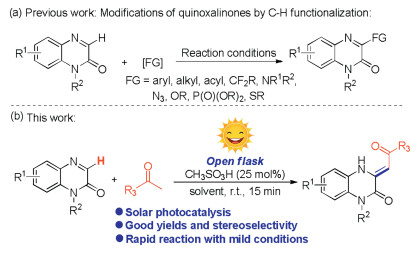
Photocatalysis has become a powerful strategy for organic synthesis due to the advantages of low energy consumption and environmental protection [13]. For example, MacMillan et al. in 2016 reported a photocatalyzed C-H arylation of aliphatic amines with aryl bromides, providing a complement to existing cross-coupling technologies [13a]. In 2021, He' group developed the first example of visible-light induced one-pot tandem reaction of arylacrylamides, CHF2CO2H and PhI(OAc)2, affording an eco-friendly and practical method to access various difluoromethylated oxindoles [13b]. The same year, Jin and coworkers developed photocatalyst-free radical tandem cyclization of quinazolinones containing an unactivated alkene moiety with difluoro bromides under illumination, giving a practical method for the synthesis of fluorine-containing ring-fused quinazolinones [13f]. In recent years, with increasing attention to renewable energy, considerable efforts have been switched to the development of photocatalytic reactions that excited by the sunlight, which is known as a renewable and simple accessible light source [14]. Our research interests focus on the development of novel and effective methodologies for the direct modification ofN-containing heterocycles [15], herein, we demonstrated a direct alkenylation reaction between quinoxalin-2(1H)-ones and methyl ketones. Compared with our previous work [15a], this transformation was achieved through a combination of Mannich-type reaction and solar photocatalysis, which could be completed within 15min, providing a green and efficient solution for the synthesis of potentially bioactive compounds that containing a 3, 4-dihydroquinoxalin-2(1H)-one structure (Scheme 1b).
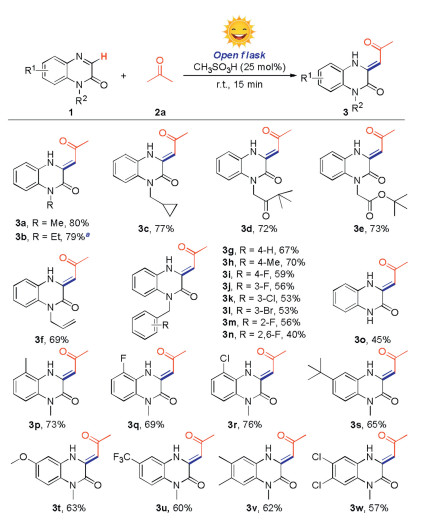
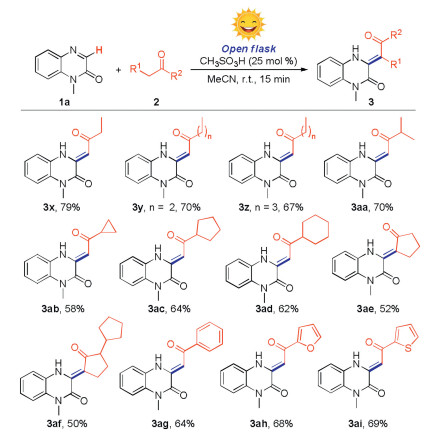
With the optimum reaction conditions in hands, we then examined the substrate scope of the reaction by employing various quinoxalin-2(1H)-ones (1) with acetone (2a) (Scheme 2). Firstly, the N-substituted groups such as N-methyl, N-ethyl, N-cyclopropylmethyl, N-keto and N-ester were well compatible under the standard conditions, giving the desired products (3a-e) in 72%–80% yields. It is worth mentioning that quinoxalin-2(1H)-one with a sensitive allyl group, which could be further functionalized, also could give the product (3f) in 69% yield. A wide range of quinoxalin-2(1H)-ones with different benzyl groups, bearing both electron-donating and electron-withdrawing substituents at ortho-, meta-, or para-position could undergo the reaction smoothly, affording the corresponding products (3g–n) in 40%–72% yields. Importantly, the N-free quinoxalin-2(1H)-one could undergo the reaction smoothly, providing the product (3o) in 45% yield. Besides, plenty of quinoxalin-2(1H)-ones that bear the functional groups including methyl, halogen, tert-butyl, methoxy or trifluoromethyl at C5-, C6- or C7-position also gave the desired products in satisfactory yield (3p-3w). To expand the substrate scope of N-heterocycles, we also tested quinoline, isoquinoline, quinoxaline, benzimidazole and benzothiazole under standard conditions, however, no corresponding product was obtained (see Supporting information).
