Sulfone-containing compounds are frequently found in pharmacologically active compounds, natural products, and materials [1]. Furthermore, they can also be employed as important intermediates in various organic transformations to access a series of useful molecules [2]. Consequently, the introduction of sulfone functionality into organic framework has attracted considerable synthetic pursuit of chemists because of their interesting biological activities and versatile synthetic applications [3]. During the past decades, a number of sulfonylating agents such as sulfonyl chlorides [4], sulfonyl hydrazides [5], sulfinates [6], sulfinic acids [7], thiophenols [8], sulfonyl cyanides [9], sulfonyl selenides [10] and SO2 surrogates [11] have been developed for the construction of organic sulfones. Nevertheless, most of these sulfonylation reactions usually require the use of catalysts or a stoichiometric amount of oxidants, or suffer from complex reaction conditions. It is still highly desirable to develop new strategies to construct sulfone-containing compounds from simple sulfonylating agents under mild conditions.
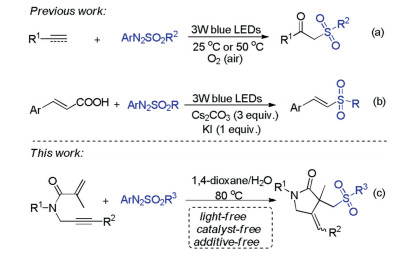
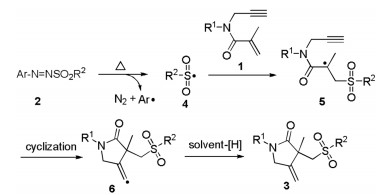
Arylazo sulfones are colored and bench stable compounds, which could generate aryl radical and sulfonyl radical along with the release of N2 through the homolytic cleavage of C-N and S-N bonds under visible-light irradiation or heating conditions [12]. Generally, arylazo sulfones are utilized as arylating reagents in photoinduced reactions in the absence of photocatalyst [13]. Recently, arylazo sulfones have alternatively emerged as sulfonylating agents for constructing sulfone-containing compounds [14]. In 2019, our group reported oxysulfonylation of alkenes or alkynes with arylazo sulfones and dioxygen leading to β-oxo sulfones under visible-light irradiation conditions (Scheme 1a) [14a, b]. In 2020, Yadav and co-workers also described an efficient visible-light mediated decarboxylative sulfonylation of cinnamic acids and arylazo sulfones for the synthesis of (E)-vinyl sulfones (Scheme 1b)
[14c]. As our ongoing interest in sulfonylation reactions [15], herein, we wish to present a catalyst- and light-free selective sulfonylation/cyclization of 1,6-enynes with arylazo sulfones to synthesize sulfonated γ-butyrolactams under simple heating conditions (Scheme 1c).
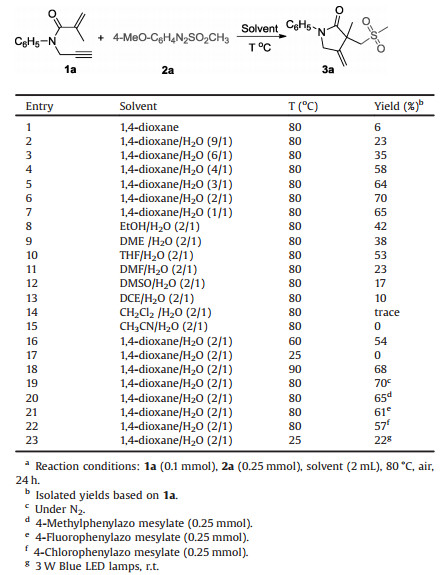
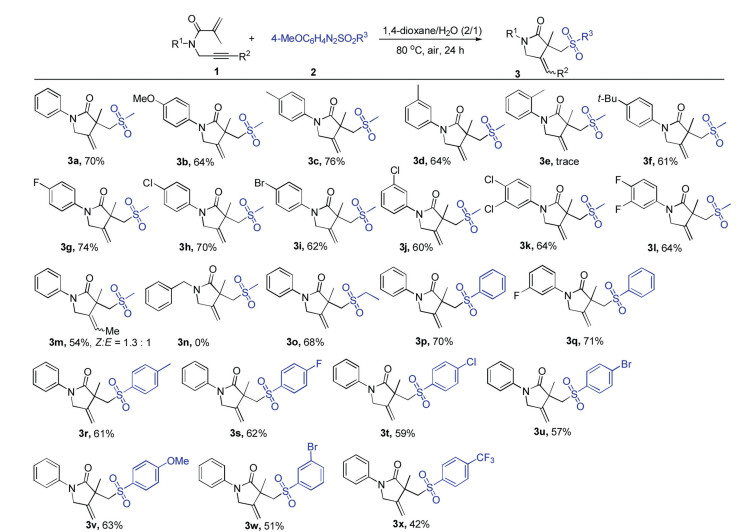
Under the optimized conditions, the scope and limitation of various 1,6-enyne derivatives and arylazo sulfones were further examined (Scheme 2). Generally, a series of 1,6-enynes with both electron-donating and electron-withdrawing groups on the N-aromatic ring could undergo the reaction efficiently to produce the desired products (3b-3l) in moderate to good yields. Notably, halogen substituents such as F, Cl and Br groups were all tolerated in this reaction system, which could be used for the further modification via coupling reactions. It was found that the reaction efficiency was greatly affected by the steric hindrance of substituent on the aromatic ring, and only a trace amount of product 3e was detected when ortho-methyl N-aromatic ring substrate was employed in this system. Unfortunately, when N-alkyl substituted 1,6-enyne such as N-benzyl-N-(prop-2-ynyl) methacrylamide was tested in the present reaction, none of the desired product 3n was detected. It should be noted that internal alkyne such as N-(but-2-yn-1-yl)-N-phenylmethacrylamide was suitable for this reaction, affording the product 3m in moderate yield. Then, the scope of different arylazo sulfones was investigated. Arylazo alkylsulfone (i.e., 4-methoxyphenylazo ethyl sulfone) could also be utilized in this system to afford the corresponding product 3o in 68% yield. Moreover, a variety of arylazo arylsulfones bearing an electron-donating (methyl and methoxy) or an electron-withdrawing group (fluoro, chloro, bromo and trifluoromethyl) on the aryl rings were suitable substrates, and the corresponding products (3r-3x) were obtained in moderate to good yields.
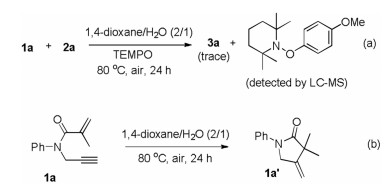
To investigate the possible reaction mechanism, two control experiments were carried out. When radical scavenger TEMPO (2, 2, 6, 6-tetramethyl-1-piperidinyloxy) was added in the model reaction system, the transformation was extremely inhibited and aryl-TEMPO adduct was observed by LC–MS. This result suggested that a radical process might be involved in the present reaction (Scheme 3a). Furthermore, the cyclization product 1a' was not generated from 1a under standard conditions in the absence of 4-methoxyphenylazo mesylate (2a) (Scheme 3b), suggesting that this cascade cyclization was triggered by in situ generated sulfonyl radical.
In summary, a convenient and efficient method has been developed for the construction of sulfonylated γ-butyrolactams through the regioselective sulfonylation/cyclization of 1,6-enynes with arylazo sulfones. The present transformation could be achieved under catalyst- and additive-free conditions via the sequential formation of C-S and C-C bonds. This protocol simply utilizes arylazo sulfones as sulfonylating agents offering a highly attractive routine to synthesize sulfonylated γ-butyrolactams, which is expected to exhibit potential application in synthetic chemistry.
The authors report no declarations of interest.
This work was supported by the International Cooperation Project of Qinghai Province (No. 2018-HZ-806), the Youth Innovation and Technology Project of Higher School in Shandong Province (No. 2019KJC021), the Natural Science Foundation of Shandong Province (No. ZR2018MB009), the Qinghai Key Laboratory of Tibetan Medicine Research (No. 2017- ZJ-Y11) and CAS "Light of West China" Program 2018, and Entrepreneurship Training Program for College Students (No. 201910049).
-
[1]
(a) M.D. McReynolds, J.M. Dougherty, P.R. Hanson, Chem. Rev. 104 (2004) 2239-2258;
(b) W. Li, G. Yin, L. Huang, et al., Green Chem. 18 (2016) 4879-4883;
(c) K. Sun, X. Wang, F. Fu, et al., Green Chem. 19 (2017) 1490-1493;
(d) K. Sun, Z. Shi, Z. Liu, et al., Org. Lett. 20 (2018) 6687-6690.
-
[2]
(a) J. Li, W. Zhang, X. Wei, et al., J. Org. Chem. 82 (2017) 6621-6628;
(b) Q. Deng, L. Tan, Y. Xu, P. Liu, P. Sun, J. Org. Chem. 83 (2018) 6151-6161;
(c) H. Li, W. Hao, M. Wang, et al., Org. Lett. 20 (2018) 4362-4366;
(d) X. Li, S. Zhuang, X. Fang, P. Liu, P. Sun, Org. Biomol. Chem. 15 (2017) 1821-1827;
(e) C. Zhang, H. Jiang, S. Zhu, Chem. Commun. 53 (2017) 2677-2680;
(f) H. Sha, T. Xu, F. Liu, et al., Chem. Commun. 54 (2018) 10415-10418.
-
[3]
(a) J. Zhu, W.C. Yang, X.D. Wang, L. Wu, Adv. Synth. Catal. 360 (2018) 386-400;
(b) L.Y. Xie, Y.J. Li, J. Qu, et al., Green Chem. 19 (2017) 5642-5646;
(c) L.Y. Xie, S. Peng, J.X. Tan, et al., ACS Sustainable Chem. Eng. 6 (2018) 16976-16981;
(d) K. Sun, X.L. Chen, S.J. Li, et al., J. Org. Chem. 83 (2018) 14419-14430.
-
[4]
(a) S.K. Kang, H.W. Seo, Y.H. Ha, Synthesis 9 (2001) 1321-1326;
(b) G.B. Deng, Z.Q. Wang, J.D. Xia, et al., Angew. Chem. Int. Ed. 52 (2013) 1535-1538;
(c) Y. Liu, J.L. Zhang, M.B. Zhou, R.J. Song, J.H. Li, Chem. Commun. 50 (2014) 14412-14414;
(d) H. Hou, H. Li, Y. Xu, et al., Adv. Synth. Catal. 360 (2018) 4325-4329;
(e) P. Bao, L. Wang, Q. Liu, et al., Tetrahedron Lett. 60 (2019) 214-218;
(f) X. Mi, Y. Kong, J. Zhang, C. Pi, X. Cui, Chin. Chem. Lett. 30 (2019) 2295-2298.
-
[5]
(a) W. Wei, C. Liu, D. Yang, et al., Chem. Commun. 49 (2013) 10239-10241;
(b) K. Sun, X. Wang, F. Fu, et al., Green Chem. 19 (2017) 1490-1493;
(c) K. Sun, X.L. Chen, S.J. Li, et al., J. Org. Chem. 83 (2018) 14419-14430;
(d) H.D. Zuo, W.J. Hao, C.F. Zhu, et al., Org. Lett. 22 (2020) 4471-4477;
(e) T.S. Zhang, W.J. Hao, R. Wang, et al., Green Chem. 22 (2020) 4259-4269.
-
[6]
(a) L.Y. Xie, S. Peng, F. Liu, et al., Org. Chem. Front. 5 (2018) 2604-2609;
(b) S. Peng, Y.X. Song, J.Y. He, et al., Chin. Chem. Lett. 30 (2019) 2287-2290;
(c) W. Li, G. Yin, L. Huang, et al., Green Chem. 18 (2016) 4879-4883.
-
[7]
(a) Q. Lu, J. Zhang, G. Zhao, et al., J. Am. Chem. Soc. 135 (2013) 11481-11484;
(b) W. Wei, J. Li, D. Yang, et al., Org. Biomol. Chem. 12 (2014) 1861-1864;
(c) T. Shen, Y. Yuan, S. Song, N. Jiao, Chem. Commun. 50 (2014) 4115-4118;
(d) G.H. Lia, Q.Q. Han, Y.Y. Sun, et al., Chin. Chem. Lett. 31 (2020) 3255-3258;
(e) C. Wu, P. Yang, Z. Fu, et al., J. Org. Chem. 81 (2016) 10664-10671;
(f) Z. Zhang, J. Yan, D. Ma, J. Sun, Chin. Chem. Lett. 30 (2019) 1509-1511.
-
[8]
(a) Q. Xue, Z. Mao, Y. Shi, et al., Tetrahedron Lett. 53 (2012) 1851-1854;
(b) L. Wang, H. Yue, D. Yang, et al., J. Org. Chem. 82 (2017) 6857-6864.
-
[9]
(a) J.M. Fang, M.Y. Chen, Tetrahedron Lett. 28 (1987) 2853-2856;
(b) L.R. Reddy, B. Hu, M. Prashad, K. Prasad, Angew. Chem. Int. Ed. 48 (2009) 172-174.
-
[10]
(a) D.H.R. Barton, M.S. Csiba, J.C. Jaszberenyi, Tetrahedron Lett. 35 (1994) 2869-2872;
(b) M. Yoshimatsu, M. Hayashi, G. Tanabe, O. Muraoka, Tetrahedron Lett. 37 (1996) 4161-4164;
(c) H. Qian, X. Huang, Synthesis 12 (2006) 1934-1936.
-
[11]
(a) K. Sun, Z. Shi, Z. Liu, et al., Org. Lett. 20 (2018) 6687-6690;
(b) S. Liu, K. Chen, W.J. Hao, et al., J. Org. Chem. 84 (2019) 1964-1971;
(c) X. Gong, X. Li, W. Xie, J. Wu, S. Ye, Org. Chem. Front. 6 (2019) 1863-1867;
(d) X. Wang, M. Yang, W. Xie, X. Fan, J. Wu, Chem. Commun. 55 (2019) 6010-6013;
(e) Y. Zong, Y. Lang, M. Yang, et al., Org. Lett. 21 (2019) 1935-1938.
-
[12]
(a) I. Sapountzis, P. Knochel, Angew. Chem., Int. Ed. 43 (2004) 897-900;
(b)M. Kobayashi, S. Fujii, H. Minato, Bull. Chem. Soc. Jpn. 45 (1972) 2039-2042;
(c) M. Kobayashi, M. Gotoh, H. Minato, J. Org. Chem. 40 (1975) 140-142;
(d) J.L. Kice, R.S. Gabrielsen, J. Org. Chem. 35 (1970) 1010-1015;
(e) M.J. Evers, L.E. Christiaens, M.R. Guillaume, M.J. Renson, J. Org. Chem. 50 (1985) 1779-1780;
(f) M.J. Evers, L.E. Christiaens, M.J. Renson, J. Org. Chem. 51 (1986) 5196-5198.
-
[13]
(a) S. Crespi, S. Protti, M. Fagnoni, J. Org. Chem. 81 (2016) 9612-9619;
(b) A. Dossena, S. Sampaolesi, A. Palmieri, S. Protti, M. Fagnoni, J. Org. Chem. 82 (2017) 10687-10692;
(c) Y. Xu, X. Yang, H. Fang, J. Org. Chem. 83 (2018) 12831-12837;
(d) M. Malacarne, S. Protti, M. Fagnoni, Adv. Synth. Catal. 359 (2017) 3826-3830;
(e) Q. Liu, L. Wang, H. Yue, et al., Green Chem. 21 (2019) 1609-1613;
(f) C. Lian, G. Yue, J. Mao, et al., Org. Lett. 21 (2019) 5187-5191.
-
[14]
(a) Q. Liu, F. Liu, H. Yue, et al., Adv. Synth. Catal. 361 (2019) 5277-5282;
(b) Y. Lv, Q. Liu, F. Liu, et al., Tetrahedron Lett. 61 (2020) 151335;
(c) R. Chawla, S. Jaiswal, P.K. Dutta, L.S. Yadav, Tetrahedron Lett. 61 (2020) 151898.
-
[15]
(a) J. Wen, W. Wei, S. Xue, et al., J. Org. Chem. 80 (2015) 4966-4972;
(b) W. Wei, J. Wen, D. Yang, et al., Chem. Commun. 51 (2015) 768-771;
(c) L. Wang, M. Zhang, Y. Zhang, et al., Chin. Chem. Lett. 31 (2020) 67-70;
(d) W. Wei, H. Cui, D. Yang, et al., Org. Chem. Front. 4 (2017) 26-30.
-
[16]
(a) J. Qiu, B. Jiang, Y. Zhu, et al., J. Am. Chem. Soc. 137 (2015) 8928-8931.
-
[17]
(b) A. Wang, Y. Zhu, S. Wang, et al., J. Org. Chem. 81 (2016) 1099-1105;
(c) Y. Zhu, B. Jiang, W. Hao, et al., Chem. Co mmun. 52 (2016) 1907-1910;
(d) P. Zhou, J. Wang, T. Zhang, et al., Chem. Commun. 54 (2018) 164-167;
(e) H. Sha, F. Liu, J. Lu, et al., Green Chem. 20 (2018) 3476-3485;
(f) J. Qi, Q. Teng, N. Thirupathi, C. Tung, Z. Xu, Org. Lett. 21 (2019) 692-695;
(g) Y. Yu, Z. Cai, W. Yuan, P. Liu, P. Sun, J. Org. Chem. 82 (2017) 8148-8156.
-
[18]
(a) J. Li, W. Zhang, X. Wei, et al., J. Org. Chem. 82 (2017) 6621-6628;
(b) Q. Deng, L. Tan, Y. Xu, P. Liu, P. Sun, J. Org. Chem. 83 (2018) 6151-6161;
(c) H. Li, W. Hao, M. Wang, et al., Org. Lett. 20 (2018) 4362-4366;
(d) X. Li, S. Zhuang, X. Fang, P. Liu, P. Sun, Org. Biomol. Chem. 15 (2017) 1821-1827;
(e) C. Zhang, H. Jiang, S. Zhu, Chem. Commun. 53 (2017) 2677-2680;
(f) H. Sha, T. Xu, F. Liu, et al., Chem. Commun. 54 (2018) 10415-10418.
-
[19]
J. Caruano, G.G. Mucciolib, R. Robiette, Org. Biomol. Chem. 14 (2016) 10134-10156.
-
[20]
(a) W.T. Wei, W.W. Ying, W.H. Bao, et al., ACS Sustainable Chem. Eng. 6 (2018) 15301-15305;
(b) Y.N. Meng, Q.Q. Kang, T.T. Cao, et al., ACS Sustainable Chem. Eng. 7 (2019) 18738-18743;
(c) X.D. Xu, T.T. Cao, Y.N. Meng, et al., ACS Sustainable Chem. Eng. 7 (2019) 13491-13496;
(d) X.J. Huang, F.H. Qin, Y. Liu, et al., Green Chem. 22 (2020) 3952-3955;
(e) X.X. Meng, Q.Q. Kang, J.Y. Zhang, et al., Green Chem. 22 (2020) 1388-1392;
(f) L.H. Gao, J.Y. Zhang, S.Z. Song, et al., Org. Biomol. Chem. 17 (2019) 7674-7678.

 Login In
Login In





 DownLoad:
DownLoad:

 DownLoad:
DownLoad: