mater. 32 (2020) 1908420.
doi: 10.1002/adma.201908420
S. Zhao, K. Yan, P. Munroe, et al., Adv. Energy Mater. 9 (2019) 1803757.
doi: 10.1002/aenm.201803757
Q. Deng, Q. Cheng, X. Liu, et al., Chem. Eng. J. 430 (2022) 132710.
doi: 10.1016/j.cej.2021.132710
H. Geng, M. Cheng, B. Wang, et al., Adv. Funct. Mater. 30 (2020) 1907684.
doi: 10.1002/adfm.201907684
S.M. Mousavi, M. Zarei, S.A. Hashemi, et al., Potassium Ion Batter. Mater. Appl. (2020) 43–98.
Y. Li, Y. Lu, C. Zhao, et al., Energy Storage Mater. 7 (2017) 130–151.
doi: 10.1016/j.ensm.2017.01.002
B. Scrosati, J. Hassoun, Y. -. K. Sun, Energy Environ. Sci. 4 (2011) 3287–3295.
doi: 10.1039/c1ee01388b
S.W. Kim, D.H. Seo, X. Ma, et al., Adv. Energy Mater. 2 (2012) 710–721.
doi: 10.1002/aenm.201200026
Q. Zhang, Z. Wang, X. Li, et al., Chem. Eng. J. 431 (2021) 133456.
X. Wu, D.P. Leonard, X. Ji, Chem. Mater. 29 (2017) 5031–5042.
doi: 10.1021/acs.chemmater.7b01764
Q. Deng, F.H. Zheng, W.T. Zhong, et al., Chem. Eng. J. 392 (2020) 12375.
Y. Liu, Q. Deng, Y. Li, et al., ACS Nano 15 (2021) 1121–1132.
doi: 10.1021/acsnano.0c08094
Y. Xia, W. Jin, Y. Qi, et al., Chin. Chem. Lett. 32 (2021) 2433–2437.
doi: 10.1016/j.cclet.2021.01.025
K. Lei, F. Li, C. Mu, et al., Energy Environ. Sci. 10 (2017) 552–557.
doi: 10.1039/C6EE03185D
S. Komaba, T. Hasegawa, M. Dahbi, et al., Electrochem. Commun. 60 (2015) 172–175.
doi: 10.1016/j.elecom.2015.09.002
W. Zhang, Y. Liu, Z. Guo, Sci. Adv. 5 (2019) eaav7412.
doi: 10.1126/sciadv.aav7412
X. Zou, P. Xiong, J. Zhao, et al., Phys. Chem. Chem. Phys. 19 (2017) 26495–26506.
doi: 10.1039/C7CP03852F
X. Min, J. Xiao, M. Fang, et al., Energy Environ. Sci. 14 (2021) 2186–2243.
doi: 10.1039/D0EE02917C
W. Zhu, A. Li, Z. Wang, et al., Small 17 (2021) 2006424.
doi: 10.1002/smll.202006424
Y. Li, W. Zhong, C. Yang, et al., Chem. Eng. J. 358 (2019) 1147–1154.
doi: 10.1016/j.cej.2018.10.135
A.P. Cohn, N. Muralidharan, R. Carter, et al., J. Mater. Chem. A 4 (2016) 14954–14959.
doi: 10.1039/C6TA06797B
B. Xu, S. Qi, F. Li, et al., Chin. Chem. Lett. 31 (2020) 217–222.
doi: 10.1016/j.cclet.2019.10.009
V. Gabaudan, R. Berthelot, L. Stievano, et al., J. Phys. Chem. C 122 (2018) 18266–18273.
doi: 10.1021/acs.jpcc.8b04575
J. Huang, X. Lin, H. Tan, et al., Adv. Energy Mater. 8 (2018) 1703496.
doi: 10.1002/aenm.201703496
W. Zhang, W. Huang, Q. Zhang, Chem. Eur. J. 27 (2021) 6131–6144.
doi: 10.1002/chem.202005259
Y. Liang, C. Luo, F. Wang, et al., Adv. Energy Mater. 9 (2019) 1802986.
doi: 10.1002/aenm.201802986
J. Zhou, H. Zhao, Q. Zhang, et al., Chem. Commun. 55 (2019) 1406–1409.
doi: 10.1039/C8CC09205B
Y. Li, Q. Zhang, Y. Yuan, et al., Adv. Energy Mater. 10 (2020) 2000717.
doi: 10.1002/aenm.202000717
D. Xu, L. Chen, X. Su, et al., Adv. Funct. Mater. 32 (2022) 2110223.
doi: 10.1002/adfm.202110223
C. Hu, K. Ma, Y. Hu, et al., Green Energy Environ. 6 (2021) 75–82.
doi: 10.1016/j.gee.2020.02.001
Y. Li, C. Yang, F. Zheng, et al., Nano Energy 59 (2019) 582–590.
doi: 10.1016/j.nanoen.2019.03.002
T. Jin, H. Li, Y. Li, et al., Nano Energy 50 (2018) 462–467.
doi: 10.1016/j.nanoen.2018.05.056
F. Chen, S. Wang, X.D. He, et al., J. Mater. Chem. A 8 (2020) 13261–13266.
doi: 10.1039/D0TA01057J
J. Hu, Y. Xie, J. Zheng, et al., ACS Appl. Mater. Interfaces 13 (2021) 12149–12158.
doi: 10.1021/acsami.1c01303
J. Lu, C. Wang, G. Xia, et al., J. Mater. Chem. A 8 (2020) 23939–23946.
doi: 10.1039/D0TA08845E
J. Cao, D. Zhang, Y. Yue, et al., Nano Energy 84 (2021) 105876.
doi: 10.1016/j.nanoen.2021.105876
X. Deng, K. Zou, R. Momen, et al., Sci. Bull. 66 (2021) 1858–1868.
doi: 10.1016/j.scib.2021.04.042
Q. Gan, H. He, Y. Zhu, et al., ACS Nano 13 (2019) 9247–9258.
doi: 10.1021/acsnano.9b03766
Y. Ding, Y. Peng, S. Chen, et al., ACS Appl. Mater. Interfaces 11 (2019) 44109–44117.
doi: 10.1021/acsami.9b13729
Y. Li, K. Xiao, C. Huang, et al., Nano Micro Lett. 13 (2021) 1.
doi: 10.1007/s40820-020-00525-y
S. Petnikota, J.J. Toh, J.Y. Li, et al., ChemElectroChem 6 (2019) 493–503.
doi: 10.1002/celc.201801244
H. Luo, B. Wang, C. Wang, et al., Energy Storage Mater. 33 (2020) 390–398.
doi: 10.1016/j.ensm.2020.08.011
N. Liu, X. Wu, L. Fan, et al., Adv. Mater. 32 (2020) 1908420.
doi: 10.1002/adma.201908420
S. Zhao, K. Yan, P. Munroe, et al., Adv. Energy Mater. 9 (2019) 1803757.
doi: 10.1002/aenm.201803757
Q. Deng, Q. Cheng, X. Liu, et al., Chem. Eng. J. 430 (2022) 132710.
doi: 10.1016/j.cej.2021.132710
H. Geng, M. Cheng, B. Wang, et al., Adv. Funct. Mater. 30 (2020) 1907684.
doi: 10.1002/adfm.201907684

Zhuangzhuang Zhang , Yaru Qiao , Jun Zhao , Dai-Huo Liu , Mengmin Jia , Hongwei Tang , Liang Wang , Dongmei Dai , Bao Li . Fluorine-doped K0.39Mn0.77Ni0.23O1.9F0.1 microspheres with highly reversible oxygen redox reaction for potassium-ion battery cathode. Chinese Chemical Letters, 2025, 36(3): 109907-. doi: 10.1016/j.cclet.2024.109907
Yanxue Wu , Xijun Xu , Shanshan Shi , Fangkun Li , Shaomin Ji , Jingwei Zhao , Jun Liu , Yanping Huo . Facile construction of Cu2-xSe@C nanobelts as anode for superior sodium-ion storage. Chinese Chemical Letters, 2025, 36(6): 110062-. doi: 10.1016/j.cclet.2024.110062
Tong Su , Yue Wang , Qizhen Zhu , Mengyao Xu , Ning Qiao , Bin Xu . Multiple conductive network for KTi2(PO4)3 anode based on MXene as a binder for high-performance potassium storage. Chinese Chemical Letters, 2024, 35(8): 109191-. doi: 10.1016/j.cclet.2023.109191
Li Lin , Song-Lin Tian , Zhen-Yu Hu , Yu Zhang , Li-Min Chang , Jia-Jun Wang , Wan-Qiang Liu , Qing-Shuang Wang , Fang Wang . Molecular crowding electrolytes for stabilizing Zn metal anode in rechargeable aqueous batteries. Chinese Chemical Letters, 2024, 35(7): 109802-. doi: 10.1016/j.cclet.2024.109802
Caili Yang , Tao Long , Ruotong Li , Chunyang Wu , Yuan-Li Ding . Pseudocapacitance dominated Li3VO4 encapsulated in N-doped graphene via 2D nanospace confined synthesis for superior lithium ion capacitors. Chinese Chemical Letters, 2025, 36(2): 109675-. doi: 10.1016/j.cclet.2024.109675
Zhanheng Yan , Weiqing Su , Weiwei Xu , Qianhui Mao , Lisha Xue , Huanxin Li , Wuhua Liu , Xiu Li , Qiuhui Zhang . Carbon-based quantum dots/nanodots materials for potassium ion storage. Chinese Chemical Letters, 2025, 36(4): 110217-. doi: 10.1016/j.cclet.2024.110217
Yunyu Zhao , Chuntao Yang , Yingjian Yu . A review on covalent organic frameworks for rechargeable zinc-ion batteries. Chinese Chemical Letters, 2024, 35(7): 108865-. doi: 10.1016/j.cclet.2023.108865
Junhan Luo , Qi Qing , Liqin Huang , Zhe Wang , Shuang Liu , Jing Chen , Yuexiang Lu . Non-contact gaseous microplasma electrode as anode for electrodeposition of metal and metal alloy in molten salt. Chinese Chemical Letters, 2024, 35(4): 108483-. doi: 10.1016/j.cclet.2023.108483
Kailong Zhang , Chao Zhang , Luanhui Wu , Qidong Yang , Jiadong Zhang , Guang Hu , Liang Song , Gaoran Li , Wenlong Cai . Chloride molten salt derived attapulgite with ground-breaking electrochemical performance. Chinese Chemical Letters, 2024, 35(10): 109618-. doi: 10.1016/j.cclet.2024.109618
Yixin Lu , Minghan Qin , Shixian Zhang , Zhen Liu , Wang Sun , Zhenhua Wang , Jinshuo Qiao , Kening Sun . Triple-conducting heterostructure anodes for electrochemical ethane nonoxidative dehydrogenation by protonic ceramic electrolysis cells. Chinese Chemical Letters, 2025, 36(4): 110567-. doi: 10.1016/j.cclet.2024.110567
Huixin Chen , Chen Zhao , Hongjun Yue , Guiming Zhong , Xiang Han , Liang Yin , Ding Chen . Unraveling the reaction mechanism of high reversible capacity CuP2/C anode with native oxidation POx component for sodium-ion batteries. Chinese Chemical Letters, 2025, 36(1): 109650-. doi: 10.1016/j.cclet.2024.109650
Mianying Huang , Zhiguang Xu , Xiaoming Lin . Mechanistic analysis of Co2VO4/X (X = Ni, C) heterostructures as anode materials of lithium-ion batteries. Chinese Journal of Structural Chemistry, 2024, 43(7): 100309-100309. doi: 10.1016/j.cjsc.2024.100309
Xinpin Pan , Yongjian Cui , Zhe Wang , Bowen Li , Hailong Wang , Jian Hao , Feng Li , Jing Li . Robust chemo-mechanical stability of additives-free SiO2 anode realized by honeycomb nanolattice for high performance Li-ion batteries. Chinese Chemical Letters, 2024, 35(10): 109567-. doi: 10.1016/j.cclet.2024.109567
Renshu Huang , Jinli Chen , Xingfa Chen , Tianqi Yu , Huyi Yu , Kaien Li , Bin Li , Shibin Yin . Synergized oxygen vacancies with Mn2O3@CeO2 heterojunction as high current density catalysts for Li–O2 batteries. Chinese Journal of Structural Chemistry, 2023, 42(11): 100171-100171. doi: 10.1016/j.cjsc.2023.100171
Run Chai , Qiujie Wu , Yongchao Liu , Xiaohui Song , Xuyong Feng , Yi Sun , Hongfa Xiang . A 3D dual layer host with enhanced sodiophilicity as stable anode for high-energy sodium metal batteries. Chinese Chemical Letters, 2025, 36(6): 110007-. doi: 10.1016/j.cclet.2024.110007
Xin-Tong Zhao , Jin-Zhi Guo , Wen-Liang Li , Jing-Ping Zhang , Xing-Long Wu . Two-dimensional conjugated coordination polymer monolayer as anode material for lithium-ion batteries: A DFT study. Chinese Chemical Letters, 2024, 35(6): 108715-. doi: 10.1016/j.cclet.2023.108715
Ruofan Yin , Zhaoxin Guo , Rui Liu , Xian-Sen Tao . Ultrafast synthesis of Na3V2(PO4)3 cathode for high performance sodium-ion batteries. Chinese Chemical Letters, 2025, 36(2): 109643-. doi: 10.1016/j.cclet.2024.109643
Xingang Kong , Yabei Su , Cuijuan Xing , Weijie Cheng , Jianfeng Huang , Lifeng Zhang , Haibo Ouyang , Qi Feng . Facile synthesis of porous TiO2/SnO2 nanocomposite as lithium ion battery anode with enhanced cycling stability via nanoconfinement effect. Chinese Chemical Letters, 2024, 35(11): 109428-. doi: 10.1016/j.cclet.2023.109428
Siqi Sun , Cheng Zhao , Zhaohuan Zhang , Ding Wang , Xinru Yin , Jingting Han , Jinlei Wei , Yong Zhao , Yongheng Zhu . Highly selective QCM sensor based on functionalized hierarchical hollow TiO2 nanospheres for detecting ppb-level 3-hydroxy-2-butanone biomarker at room temperature. Chinese Chemical Letters, 2025, 36(5): 109939-. doi: 10.1016/j.cclet.2024.109939
Yang Li , Xiaoxu Liu , Tianyi Ji , Man Zhang , Xueru Yan , Mengjie Yao , Dawei Sheng , Shaodong Li , Peipei Ren , Zexiang Shen . Potassium ion doped manganese oxide nanoscrolls enhanced the performance of aqueous zinc-ion batteries. Chinese Chemical Letters, 2025, 36(1): 109551-. doi: 10.1016/j.cclet.2024.109551