Nitrogen-bridged Ni(Ⅱ) porphyrinoid trimers with a central quinodiimine unit
-
* Corresponding author.
E-mail address: jxsong@hunnu.edu.cn (J. Song).
Citation:
Kaisheng Wang, Boyu Xiao, Ling Xu, Mingbo Zhou, Takayuki Tanaka, Atsuhiro Osuka, Jianxin Song. Nitrogen-bridged Ni(Ⅱ) porphyrinoid trimers with a central quinodiimine unit[J]. Chinese Chemical Letters,
;2022, 33(10): 4545-4548.
doi:
10.1016/j.cclet.2022.01.061

Porphyrin arrays are organic functional molecules with large π-conjugated systems and have potential applications in optoelectronic devices [1-11], sensors [12-15] and photodynamic therapy (PDT) [16-18]. In the last decade, porphyrin arrays with alkynes [19, 20], benzene [21] or heterocycles (such as thiophene [22], pyridine [23], pyrrole [24, 25]) as bridging units have been intensively studied. Porphyrin dimers with a single carbon or heteroatom bridging unit have received much attention due to their unique photophysical properties, chemical properties, and characteristic electronic delocalization [26-37]. In 2006, Arnold et al. reported the first isolation of meso-meso nitrogen-bridged diporphyrinylamine 1, which showed a broadened Soret band and red shift Q bands, indicating substantial electronic interaction between the porphyrins [27]. Ruppert et al. reported meso-meso, β-meso, β-β-nitrogen-bridged diporphyrinylamines [29], which were all synthesized by Buchwald-Hartwig amination. Later, Osuka et al. reported that meso-meso nitrogen-bridged Ni(Ⅱ) porphyrin dimer was cleanly converted into aminyl radical 2 and nitrenium cation 3 by oxidation with PbO2 and tris(4-bromophenyl)aminiumyl hexachloroantimonate (Magic Blue), respectively (Fig. 1) [34]. As an extension, we report here the synthesis of nitrogen-atom bridged Ni(Ⅱ) porphyrin trimers.
First we attempted to synthesize linear NH-bridged porphyrin trimer 4Ni-2H by the similar Buchwald-Hartwig amination of 5, 15-dibromo Ni(Ⅱ)porphyrin 7Ni with 5-amino Ni(Ⅱ)porphyrin 6Ni [34]. A 4:1 solution of 6Ni and 7Ni in toluene was heated at 100 ℃ for 12 h in the presence of 0.4 equiv. Pd(OAc)2, 0.4 equiv. BINAP, and 7 equiv. t-BuOK (Scheme 1). To our surprise, only a linear trimer 4Ni bearing a central quinodiimine-type porphyrinoid unit was obtained in 38% yield. The matrix-assisted laser desorption ionization time-of-flight (MALDI-TOF) mass spectrum showed the parent ion of 4Ni at m/z 2627.3453 [M]+ (calcd. for (C172H192N14Ni3)+ = 2627.3509) (Fig. S13 in Supporting information), which is smaller by two than the expected parent ion peak of 4Ni-2H. The structure of 4Ni has been revealed by X-ray diffraction structural analysis (Fig. 2 and Fig. S17 in Supporting information). The bond lengths of C2meso-N (1.300(6) Å and 1.304(6) Å) are distinctly shorter than those of C1meso-N (1.412(6) Å and 1.395(7) Å). The 1H NMR spectrum of 4Ni showed broadened signals at room temperature in CDCl3 (Fig. S3 in Supporting information), which gradually changed to sharp peaks upon cooling down to −60 ℃ (Fig. S4 in Supporting information) [38], suggesting conformational motions at room temperature, which are comparable or faster than 1H NMR timescale. It is noteworthy that four doublets due to the b-protons of the central quinodiimine unit were observed in the up-field shifted region at 7.77, 6.76, 5.70 and 3.99 ppm.
Similarly, Buchwald-Hartwig amination of 5, 10-dibromo Ni(Ⅱ)porphyrin 8Ni with 6Ni afforded l-shaped bent trimer 5Ni in 25% yield. The quinodiimine structure of 5Ni has been also confirmed by X-ray analysis. 5Ni shows that the bond lengths of C2meso-N (1.299(5) Å and 1.302(6) Å) are shorter than those of C1meso-N bonds (1.399(5) Å and 1.413(6) Å) (Fig. 2 and Fig. S18 in Supporting information). The 1H NMR spectrum of 5Ni showed broadened signals at room temperature that became sharp and complicated signals at −60 ℃ in CDCl3 (Figs. S5 and S6 in Supporting information). In line with the quinodiimine structure, the corresponding β-protons were observed in the high field at 7.07, 6.73, 6.42, 6.33, 5.66, 4.33, and 3.74 ppm.
The structural data of 4Ni shows that lengths of C1meso-N bonds (1.412(6) Å and 1.395(7) Å) bond to the terminal porphyrin units are longer than C2meso-N (1.300(6) Å and 1.304(6) Å) attached to the central quinodiimine units. Similarly, 5Ni shows that lengths of C1meso-N bonds (1.399(5) Å and 1.413(6) Å) bond to the terminal porphyrin units are longer than C2meso-N (1.299(5) Å and 1.302(6) Å) attached to the central quinodiimine units. The observed short C2meso-N bond lengths in 4Ni and 5Ni indicated its double bond characters significantly [34], which further proved the structure of 4Ni and 5Ni to be N-bridged (rather than NH-bridged) porphyrin trimer. The dihedral angles between the terminal porphyrins and terminal porphyrin, terminal porphyrin and central quinodiimine are 66.81(3)°, 56.34(3)° and 58.06(3)° in 4Ni, and 6.83(3)°, 42.67(3)° and 39.34(3)° in 5Ni (Fig. 2 and Figs. S17 and S18 in Supporting information).
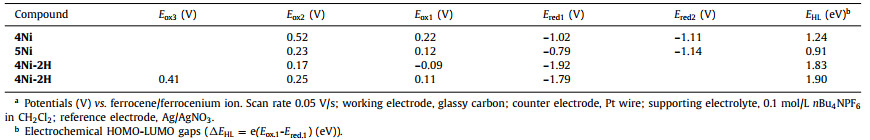
Electrochemical properties of 4Ni and 5Ni were examined by cyclic voltammetry and differential-pulse voltammetry in CH2Cl2 against a ferrocene/ferrocenium ion couple (Table 1 and Table S4 in Supporting information). Reversible oxidation waves were recorded at 0.22 and 0.52 V for 4Ni, and at 0.12 and 0.23 V for 5Ni. Reversible reduction waves were observed at −1.02 and −1.11 V for 4Ni, and at −0.79 and −1.14 V for 5Ni (Figs. S20 and S21 in Supporting information). As a result, the electrochemical HOMO-LUMO gaps of 4Ni and 5Ni were determined to be 1.24 and 0.91 eV, respectively. The observed reversible reduction waves of 4Ni and 5Ni encouraged us to examine their chemical reduction. After many attempts, we found that reduction of 5Ni with aqueous hydrazine in CH2Cl2 afforded 5Ni-2H quantitatively (Scheme 2). Curiously, 4Ni was not reduced with aqueous hydrazine but was reduced quantitatively to give 4Ni-2H with NaBH4 and Pd/C in CH2Cl2/CH3OH. 1H NMR spectra of both 4Ni-2H and 5Ni-2H are very simple, reflecting their symmetric structures with signals of the β-protons appearing in the range of 8–9 ppm (Fig. 3 and Figs. S7 and S8 in Supporting information). The structure of 5Ni-2H has been confirmed by single crystal X-ray diffraction analysis (Fig. 4 and Fig. S19 in Supporting information). In 5Ni-2H, the bond lengths of the C2meso-N bond and the C1meso-N bond are similar, being 1.409(8) Å, 1.406(8) Å and 1.393(7) Å, 1.434(11) Å, respectively, in line with the assigned structures. In addition, the dihedral angles between the terminal porphyrins and the central porphyrin are 58.29(7)° and 58.15(7)°, which are larger than those on 5Ni (42.67(3)° and 39.34(3)°).
|
The unexpected formation of 4Ni and 5Ni may be ascribed to the facile oxidation of 4Ni-2H and 5Ni-2H under the amination reaction conditions. These trimers have the central electron-rich Ni(Ⅱ) porphyrin bearing 5, 15 or 5, 10-aminoporphyrin units. Thus, we examined the electrochemical properties of 4Ni-2H and 5Ni-2H (Table 1 and Table S4 in Supporting information). Actually, the reversible oxidation waves were observed at −0.09 and 0.17 V for 4Ni-2H, and at 0.11, 0.25 and 0.41 V for 5Ni-2H (Figs. S22 and S23 in Supporting information). It is thus conceivable that 4Ni-2H and 5Ni-2H are oxidized under the amination conditions with air. So, when we try to oxidized them with PbO2 and Magic Blue, neither aminyl radical nor nitrenium cation was found. The possible reason may be that the quinodiimine unit is more stable than other species.
The UV–vis-NIR absorption spectra of 4Ni, 5Ni, 4Ni-2H and 5Ni-2H in CH2Cl2 are shown in Fig. 5. 4Ni shows two split Soret bands at 426 and 472 nm, a Q-band at 537 nm, and a broadened Q-like band at 915 nm. 5Ni shows a Soret band at 429 nm, Q-bands at 540 and 581 nm, and a broadened Q-like band at 892 nm. Both 4Ni and 5Ni exhibit characteristic absorption spectra of quinonoidal porphyrinoid arrays [39-42]. 4Ni-2H shows a Soret band at 423 nm, and a Q-band at 627 nm. Similarly to 4Ni-2H, the absorption spectrum of 5Ni-2H shows a Soret band at 418 nm, and a Q-band at 664 nm. In particular, 4Ni and 5Ni display the lowest energy band reaching to 1200 nm and 1400 nm, respectively.
Density functional theory (DFT) calculations clearly indicated that both the HOMO of 4Ni and HOMO-1 5Ni were localized at terminal porphyrin units, whereas both LUMOs of 4Ni and 5Ni were localized at the central quinodiimine units (Figs. S28 and S29 in Supporting information). Time-dependent density functional theory (TD-DFT) calculations indicated that the absorption bands around 1000 nm of trimers 4Ni and 5Ni resulted from the transition from HOMO to LUMO of 4Ni and HOMO-1 to LUMO of 5Ni, respectively (Figs. S24 and S25 in Supporting information). These results show that both absorption bands around 1000 nm of 4Ni and 5Ni could be assigned to charge transfer (CT) band.
In summary, we synthesized N-bridged porphyrinoid trimers 4Ni and 5Ni having the central quinodiimine through Buchwald-Hartwig amination, under which the oxidations of the NH-bridged porphyrin trimers 4Ni-2H and 5Ni-2H proceeded smoothly. The trimer 4Ni-2H was obtained by reduction with NaBH4 and Pd/C, while 5Ni-2H was obtained by reduction with aqueous hydrazine. The structures of 4Ni, 5Ni and 5Ni-2H were determined by X-ray diffraction analysis. The UV–vis-NIR absorption spectra showed that the trimers 4Ni and 5Ni have the lowest energy band reaching to 1200 nm and 1400 nm, respectively. These N-bridged porphyrinoid trimers exhibited interesting spectral properties. Further exploration of cyclic or larger N-bridged porphyrinoid arrays is ongoing in our laboratory.
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
The work at Hunan Normal University was supported by the National Natural Science Foundation of China (Nos. 21772036, 22071052, 21602058, 21702057), the Science and Technology Planning Project of Hunan Province (No. 2018TP1017), and the Scientific Research Fund of Hunan Provincial Education Department (No. 19A331), and Hunan Provincial Innovation Foundation for Postgraduate (No. CX20210473).
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.cclet.2022.01.061.
A. Tsuda, A. Osuka, Science 293 (2001) 79–82.
doi: 10.1126/science.1059552
D. Holten, D.F. Bocian, J.S. Lindsey, Acc. Chem. Res. 35 (2002) 57–69.
doi: 10.1021/ar970264z
N. Aratani, D. Kim, A. Osuka, Chem. Asian J. 4 (2009) 1172–1182.
doi: 10.1002/asia.200900045
N. Aratani, A. Osuka, H.S. Cho, D. Kim, J. Photochem. Photobiol. C 3 (2002) 25–52.
doi: 10.1016/S1389-5567(02)00003-5
K.S. Kim, J.M. Lim, A. Osuka, D. Kim, J. Photochem. Photobiol. C 9 (2008) 13–28.
doi: 10.5467/JKESS.2008.29.1.013
K. Zeng, Z. Tong, L. Ma, et al., Energy Environ. Sci. 13 (2020) 1617–1657.
doi: 10.1039/c9ee04200h
Y. Rio, P. Vázquez, E. Palomares, J. Porphyrins Phthalocyanines 13 (2009) 645–651.
doi: 10.1142/S1088424609000826
L.L. Li, E.W.G. Diau, Chem. Soc. Rev. 42 (2013) 291–304.
doi: 10.1039/C2CS35257E
M. Urbani, M. Grätzel, M.K. Nazeeruddin, T. Torres, Chem. Rev. 114 (2014) 12330–12396.
doi: 10.1021/cr5001964
A. Mahmood, J.Y. Hu, B. Xiao, et al., J. Mater. Chem. A 6 (2018) 16769–16797.
doi: 10.1039/C8TA06392C
Q. Li, C. Li, J. Kim, et al., J. Am. Chem. Soc. 141 (2019) 5294–5302.
doi: 10.1021/jacs.8b13148
J. Yang, M.C. Yoon, H. Yoo, P. Kim, D. Kim, Chem. Soc. Rev. 41 (2012) 4808–4826.
doi: 10.1039/c2cs35022j
V.S.Y. Lin, S.G. DiMagno, M.J. Therien, Science 264 (1994) 1105–1111.
doi: 10.1126/science.8178169
T. Tanaka, A. Osuka, Chem. Soc. Rev. 44 (2015) 943–969.
doi: 10.1039/C3CS60443H
Q. Li, C. Li, G. Baryshnikov, et al., Nat. Commun. 11 (2020) 5289.
doi: 10.1038/s41467-020-19118-9
J. Tian, B. Huang, M.H. Nawaz, W. Zhang, Coord. Chem. Rev. 420 (2020) 213410–213429.
doi: 10.1016/j.ccr.2020.213410
M. Ethirajan, Y. Chen, P. Joshi, R.K. Pandey, Chem. Soc. Rev. 40 (2011) 340–362.
doi: 10.1039/B915149B
R.D. Teo, J.Y. Hwang, J. Termini, Z. Gross, H.B. Gray, Chem. Rev. 117 (2017) 2711–2729.
doi: 10.1021/acs.chemrev.6b00400
M. Rickhaus, A.V. Jentzsch, L. Tejerina, et al., J. Am. Chem. Soc. 139 (2017) 16502–16505.
doi: 10.1021/jacs.7b10710
P.S. Bols, H.L. Anderson, Acc. Chem. Res. 51 (2018) 2083–2092.
doi: 10.1021/acs.accounts.8b00313
O. Wennerström, H. Ericsson, I. Raston, S. Svensson, W. Pimlott, Tetrahedron Lett. 30 (1989) 1129–1132.
doi: 10.1016/S0040-4039(01)80378-6
J. Song, S.Y. Jang, S. Yamaguchi, et al., Angew. Chem. Int. Ed. 47 (2008) 6004–6007.
doi: 10.1002/anie.200802026
J. Song, N. Aratani, J.H. Heo, et al., J. Am. Chem. Soc. 132 (2010) 11868–11869.
doi: 10.1021/ja1046654
C. Maeda, H. Shinokubo, A. Osuka, Org. Lett. 12 (2010) 1820–1823.
doi: 10.1021/ol100448x
Y. Rao, J.O. Kim, W. Kim, et al., Chem. Eur. J. 22 (2016) 8801–8804.
doi: 10.1002/chem.201601306
M.O. Senge, M.G.H. Vicente, K.R. Gerzevske, T.P. Forsyth, K.M. Smith, Inorg. Chem. 33 (1994) 5625–5638.
doi: 10.1021/ic00103a006
L.J. Esdaile, M.O. Senge, D.P. Arnold, Chem. Commun. (2006) 4192–4194.
doi: 10.1039/b608365j
L.J. Esdaile, P. Jensen, J.C. McMurtrie, D.P. Arnold, Angew. Chem. Int. Ed. 46 (2007) 2090–2093.
doi: 10.1002/anie.200604658
A.M.V.M. Pereira, M.G.P.M.S. Neves, J.A.S. Cavaleiro, et al., Org. Lett. 13 (2011) 4742–4745.
doi: 10.1021/ol2020658
C.H. Devillers, S. Hebié, D. Lucas, et al., J. Org. Chem. 79 (2014) 6424–6434.
doi: 10.1021/jo5005586
A.A. Ryan, S. Plunkett, A. Casey, T. McCabe, M.O. Senge, Chem. Commun. 50 (2014) 353–355.
doi: 10.1039/C3CC46828C
K. Fujimoto, H. Yorimitsu, A. Osuka, Chem. Eur. J. 21 (2015) 11311–11314.
doi: 10.1002/chem.201502215
K. Merahi, A.M.V.M. Pereira, C. Jeandon, et al., J. Porphyrins Phthalocyanines 20 (2016) 1233–1243.
doi: 10.1142/S1088424616500954
D. Shimizu, K. Fujimoto, A. Osuka, Angew. Chem. Int. Ed. 57 (2018) 9434–9438.
doi: 10.1002/anie.201805385
N. Fukui, A. Osuka, Bull. Chem. Soc. Jpn. 91 (2018) 1131–1137.
doi: 10.1246/bcsj.20180103
D. Shimizu, Y. Ide, T. Ikeue, A. Osuka, Angew. Chem. Int. Ed. 58 (2019) 5023–5027.
doi: 10.1002/anie.201900792
K. Wang, A. Osuka, J. Song, ACS Cent. Sci. 6 (2020) 2159–2178.
doi: 10.1021/acscentsci.0c01300
H. Mori, J.M. Lim, D. Kim, A. Osuka, Angew. Chem. Int. Ed. 52 (2013) 12997–13001.
doi: 10.1002/anie.201308545
I.M. Blake, L.H. Rees, T.D.W. Claridge, H.L. Anderson, Angew. Chem. Int. Ed. 39 (2000) 1818–1821.
doi: 10.1002/(SICI)1521-3773(20000515)39:10<1818::AID-ANIE1818>3.0.CO;2-E
I.M. Blake, A. Krivokapic, M. Katterle, H.L. Anderson, Chem. Commun. (2002) 1662–1663.
L.J. Esdaile, L. Rintoul, M.S. Goh, et al., Chem. Eur. J. 22 (2016) 3430–3446.
doi: 10.1002/chem.201504252
Y. Jun-i, N. Fukui, K. Furukawa, A. Osuka, Chem. Eur. J. 24 (2018) 1528–1532.
doi: 10.1002/chem.201705769
A. Tsuda, A. Osuka, Science 293 (2001) 79–82.
doi: 10.1126/science.1059552
D. Holten, D.F. Bocian, J.S. Lindsey, Acc. Chem. Res. 35 (2002) 57–69.
doi: 10.1021/ar970264z
N. Aratani, D. Kim, A. Osuka, Chem. Asian J. 4 (2009) 1172–1182.
doi: 10.1002/asia.200900045
N. Aratani, A. Osuka, H.S. Cho, D. Kim, J. Photochem. Photobiol. C 3 (2002) 25–52.
doi: 10.1016/S1389-5567(02)00003-5
K.S. Kim, J.M. Lim, A. Osuka, D. Kim, J. Photochem. Photobiol. C 9 (2008) 13–28.
doi: 10.5467/JKESS.2008.29.1.013
K. Zeng, Z. Tong, L. Ma, et al., Energy Environ. Sci. 13 (2020) 1617–1657.
doi: 10.1039/c9ee04200h
Y. Rio, P. Vázquez, E. Palomares, J. Porphyrins Phthalocyanines 13 (2009) 645–651.
doi: 10.1142/S1088424609000826
L.L. Li, E.W.G. Diau, Chem. Soc. Rev. 42 (2013) 291–304.
doi: 10.1039/C2CS35257E
M. Urbani, M. Grätzel, M.K. Nazeeruddin, T. Torres, Chem. Rev. 114 (2014) 12330–12396.
doi: 10.1021/cr5001964
A. Mahmood, J.Y. Hu, B. Xiao, et al., J. Mater. Chem. A 6 (2018) 16769–16797.
doi: 10.1039/C8TA06392C
Q. Li, C. Li, J. Kim, et al., J. Am. Chem. Soc. 141 (2019) 5294–5302.
doi: 10.1021/jacs.8b13148
J. Yang, M.C. Yoon, H. Yoo, P. Kim, D. Kim, Chem. Soc. Rev. 41 (2012) 4808–4826.
doi: 10.1039/c2cs35022j
V.S.Y. Lin, S.G. DiMagno, M.J. Therien, Science 264 (1994) 1105–1111.
doi: 10.1126/science.8178169
T. Tanaka, A. Osuka, Chem. Soc. Rev. 44 (2015) 943–969.
doi: 10.1039/C3CS60443H
Q. Li, C. Li, G. Baryshnikov, et al., Nat. Commun. 11 (2020) 5289.
doi: 10.1038/s41467-020-19118-9
J. Tian, B. Huang, M.H. Nawaz, W. Zhang, Coord. Chem. Rev. 420 (2020) 213410–213429.
doi: 10.1016/j.ccr.2020.213410
M. Ethirajan, Y. Chen, P. Joshi, R.K. Pandey, Chem. Soc. Rev. 40 (2011) 340–362.
doi: 10.1039/B915149B
R.D. Teo, J.Y. Hwang, J. Termini, Z. Gross, H.B. Gray, Chem. Rev. 117 (2017) 2711–2729.
doi: 10.1021/acs.chemrev.6b00400
M. Rickhaus, A.V. Jentzsch, L. Tejerina, et al., J. Am. Chem. Soc. 139 (2017) 16502–16505.
doi: 10.1021/jacs.7b10710
P.S. Bols, H.L. Anderson, Acc. Chem. Res. 51 (2018) 2083–2092.
doi: 10.1021/acs.accounts.8b00313
O. Wennerström, H. Ericsson, I. Raston, S. Svensson, W. Pimlott, Tetrahedron Lett. 30 (1989) 1129–1132.
doi: 10.1016/S0040-4039(01)80378-6
J. Song, S.Y. Jang, S. Yamaguchi, et al., Angew. Chem. Int. Ed. 47 (2008) 6004–6007.
doi: 10.1002/anie.200802026
J. Song, N. Aratani, J.H. Heo, et al., J. Am. Chem. Soc. 132 (2010) 11868–11869.
doi: 10.1021/ja1046654
C. Maeda, H. Shinokubo, A. Osuka, Org. Lett. 12 (2010) 1820–1823.
doi: 10.1021/ol100448x
Y. Rao, J.O. Kim, W. Kim, et al., Chem. Eur. J. 22 (2016) 8801–8804.
doi: 10.1002/chem.201601306
M.O. Senge, M.G.H. Vicente, K.R. Gerzevske, T.P. Forsyth, K.M. Smith, Inorg. Chem. 33 (1994) 5625–5638.
doi: 10.1021/ic00103a006
L.J. Esdaile, M.O. Senge, D.P. Arnold, Chem. Commun. (2006) 4192–4194.
doi: 10.1039/b608365j
L.J. Esdaile, P. Jensen, J.C. McMurtrie, D.P. Arnold, Angew. Chem. Int. Ed. 46 (2007) 2090–2093.
doi: 10.1002/anie.200604658
A.M.V.M. Pereira, M.G.P.M.S. Neves, J.A.S. Cavaleiro, et al., Org. Lett. 13 (2011) 4742–4745.
doi: 10.1021/ol2020658
C.H. Devillers, S. Hebié, D. Lucas, et al., J. Org. Chem. 79 (2014) 6424–6434.
doi: 10.1021/jo5005586
A.A. Ryan, S. Plunkett, A. Casey, T. McCabe, M.O. Senge, Chem. Commun. 50 (2014) 353–355.
doi: 10.1039/C3CC46828C
K. Fujimoto, H. Yorimitsu, A. Osuka, Chem. Eur. J. 21 (2015) 11311–11314.
doi: 10.1002/chem.201502215
K. Merahi, A.M.V.M. Pereira, C. Jeandon, et al., J. Porphyrins Phthalocyanines 20 (2016) 1233–1243.
doi: 10.1142/S1088424616500954
D. Shimizu, K. Fujimoto, A. Osuka, Angew. Chem. Int. Ed. 57 (2018) 9434–9438.
doi: 10.1002/anie.201805385
N. Fukui, A. Osuka, Bull. Chem. Soc. Jpn. 91 (2018) 1131–1137.
doi: 10.1246/bcsj.20180103
D. Shimizu, Y. Ide, T. Ikeue, A. Osuka, Angew. Chem. Int. Ed. 58 (2019) 5023–5027.
doi: 10.1002/anie.201900792
K. Wang, A. Osuka, J. Song, ACS Cent. Sci. 6 (2020) 2159–2178.
doi: 10.1021/acscentsci.0c01300
H. Mori, J.M. Lim, D. Kim, A. Osuka, Angew. Chem. Int. Ed. 52 (2013) 12997–13001.
doi: 10.1002/anie.201308545
I.M. Blake, L.H. Rees, T.D.W. Claridge, H.L. Anderson, Angew. Chem. Int. Ed. 39 (2000) 1818–1821.
doi: 10.1002/(SICI)1521-3773(20000515)39:10<1818::AID-ANIE1818>3.0.CO;2-E
I.M. Blake, A. Krivokapic, M. Katterle, H.L. Anderson, Chem. Commun. (2002) 1662–1663.
L.J. Esdaile, L. Rintoul, M.S. Goh, et al., Chem. Eur. J. 22 (2016) 3430–3446.
doi: 10.1002/chem.201504252
Y. Jun-i, N. Fukui, K. Furukawa, A. Osuka, Chem. Eur. J. 24 (2018) 1528–1532.
doi: 10.1002/chem.201705769

Haiyan Yin , Abdusalam Ablez , Zhuangzhuang Wang , Weian Li , Yanqi Wang , Qianqian Hu , Xiaoying Huang . Novel open-framework chalcogenide photocatalysts: Cobalt cocatalyst valence state modulating critical charge transfer pathways towards high-efficiency hydrogen evolution. Chinese Journal of Structural Chemistry, 2025, 44(4): 100560-100560. doi: 10.1016/j.cjsc.2025.100560
Shu-Ran Xu , Fang-Xing Xiao . Metal halide perovskites quantum dots: Synthesis, and modification strategies for solar CO2 conversion. Chinese Journal of Structural Chemistry, 2023, 42(12): 100173-100173. doi: 10.1016/j.cjsc.2023.100173
Shuqi Yu , Yu Yang , Keisuke Kuroda , Jian Pu , Rui Guo , Li-An Hou . Selective removal of Cr(Ⅵ) using polyvinylpyrrolidone and polyacrylamide co-modified MoS2 composites by adsorption combined with reduction. Chinese Chemical Letters, 2024, 35(6): 109130-. doi: 10.1016/j.cclet.2023.109130
Yuchen Wang , Yaoyu Liu , Xiongfei Huang , Guanjie He , Kai Yan . Fe nanoclusters anchored in biomass waste-derived porous carbon nanosheets for high-performance supercapacitor. Chinese Chemical Letters, 2024, 35(8): 109301-. doi: 10.1016/j.cclet.2023.109301
Guodong Xu , Chengcai Sheng , Xiaomeng Zhao , Tuojiang Zhang , Zongtang Liu , Jun Dong . Reform of Comprehensive Organic Chemistry Experiments in the Context of Emerging Engineering Education: A Case Study on the Improved Preparation of Benzocaine. University Chemistry, 2024, 39(11): 286-295. doi: 10.12461/PKU.DXHX202403094
Jianyin He , Liuyun Chen , Xinling Xie , Zuzeng Qin , Hongbing Ji , Tongming Su . ZnCoP/CdLa2S4肖特基异质结的构建促进光催化产氢. Acta Physico-Chimica Sinica, 2024, 40(11): 2404030-. doi: 10.3866/PKU.WHXB202404030
Xiutao Xu , Chunfeng Shao , Jinfeng Zhang , Zhongliao Wang , Kai Dai . Rational Design of S-Scheme CeO2/Bi2MoO6 Microsphere Heterojunction for Efficient Photocatalytic CO2 Reduction. Acta Physico-Chimica Sinica, 2024, 40(10): 2309031-. doi: 10.3866/PKU.WHXB202309031
Fang Niu , Rong Li , Qiaolan Zhang . Analysis of Gas-Solid Adsorption Behavior in Resistive Gas Sensing Process. University Chemistry, 2024, 39(8): 142-148. doi: 10.3866/PKU.DXHX202311102
Quanyou Guo , Yue Yang , Tingting Hu , Hongqi Chu , Lijun Liao , Xuepeng Wang , Zhenzi Li , Liping Guo , Wei Zhou . Regulating local electron transfer environment of covalent triazine frameworks through F, N co-modification towards optimized oxygen reduction reaction. Chinese Chemical Letters, 2025, 36(1): 110235-. doi: 10.1016/j.cclet.2024.110235
Yihu Ke , Shuai Wang , Fei Jin , Guangbo Liu , Zhiliang Jin , Noritatsu Tsubaki . Charge transfer optimization: Role of Cu-graphdiyne/NiCoMoO4 S-scheme heterojunction and Ohmic junction. Chinese Journal of Structural Chemistry, 2024, 43(12): 100458-100458. doi: 10.1016/j.cjsc.2024.100458
Xiang Li , Beibei Zhang , Zhixiang Wang , Xiangyu Chen . Organocatalyzed iodine-mediated reversible-deactivation radical polymerization via photoinduced charge transfer complex catalysis. Chinese Chemical Letters, 2025, 36(6): 110383-. doi: 10.1016/j.cclet.2024.110383
Xiuzheng Deng , Yi Ke , Jiawen Ding , Yingtang Zhou , Hui Huang , Qian Liang , Zhenhui Kang . Construction of ZnO@CDs@Co3O4 sandwich heterostructure with multi-interfacial electron-transfer toward enhanced photocatalytic CO2 reduction. Chinese Chemical Letters, 2024, 35(4): 109064-. doi: 10.1016/j.cclet.2023.109064
Yuwei Liu , Yihui Zhu , Weijian Duan , Yizhuo Yang , Haorui Tuo , Chunhua Feng . Electrocatalytic nitrate reduction on Fe, Fe3O4, and Fe@Fe3O4 cathodes: Elucidating structure-sensitive mechanisms of direct electron versus hydrogen atom transfer. Chinese Chemical Letters, 2025, 36(6): 110347-. doi: 10.1016/j.cclet.2024.110347
Jieqiong Xu , Wenbin Chen , Shengkai Li , Qian Chen , Tao Wang , Yadong Shi , Shengyong Deng , Mingde Li , Peifa Wei , Zhuo Chen . Organic stoichiometric cocrystals with a subtle balance of charge-transfer degree and molecular stacking towards high-efficiency NIR photothermal conversion. Chinese Chemical Letters, 2024, 35(10): 109808-. doi: 10.1016/j.cclet.2024.109808
Xiao Yu , Dongyue Cui , Mengmeng Wang , Zhaojin Wang , Mengzhu Wang , Deshuang Tu , Vladimir Bregadze , Changsheng Lu , Qiang Zhao , Runfeng Chen , Hong Yan . Boron cluster-based TADF emitter via through-space charge transfer enabling efficient orange-red electroluminescence. Chinese Chemical Letters, 2025, 36(3): 110520-. doi: 10.1016/j.cclet.2024.110520
Manlin Lu , Sheng Liao , Jiayu Li , Zidong Yu , Ningjiu Zhao , Zuoti Xie , Shunli Chen , Li Dang , Ming-De Li . Face-to-face π-π interactions and electron communication boosting efficient reverse intersystem crossing in through-space charge transfer molecules. Chinese Chemical Letters, 2025, 36(6): 110066-. doi: 10.1016/j.cclet.2024.110066
Huizhong Wu , Ruiheng Liang , Ge Song , Zhongzheng Hu , Xuyang Zhang , Minghua Zhou . Enhanced interfacial charge transfer on Bi metal@defective Bi2Sn2O7 quantum dots towards improved full-spectrum photocatalysis: A combined experimental and theoretical investigation. Chinese Chemical Letters, 2024, 35(6): 109131-. doi: 10.1016/j.cclet.2023.109131
Ying Hou , Zhen Liu , Xiaoyan Liu , Zhiwei Sun , Zenan Wang , Hong Liu , Weijia Zhou . Laser constructed vacancy-rich TiO2-x/Ti microfiber via enhanced interfacial charge transfer for operando extraction-SERS sensing. Chinese Chemical Letters, 2024, 35(9): 109634-. doi: 10.1016/j.cclet.2024.109634
Hui Liu , Xiangyang Tang , Zhuang Cheng , Yin Hu , Yan Yan , Yangze Xu , Zihan Su , Futong Liu , Ping Lu . Constructing multifunctional deep-blue emitters with weak charge transfer excited state for high-performance non-doped blue OLEDs and single-emissive-layer hybrid white OLEDs. Chinese Chemical Letters, 2024, 35(10): 109809-. doi: 10.1016/j.cclet.2024.109809
Xin Jiang , Han Jiang , Yimin Tang , Huizhu Zhang , Libin Yang , Xiuwen Wang , Bing Zhao . g-C3N4/TiO2-X heterojunction with high-efficiency carrier separation and multiple charge transfer paths for ultrasensitive SERS sensing. Chinese Chemical Letters, 2024, 35(10): 109415-. doi: 10.1016/j.cclet.2023.109415