Subsequently, we evaluated the substrate scope of methyl ketones for the reaction (Scheme 3). To reduce the dosage of reactant, the reactions were performed with 2.0 equiv. of methyl ketones by using acetonitrile as solvent. To our delight, both long-chain and cycloalkyl methyl ketones could undergo the reaction smoothly, giving the corresponding products (3x–3ad) in 58%–79% yields. The molecular structure of 3y was confirmed by X-ray crystallographic analysis (CCDC: 2060383). It was found that the molecular structure was more stable in (Z)-configuration probably because the effect of hydrogen bond interaction between amine and carbonyl group. Then, we found that cyclopentanone skeleton could also react with quinoxalin-2(1H)-one smoothly to deliver the target products (3ae and 3af) in moderate yield. The subsequent exploration found that the aryl methyl ketones, such as acetophenone, 1-(furan-2-yl)ethan-1-one and 1-(thiophen-2-yl)ethan-1-one were also could be converted into target products (3ag-3ai) in acceptable yields. Unfortunately, the substrates like ethyl acetate, acetonitrile, nitromethane, ethyl acetoacetate, and acetylacetone were not compatible under standard conditions (Supporting information).
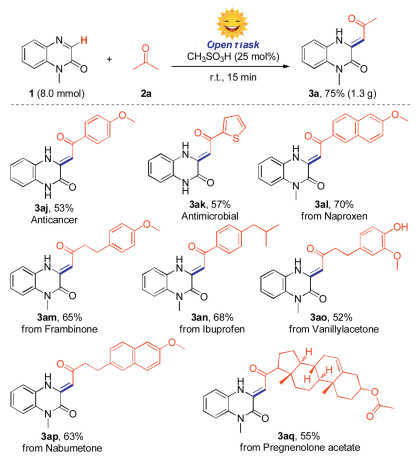
To show the synthetic utility of this protocol, a gram-scale synthesis experiment was performed to give the target product (3a) in 75% yield (Scheme 4). Interestingly, the anticancer compound (3aj) and antimicrobial compound (3ak) were obtained in moderate yields by using our strategy [16]. Moreover, since the molecules that bearing a 3, 4 dihydroquinoxalin-2(1H)-one framework are a promising class of biologically active compounds, in this regard, several bioactive molecules such as naproxen derivative, frambinone, ibuprofen derivative, vanillylacetone, nabumetone and pregnenolone acetate were selected to react with 1-methylquinoxalin-2(1H)-one directly, providing the potentially active molecules (3al-3aq) in 52%–70% yields.
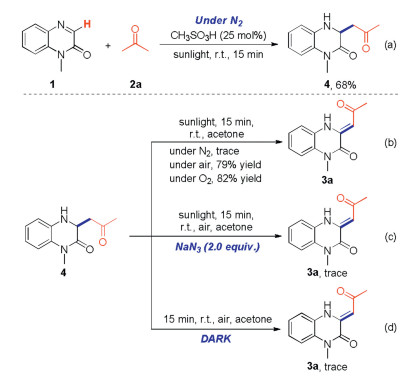
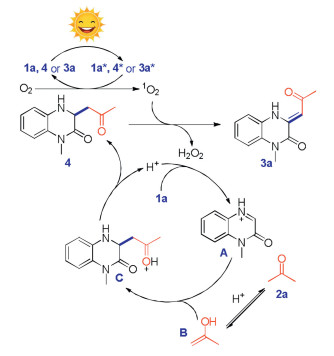
To study the reaction mechanism, a series of control experiments were carried out. Product 4 was generated instead of target product 3a when the reaction was performed under nitrogen atmosphere (Scheme 5). This result showed that oxygen in air was included in the subsequent oxidation process. To confirm the assumption, the oxidation process of compound 4 was studied. First, target product 3 was formed in 0%, 79% and 82% yields when the reaction performed under nitrogen, air or oxygen atmosphere respectively (Scheme 5). Second, the reaction was inhibited when singlet oxygen inhibitor (NaN3) was involved in the transformation (Scheme 5). Furthermore, compound 4 could not be converted into target product 3 when the reaction was performed in dark condition (Scheme 5). These experimental results strongly supported that the singlet oxygen 1O2, which was generated from triplet oxygen 3O2 through photocatalysis, serves as the real oxidant.
In conclusion, this study described a novel strategy for the olefination of quinoxalin-2(1H)-ones with methyl ketones. Various substrates were compatible under standard condition, providing the corresponding products in moderate to good yields. Control experiments revealed that a Mannich-type reaction and oxidative process were involved in the transformation.
The authors declare that they have no conflict of interest.
We thank the Natural Science Foundation of Zhejiang Province (No. LY21B060009) and the National Natural Science Foundation of China (No. 21871071) for financial support.
-
[1]
(a) R.E. TenBrink, W.B. Im, V.H. Sethy, et al., J. Med. Chem. 37 (1994) 758-768;
(b) A. Monge, F.J. Martinez-Crespo, A.L. Cerai, et al., J. Med. Chem. 38 (1995) 4488-4494;
(c) M.M. Badran, K.A.M. Abouzid, M.H.M. Hussein, Arch. Pharmacal Res. 26 (2003) 107-113;
(d) H.M. Refaat, A.A. Moneer, O.M. Khalil, Arch. Pharmacal Res. 27 (2004) 1093-1098;
(e) A. Carta, S. Piras, G. Loriga, G. Paglietti, Mini-Rev. Med. Chem. 6 (2006) 1179-1200;
(f) J.H. Fu, J.W. Yuan, Y. Zhang, et al., Org. Chem. Front. 5 (2018) 3382-3390;
(g) W. Wei, L.L. Wang, H.L. Yue, et al., ACS Sustain. Chem. Eng. 6 (2018) 17252-17257;
(h) J.W. Yuan, J.H. Fu, S.N. Liu, et al., Org. Biomol. Chem. 16 (2018) 3203-3212.
-
[2]
X.B. Zeng, C.L. Liu, X.Y. Wang, et al., Org. Biomol. Chem. 15 (2017) 8929-8935.
doi: 10.1039/C7OB02187A
-
[3]
(a) J.A. Willardsen, D.A. Dudley, W.L. Cody, et al., J. Med. Chem. 47 (2004) 4089-4099;
(b) S.Y. Zhang, F.M. Zhang, Y.Q. Tu, Chem. Soc. Rev. 40 (2011) 1937-1949;
(c) J.R. Zbieg, E. Yamaguchi, E.L. Mclnturff, M.J. Krische, Science 336 (2012) 324-327;
(d) T.Y. Chen, M.J. Krische, Org. Lett. 15 (2013) 2994-2997;
(e) D. Liu, C. Liu, H. Li, A. Lei, Angew. Chem. Int. Ed. 52 (2013) 4453-4456;
(f) X.Q. Chu, H. Meng, Y. Zi, X.P. Xu, S.J. Ji, Chem. Commun. 50 (2014) 9718-9721;
(g) X. Qin, X. Hao, H. Han, et al., J. Med. Chem. 58 (2015) 1254-1267;
(h) J.K. Cheng, T.P. Loh, J. Am. Chem. Soc. 137 (2015) 42-45.
-
[4]
(a) K. Yin, R. Zhang, Org. Lett. 19 (2017) 1530-1533;
(b) A. Gupta, M.S. Deshmukh, N. Jain, J. Org. Chem. 82 (2017) 4784-4792;
(c) Q.M. Yang, Z.B. Yang, Y.S. Tan, et al., Adv. Synth. Catal. 361 (2019) 1662-1667.
-
[5]
X. Li, K.H. Yang, W.L. Li, W.F. Xu, Drugs Fut. 31 (2006) 979.
doi: 10.1358/dof.2006.031.11.1037128
-
[6]
J. Lu, X.K. He, X. Cheng, et al., Adv. Synth. Catal. 362 (2020) 2178-2182.
doi: 10.1002/adsc.202000116
-
[7]
Q. Ke, G. Yan, J. Yu, X. Wu, Org. Biomol. Chem. 17 (2019) 5863-5881.
doi: 10.1039/C9OB00782B
-
[8]
(a) J.Z. Jin, J.Y. Tong, W.B. Yu, J. Qiao, C. Shen, Catal. Commun. 141 (2020) 106008;
(b) J. Xu, H. Yang, H. Cai, et al., Org. Lett. 21 (2019) 4698-4702;
(c) J. Zhou, P. Zhou, T. Zhao, Q. Ren, J. Li, Adv. Synth. Catal. 361 (2019) 5371-5382;
(d) S. Peng, D. Hu, J.L. Hu, et al., Adv. Synth. Catal. 361 (2019) 5721-5726;
(e) L. Zhao, L. Wang, Y. Gao, Z. Wang, P. Li, Adv. Synth. Catal. 361 (2019) 5363-5370;
(f) Q. Yang, X. Han, J. Zhao, H.Y. Zhang, Y. Zhang, J. Org. Chem. 84 (2019) 11417-11424.
-
[9]
(a) K.J. Li, Y.Y. Jiang, K. Xu, C.C. Zeng, B.G. Sun, Green Chem. 21 (2019) 4412-4421;
(b) W.P. Mai, J.W. Yuan, J.L. Zhu, et al., ChemistrySelect 4 (2019) 11066-11070;
(c) J. Wang, J. Li, Y. Wei, J. Yang, C. Huo, Org. Chem. Front. 5 (2018) 3534-3537;
(d) Y. Kim, D.Y. Kim, Tetrahedron Lett. 59 (2018) 2443-2446;
(e) M. Gao, Y. Li, L. Xie, R. Chauvin, X. Cui, Chem. Commun. 52 (2016) 2846-2849.
-
[10]
(a) L.Y. Xie, Y.L. Chen, L. Qin, et al., Org. Chem. Front. 6 (2019) 3950-3955;
(b) Q.H. Teng, Y. Yao, W.X. Wei, et al., Green Chem. 21 (2019) 6241-6245.
-
[11]
(a) J. Yuan, J. Zhu, J. Fu, et al., Org. Chem. Front. 6 (2019) 925-935;
(b) L.Y. Xie, J.L. Hu, Y.X. Song, et al., ACS Sustain. Chem. Eng. 7 (2019) 19993-19999;
(c) J.W. Yuan, J.L. Zhu, B. Li, et al., Org. Biomol. Chem. 17 (2019) 10178-10187;
(d) Q. Yang, Z. Yang, Y. Tan, et al., Adv. Synth. Catal. 361 (2019) 1662-1667;
(e) Q. Yang, Y. Zhang, Q. Sun, et al., Adv. Synth. Catal. 360 (2018) 4509-4514;
(f) W. Wei, L. Wang, P. Bao, et al., Org. Lett. 20 (2018) 7125-7130;
(g) T.T. Hoang, T.A. To, V.T.T. Cao, et al., Catal. Commun. 101 (2017) 20-25;
(h) A. Gupta, M.S. Deshmukh, N. Jain, J. Org. Chem. 82 (2017) 4784-4792.
-
[12]
(a) L.Y. Xie, Y.S. Bai, X.Q. Xu, et al., Green Chem. 22 (2020) 1720-1725;
(b) P. Bao, F. Liu, Y. Lv, et al., Org. Chem. Front. 7 (2020) 492-498;
(c) J. Xu, H. Yang, L. He, et al., Org. Lett. 23 (2021) 195-201;
(d) J. Wang, B. Sun, L. Zhang, et al., Org. Chem. Front. 7 (2020) 113-118;
(e) J. Xu, H. Zhang, J. Zhao, et al., Org. Chem. Front. 7 (2020) 4031-4042;
(f) J. Shen, J. Xu, L. Huang, Q. Zhu, P. Zhang, Adv. Synth. Catal. 362 (2020)230-241;
(g) H. Zhang, J. Xu, M. Zhou, et al., Org. Biomol. Chem. 17 (2019) 10201-10208;
(h) L.Y. Xie, S. Peng, T.G. Fan, et al., Sci. Chin. Chem. 62 (2019) 460-464;
(i) L.X. Liu, N. Pan, W. Sheng, et al., Adv. Synth. Catal. 361 (2019) 4126-4132;
(j) W. Zhang, Y.L. Pan, C. Yang, et al., J. Org. Chem. 84 (2019) 7786-7795;
(k) L.Y. Xie, L.L. Jiang, J.X. Tan, etal., ACSSustain. Chem. Eng. 7 (2019)14153-14160;
(l) G. Hong, J. Yuan, J. Fu, et al., Org. Chem. Front. 6 (2019) 1173-1182;
(m) L. Wang, H. Liu, F. Li, et al., Adv. Synth. Catal. 361 (2019) 2354-2359;
(n) C. Jin, X. Zhuang, B. Sun, D. Li, R. Zhu, AsianJ. Org. Chem. 8 (2019) 1490-1494;
(o) W. Xue, Y. Su, K.H. Wang, et al., Asian J. Org. Chem. 8 (2019) 887-892;
(p) J. Wang, B. Sun, L. Zhang, et al., Asian J. Org. Chem. 8 (2019) 1942-1946;
(q) W. Wei, L. Wang, H. Yue, etal., ACSSustain. Chem. Eng. 6 (2018)17252-17257;
(r) S. Liu, Y. Huang, F.L. Qing, X.H. Xu, Org. Lett. 20 (2018) 5497-5501;
(s) L. Hu, J. Yuan, J. Fu, et al., Eur. J. Org. Chem. 2018 (2018) 4113-4120;
(t) J. Fu, J. Yuan, Y. Zhang, Org. Chem. Front. 5 (2018) 3382-3390;
(u) J. Yuan, J. Fu, J. Yin, et al., Org. Chem. Front. 5 (2018) 2820-2828;
(v) K. Yin, R. Zhang, Synlett 29 (2018) 597-602;
(w) B. Ramesh, C.R. Reddy, G.R. Kumar, B.V.S. Reddy, Tetrahedron Lett. 59 (2018) 628-631;
(x) L. Wang, Y. Zhang, F. Li, Adv. Synth. Catal. 360 (2018) 3969-3977;
(y) K. Yin, R. Zhang, Org. Lett. 19 (2017) 1530-1533;
(z) J. Yuan, S. Liu, L. Qu, Adv. Synth. Catal. 359 (2017) 4197-4207.
-
[13]
(a) M.H. Shaw, V.W. Shurtleff, J.A. Terrett, J.D. Cuthbertson, D.W.C. MacMillan, Science 352 (2016) 1304-1308;
(b) Q.W. Gui, F. Teng, Z.C. Li, et al., Chin. Chem. Lett. 32 (2021)1907-1910;
(c) B. Sun, P. Huang, Z. Yan, et al., Org. Lett. 23 (2021) 1026-1031;
(d) L.Y. Xie, S. Peng, L.H. Yang, et al., Green Chem. 23 (2021) 374-378;
(e) K.J. Liu, Z. Wang, L.H. Lu, et al., Green Chem. 23 (2021) 496-500;
(f) J. Yang, B. Sun, H. Ding, et al., Green Chem. 23 (2021) 575-581;
(g) G.H. Li, Q.Q. Han, Y.Y. Sun, et al., Chin. Chem. Lett. 31 (2020) 3255-3258;
(h) W. Ou, R. Zou, M. Han, L. Yu, C. Su, Chin. Chem. Lett. 31 (2020) 1899-1902;
(i) S. He, X. Chen, F. Zeng, et al., Chin. Chem. Lett. 31 (2020) 1863-1867;
(j) L. Wang, M. Zhang, Y. Zhang, et al., Chin. Chem. Lett. 31 (2020) 67-70;
(k) L. Zou, P. Li, B. Wang, L. Wang, Green Chem. 21 (2019) 3362-3369;
(l) X. Mi, Y. Kong, J. Zhang, C. Pi, X. Cui, Chin. Chem. Lett. 30 (2019) 2295-2298;
(m) J. Shen, J. Xu, L. He, Y. Ouyang, et al., Org. Lett. 23 (2021) 1204-1208;
(n) J.M.R. Narayanam, C.R.J. Stephenson, Chem. Soc. Rev. 40 (2011) 102-113.
-
[14]
(a) P. Esser, B. Pohlmann, H.D. Scharf, Angew. Chem. Int. Ed. 33 (1994) 2009-2023;
(b) M. Okada, T. Fukuyama, K. Yamada, et al., Chem. Sci. 5 (2014) 2893-2898;
(c) S. Park, W.H. Jeon, W.S. Yong, P.H. Yong, Org. Lett. 17 (2015) 5060-5063;
(d) S.Y. Ni, J. Cao, H.B. Mei, et al., Green Chem. 18 (2016) 3935-3939.
-
[15]
(a) J. Xu, L. Huang, L. He, et al., Green Chem. 23 (2021) 2123-2129;
(b) C. Shen, A. Wang, J. Xu, et al., Chem 5 (2019) 1059-1107;
(c) J. Xu, K. Du, J. Shen, et al., ChemCatChem 10 (2018) 3675-3679;
(d) J. Xu, K. Cheng, C. Shen, et al., ChemCatChem 10 (2018) 965-970;
(e) C. Shen, M. Yang, J. Xu, et al., RSC Adv. 7 (2017) 49436-49439;
(f) J. Xu, C. Shen, X. Zhu, et al., Chem. Asian J. 11 (2016) 882-892;
(g) J. Xu, X. Zhu, G. Zhou, et al., Org. Biomol. Chem. 14 (2016) 3016-3021.
-
[16]
E.E. Stepanova, D.N. Lukmanova, S.O. Kasatkina, M.V. Dmitriev, A.N. Maslivets, ChemistrySelect 4 (2019) 12774-12778.
doi: 10.1002/slct.201902900
-
[17]
(a) L.Y. Xie, Y.S. Liu, H.R. Ding, et al., Chin. J. Catal. 41 (2020) 1168-1173;
(b) D. Rawat, R. Kumar, A. Subbarayappa, Green Chem. 22 (2020) 6170-6175.

 Login In
Login In





 DownLoad:
DownLoad:

 DownLoad:
DownLoad: