High-yield synthesis of a novel water-soluble macrocycle for selective recognition of naphthalene
-
* Corresponding author.
E-mail address: zengfei@iccas.ac.cn (F. Zeng).
Citation:
Man-Hua Ding, Juan Liao, Lin-Li Tang, Guang-Chuan Ou, Fei Zeng. High-yield synthesis of a novel water-soluble macrocycle for selective recognition of naphthalene[J]. Chinese Chemical Letters,
;2021, 32(5): 1665-1668.
doi:
10.1016/j.cclet.2020.11.019

Efficient synthesis of novel macrocyclic hosts with unique structures and good host–guest properties is a permanent and challenging topic in the field of supramolecular chemistry [1-7]. During the last decade, considerable effort has been devoted to the development of macrocyclic molecular and a number of new macrocyclic receptors with novel properties have been reported, such as heterocalix[n]aromatics [8-10], pillar[n]arene [11-16], helicarenes [17-19], naphthotubes [20-24], Ex-box and Ex-cage [25-32], and others [33-37]. However, most of the reported macrocyclic hosts showed poor solubility and weak host–guest interactions in water. To develop a new macrocyclic molecule with good water solubility and molecular recognition properties not only helps us to understand and mimic the biological processes, but also enriches the toolbox of supramolecular chemists. Water soluble groups including sulfonate [38-40], carboxylate [41-43] and quaternary amine groups [44, 45] have been modified onto the macrocyclic hosts to increase their water solubility. These approaches often suffer from long synthetic steps and low yields, which restrict their further application in the complicated supramolecular self-assembly. Undoubtedly, it is important to develop novel water-soluble macrocyclic hosts that could be obtained in high yields and show good host–guest properties in water. Recently, Li and coworkers [46, 47] reported the efficient synthesis of water-soluble macrocyclic hosts using dynamic covalent chemistry (DCC) approach. In their method, both macrocycles and [2] catenanes could be obtained in high yields under the thermodynamic control. However, the purification of [2] catenanes need chromatographic. Previously, our group [42, 43] prepared a novel water-soluble cylindrical macrotricyclic host, and found that the host could bind two N-methylquinolinium salts to form 1:2 complexes in water. Inspired by these results, we deduced that whether we could find a new strategy to construct novel water-soluble macrocyclic host with significant host–guest properties in high yields.
So far as we know, 1, 8-bis(4-pyridylethynyl)anthracene, which looks like a molecule "clip", has been used as donor building block to prepare trigonal prisms [48]. Thus, the anthracene-based "clip" could also serve as half part of a macrocycle and a macrocyclic host could be obtained when a suitable building block was introduced to this clip. Herein, we report the efficient synthesis of a novel water soluble macrocyclic host 12+·2Br− and its complexation with neutral guests in water. By the utilization of the anthracene-based "clip" to react with 1, 4-bis(bromomethyl)benzene, host 12+·2Br− can be obtain via simple filtration in a high yield of 82%. Moreover, it was found that host 12+·2Br− could form 1:1 complexes with guests 2 and 3 in water solution (Fig. 1). Interestingly, we discovered that host 12+·2Br− could only selectively accommodate naphthalene among a variety of polycyclic aromatic hydrocarbons in water. This selective host-guest recognition could be employed for the further removal of naphthalene from sewage.
Synthesis of host 12+·2Br− was outlined in Scheme 1. Compound 4 was first prepared according to the literature procedure [48]. By the reaction of 4 and commercially available 1, 4-bis(bromomethyl)benzene in acetonitrile at 90 ℃, host 12+·2Br− could be easily synthesized in 82% yield. Macrocyclic host 12+·2Br− showed moderate solubility in water, and its structure was confirmed by 1H NMR, 13C NMR, HRMS spectra and crystal structure analysis (Supporting information).
Firstly, the binding properties of host 12+·2Br− toward neutral guests 2 and 3 were investigated by 1H NMR spectra in water solution. Unlike the previous results that reported by our group [43], after electron-poor host 12+·2Br− (4.0 mmol/L) and electron-rich guest 2 with 1:1 molar ratio were mixed in water, no obviously color change was observed. The similar phenomenon was also observed for the aqueous solution between host 12+·2Br− and guests 3. These results led us to doubt that whether host 12+·2Br− could form of complexes with guests 2 and 3. Consequently, the 1H NMR experiments were carried out to further investigate the complexation between host 12+·2Br− and guest 2 in water. As shown in Fig. 2, the 1H NMR spectrum of a 1:1 mixture of 12+·2Br− and 2 in D2O showed a great difference with those for free host 12+·2Br− and free guest 2. Upfield shifts of the resonances of protons H1, H2 and H3 corresponding to guest 2 were observed, which indicated that the naphthalene unit of 2 experienced a shielded magnetic environment in the aromatic cavity of 12+·2Br−. Moreover, the signal of protons Hf and Hg in host 12+·2Br− also shifted upfield, implying that the electron-poor pyridine unit of 12+·2Br− was in shielded magnetic environment and a new complex 1·2 could be formed. Meanwhile, by increasing the amount of guest 2, the spectrum of complex 1·2 showed only one set of resonances, which indicated that the complexation and decomplexation between host 12+·2Br− and guest 2 were a fast exchange process on the NMR time scale at room temperature. The formation of complex 1·2 was also supported by 2D NOESY spectral experiment. As shown in the Fig. S4 (Supporting information), the clear correlation signals between protons H1-Hg, H2-Hg and the protons of crown ether units of host 12+·2Br− were observed, which was also consistent with the formation of complex 1·2. Moreover, 1H NMR titrations and nonlinear fitting were then performed to quantitatively estimate the 1:1 binding manner between host 12+·2Br− and guest 2. Consequently, it was found that 1:1 complex 1·2 were formed by the mole ratio plot. The binding constant (K) of the complex 2⊂12+·2Br− was calculated to be 184±4 L/mol by the Scatchard plot [49]. By the counteranion exchange of 12+·2Br−, we also prepared oil-soluble 12+·2PF6− and investigated its complexation with 2 in MeCN. As shown in Fig. S3 (Supporting information), the 1H NMR spectrum of the 1:1 mixture of 12+·2PF6− and 2 was essentially the sum of the two components, indicating that no obvious complexation between 12+·2PF6− and 2 occurred and the major driving force for the formation of 2⊂12+·2Br− in water could be hydrophobic interactions. In addition, the binding constant of the complex 3⊂12+·2Br− was measured to be around 64±2 L/mol in D2O at 298 K. Compared to guest 2 containing a naphthalene unit, guest 3 has a smaller phenyl hydrophobic moiety, which accounted for its lower binding constant within host 12+·2Br−.
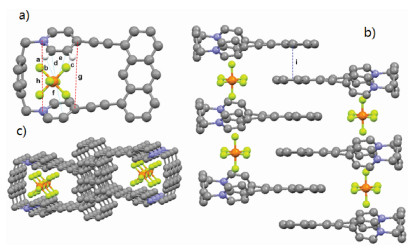
The attempts to obtain the single crystal of 2⊂12+·2Br− and 12+·2Br− were unsuccessful. Fortunately, a yellow single crystal of 12+·2PF6− was obtained by slow vapor diffusion of ether to a solution of 12+·2PF6− in CH3CN, providing unambiguous evidence for the formation of 12+·2Br−. As shown in Fig. 3a, the two pyridinium residues orientate in a face-to-face nanner and the distance between two N and C atoms of pyridinium residues are measured to be 5.812 Å (h) and 5.647 Å (g), indicating the moderate-sized cavity of host 12+·2PF6−. Interestingly, it was found that PF6− ion showed not only multiple CH···F hydrogen bonds but also anion···π interactions with pyridinium rings with the distance of 2.989 (d), 3.062 (e) and 3.159 (f), respectively. Moreover, π···π interaction between two anthracene groups of host 12+·2PF6− with a distance of 3.846 Å (i) was also observed (Fig. 3b). Because of these multiple noncovalent interactions, the macrocyclic molecule 12+·2PF6− could self-assemble to form a 1D tubular channel with PF6− ions inside the channels.
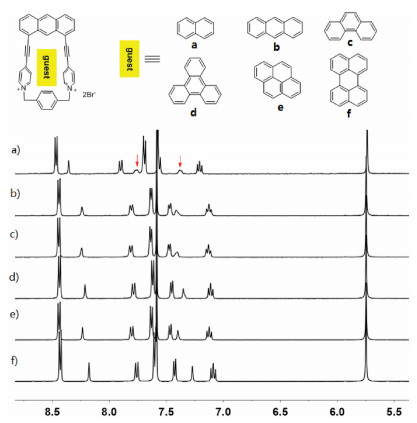
Inspired by the formation of complex 1·2 in water, we further investigated the capability of 12+·2Br− to accommodate a variety of polycyclic aromatic hydrocarbons in water. As shown in Fig. 4a, 1H NMR spectroscopy revealed that 12+·2Br− can encapsulate naphthalene to formation of 1:1 complex. However, the formation of 1:1 complexes between 12+·2Br− and anthracene, phenanthrene, triphenylene, pyrene and perylene were not observed (Figs. 4b–f). These observations could be explained by the fact that host 12+·2Br− had a small cavity and could only recognize small polycyclic aromatic hydrocarbons such as naphthalene. However, because of the extremely poor solubility of naphthalene in water, the corresponding binding constants of host 12+·2Br− and naphthalene could not be determined. The highly selective binding behavior of host 12+·2Br− toward naphthalene could be further used for the separation of naphthalene from a variety of polycyclic aromatic hydrocarbons.
In conclusion, by taking advantage of anthracene-based "clip" structure, we developed an efficient approach to construct a water-soluble macrocycle and studied its binding ability toward neutral guests containing naphthalene or phenyl units in water. The formation of 1:1 complexes between host 12+·2Br− and 2 or 3 were confirmed by the 1H NMR titrations experiment. Additionally, we demonstrated that the major driving force for the formation of 2⊂12+·2Br− or 3⊂12+·2Br− in water might be hydrophobic interactions. We further investigated its ability to host a variety of polycyclic aromatic hydrocarbons in aqueous solution. It was found that host 12+·2Br− could selectively encapsulate of naphthalene to formation of 1:1 complexes over a variety of polycyclic aromatic hydrocarbons. This highly selective accommodation hydrophobic guest in water could be explained by the fact that host 12+·2Br− had a relatively small hydrophobic cavity. The application of this novel water-soluble host for the separation of naphthalene from a variety of polycyclic aromatic hydrocarbons and removal of naphthalene from sewage, are underway in our laboratory.
The authors report no declarations of interest.
The authors are grateful for the financial support from the National Natural Science Foundation of China (Nos. 21602055 and 51772091); Natural Science Foundation of Hunan Province (No. 2017JJ3094) and Research Foundation of Education Bureau of Hunan Province (No. 18C1072).
Supplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.cclet.2020.11.019.
K. Gloe, Macrocyclic Chemistry: Current Trends and Future Perspectives, Springer, Dordrecht, 2005.
F. Davis, S. Higson, Macrocycles: Construction, Chemistry and Nanotechnology Applications, Wiley, Chichester, 2011.
J.W. Steed, J.L. Atwood, Supramolecular Chemistry, 2nd ed., Wiley, Chichester, 2009.
C.F. Chen, Chem. Commun. 47(2011) 1674-1688.
doi: 10.1039/c0cc04852f
T. Ogoshi, T.A. Yamagishi, Y. Nakamoto, Chem. Rev. 116(2016) 7937-8002.
doi: 10.1021/acs.chemrev.5b00765
Y. Han, Z. Meng, Y.X. Ma, C.F. Chen, Acc. Chem. Res. 47(2014) 2026-2040.
doi: 10.1021/ar5000677
G. Yu, K. Jie, F. Huang, Chem. Rev. 115(2015) 7240-7303.
doi: 10.1021/cr5005315
M.X. Wang, Acc. Chem. Res. 45(2012) 182-195.
doi: 10.1021/ar200108c
E.X. Zhang, D.X. Wang, Q.Y. Zheng, M.X. Wang, Org. Lett. 10(2008) 2565-2568.
doi: 10.1021/ol800840m
R.B. Xu, Q.Q. Wang, Y.F. Ao, et al., Org. Lett. 19(2017) 738-741.
doi: 10.1021/acs.orglett.7b00070
T. Ogoshi, S. Kanai, S. Fujinami, T.A. Yamagishi, J. Am. Chem. Soc. 130(2008) 5022-5023.
doi: 10.1021/ja711260m
M. Xue, Y. Yang, X.D. Chi, Z.B. Zhang, F. Huang, Acc. Chem. Res. 45(2012) 1294-1308.
doi: 10.1021/ar2003418
N.L. Strutt, H. Zhang, S.T. Schneebeli, J.F. Stoddart, Acc. Chem. Res. 47(2014) 2631-2642.
doi: 10.1021/ar500177d
H. Chen, J. Fan, X. Hu, et al., Chem. Sci. 6(2015) 197-202.
doi: 10.1039/C4SC02422B
J. Ma, H. Deng, S. Ma, et al., Chem. Commun. 51(2015) 6621-6624.
doi: 10.1039/C5CC01470K
J. Ye, R. Zhang, W. Yang, et al., Chin. Chem. Lett. 31(2020) 1550-1553.
doi: 10.1016/j.cclet.2019.11.041
G.W. Zhang, P.F. Li, Z. Meng, et al., Angew. Chem. Int. Ed. 55(2016) 5304-5308.
doi: 10.1002/anie.201600911
Q. Shi, C.F. Chen, Org. Lett. 19(2017) 3175-3178.
doi: 10.1021/acs.orglett.7b01296
G.W. Zhang, Y. Han, Y. Wang, C.F. Chen, Chem. Commun. 53(2017) 10433-10436.
doi: 10.1039/C7CC05489K
G.B. Huang, S.H. Wang, H. Ke, et al., J. Am. Chem. Soc. 138(2016) 14550-14553.
doi: 10.1021/jacs.6b09472
L.L. Wang, Z. Chen, W.E. Liu, et al., J. Am. Chem. Soc. 139(2017) 8436-8439.
doi: 10.1021/jacs.7b05021
Y.L. Ma, H. Ke, A. Valkonen, K. Rissanen, W. Jiang, Angew. Chem. Int. Ed. 57(2018) 709-713.
doi: 10.1002/anie.201711077
Z. He, G. Ye, W. Jiang, Chem. Eur. J. 21(2015) 3005-3012.
doi: 10.1002/chem.201405912
J.S. Cui, Q.K. Ba, H. Ke, et al., Angew. Chem. Int. Ed. 57(2018) 7809-7814.
doi: 10.1002/anie.201803349
Z. Liu, S.K.M. Nalluri, J.F. Stoddart, Chem. Soc. Rev. 46(2017) 2459-2478.
doi: 10.1039/C7CS00185A
J.C. Barnes, M. Juríček, N.L. Strutt, et al., J. Am. Chem. Soc. 135(2013) 183-192.
doi: 10.1021/ja307360n
S.T.J. Ryan, J.D. Barrio, I. Ghosh, et al., J. Am. Chem. Soc. 136(2014) 9053-9060.
doi: 10.1021/ja5032437
I. Roy, S. Bobbala, R.M. Young, et al., J. Am. Chem. Soc. 141(2019) 12296-12304.
doi: 10.1021/jacs.9b03990
J. Zhou, Y. Wu, I. Roy, et al., Chem. Sci. 10(2019) 4282-4292.
doi: 10.1039/C8SC05514A
M. Juríček, J.C. Barnes, N.L. Strutt, et al., Chem. Sci. 5(2014) 2724-2731.
doi: 10.1039/c4sc00488d
M. Juríček, J.C. Barnes, E.J. Dale, et al., J. Am. Chem. Soc. 135(2013) 12736-12746.
doi: 10.1021/ja4052763
E.J. Dale, N.A. Vermeulen, A.A. Thomas, et al., J. Am. Chem. Soc. 136(2014) 10669-10682.
doi: 10.1021/ja5041557
Y. Chen, C. Qian, Q. Zhao, et al., Chem. Commun. 55(2019) 8072-8075.
doi: 10.1039/C9CC03577J
M. Cheng, J. Zhang, X. Ren, et al., Chem. Commun. 53(2017) 11838-11841.
doi: 10.1039/C7CC07469G
C. Zhang, Z. Wang, L. Tan, et al., Angew. Chem. Int. Ed. 54(2015) 9244-9248.
doi: 10.1002/anie.201502912
Z. Wang, Y. Luo, T.L. Zhai, et al., Org. Lett. 18(2016) 4574-4577.
doi: 10.1021/acs.orglett.6b02219
C. Zhang, Z. Wang, S. Song, et al., J. Org. Chem. 79(2014) 2729-2732.
doi: 10.1021/jo402884a
D.J. Hoffart, J. Tiburcio, A. de la Torre, L.K. Knight, S.J. Loeb, Angew. Chem. Int. Ed. 47(2008) 97-101.
doi: 10.1002/anie.200703019
L. Chen, Y.M. Zhang, Y. Liu, J. Phys. Chem. B 116(2012) 9500-9506.
doi: 10.1021/jp305503e
L. Chen, H.Y. Zhang, Y. Liu, J. Org. Chem. 77(2012) 9766-9773.
doi: 10.1021/jo301911w
X. Ji, M. Zhang, X. Yan, J. Li, Chem. Commun. 49(2013) 1178-1180.
doi: 10.1039/c2cc38472h
F. Zeng, C.F. Chen, Org. Boimol. Chem. 13(2015) 1988-1991.
doi: 10.1039/C4OB02533D
F. Zeng, L.L. Tang, X.M. Chen, et al., J. Org. Chem. 36(2016) 1937-1941.
Y. Ma, X. Ji, F. Xiang, et al., Chem. Commun. 47(2011) 12340-12342.
doi: 10.1039/c1cc15660h
B. Gómez, V. Francisco, F. Fernández-Nieto, et al., Chem. Eur. J. 20(2014) 12123-12132.
doi: 10.1002/chem.201403194
G. Wu, C.Y. Wang, T. Jiao, et al., J. Am. Chem. Soc. 140(2018) 5955-5961.
doi: 10.1021/jacs.8b01651
L. Shen, N. Cao, L. Tong, et al., Angew. Chem. Int. Ed. 57(2018) 16486-16490.
doi: 10.1002/anie.201811025
Y.K. Kryschenko, S.R. Seidel, D.C. Muddiman, et al., J. Am. Chem. Soc. 125(2003) 9647-9652.
doi: 10.1021/ja030209n
K.A. Connors, Binding Constants, J. Wiley and Sons, New York, 1987.
K. Gloe, Macrocyclic Chemistry: Current Trends and Future Perspectives, Springer, Dordrecht, 2005.
F. Davis, S. Higson, Macrocycles: Construction, Chemistry and Nanotechnology Applications, Wiley, Chichester, 2011.
J.W. Steed, J.L. Atwood, Supramolecular Chemistry, 2nd ed., Wiley, Chichester, 2009.
C.F. Chen, Chem. Commun. 47(2011) 1674-1688.
doi: 10.1039/c0cc04852f
T. Ogoshi, T.A. Yamagishi, Y. Nakamoto, Chem. Rev. 116(2016) 7937-8002.
doi: 10.1021/acs.chemrev.5b00765
Y. Han, Z. Meng, Y.X. Ma, C.F. Chen, Acc. Chem. Res. 47(2014) 2026-2040.
doi: 10.1021/ar5000677
G. Yu, K. Jie, F. Huang, Chem. Rev. 115(2015) 7240-7303.
doi: 10.1021/cr5005315
M.X. Wang, Acc. Chem. Res. 45(2012) 182-195.
doi: 10.1021/ar200108c
E.X. Zhang, D.X. Wang, Q.Y. Zheng, M.X. Wang, Org. Lett. 10(2008) 2565-2568.
doi: 10.1021/ol800840m
R.B. Xu, Q.Q. Wang, Y.F. Ao, et al., Org. Lett. 19(2017) 738-741.
doi: 10.1021/acs.orglett.7b00070
T. Ogoshi, S. Kanai, S. Fujinami, T.A. Yamagishi, J. Am. Chem. Soc. 130(2008) 5022-5023.
doi: 10.1021/ja711260m
M. Xue, Y. Yang, X.D. Chi, Z.B. Zhang, F. Huang, Acc. Chem. Res. 45(2012) 1294-1308.
doi: 10.1021/ar2003418
N.L. Strutt, H. Zhang, S.T. Schneebeli, J.F. Stoddart, Acc. Chem. Res. 47(2014) 2631-2642.
doi: 10.1021/ar500177d
H. Chen, J. Fan, X. Hu, et al., Chem. Sci. 6(2015) 197-202.
doi: 10.1039/C4SC02422B
J. Ma, H. Deng, S. Ma, et al., Chem. Commun. 51(2015) 6621-6624.
doi: 10.1039/C5CC01470K
J. Ye, R. Zhang, W. Yang, et al., Chin. Chem. Lett. 31(2020) 1550-1553.
doi: 10.1016/j.cclet.2019.11.041
G.W. Zhang, P.F. Li, Z. Meng, et al., Angew. Chem. Int. Ed. 55(2016) 5304-5308.
doi: 10.1002/anie.201600911
Q. Shi, C.F. Chen, Org. Lett. 19(2017) 3175-3178.
doi: 10.1021/acs.orglett.7b01296
G.W. Zhang, Y. Han, Y. Wang, C.F. Chen, Chem. Commun. 53(2017) 10433-10436.
doi: 10.1039/C7CC05489K
G.B. Huang, S.H. Wang, H. Ke, et al., J. Am. Chem. Soc. 138(2016) 14550-14553.
doi: 10.1021/jacs.6b09472
L.L. Wang, Z. Chen, W.E. Liu, et al., J. Am. Chem. Soc. 139(2017) 8436-8439.
doi: 10.1021/jacs.7b05021
Y.L. Ma, H. Ke, A. Valkonen, K. Rissanen, W. Jiang, Angew. Chem. Int. Ed. 57(2018) 709-713.
doi: 10.1002/anie.201711077
Z. He, G. Ye, W. Jiang, Chem. Eur. J. 21(2015) 3005-3012.
doi: 10.1002/chem.201405912
J.S. Cui, Q.K. Ba, H. Ke, et al., Angew. Chem. Int. Ed. 57(2018) 7809-7814.
doi: 10.1002/anie.201803349
Z. Liu, S.K.M. Nalluri, J.F. Stoddart, Chem. Soc. Rev. 46(2017) 2459-2478.
doi: 10.1039/C7CS00185A
J.C. Barnes, M. Juríček, N.L. Strutt, et al., J. Am. Chem. Soc. 135(2013) 183-192.
doi: 10.1021/ja307360n
S.T.J. Ryan, J.D. Barrio, I. Ghosh, et al., J. Am. Chem. Soc. 136(2014) 9053-9060.
doi: 10.1021/ja5032437
I. Roy, S. Bobbala, R.M. Young, et al., J. Am. Chem. Soc. 141(2019) 12296-12304.
doi: 10.1021/jacs.9b03990
J. Zhou, Y. Wu, I. Roy, et al., Chem. Sci. 10(2019) 4282-4292.
doi: 10.1039/C8SC05514A
M. Juríček, J.C. Barnes, N.L. Strutt, et al., Chem. Sci. 5(2014) 2724-2731.
doi: 10.1039/c4sc00488d
M. Juríček, J.C. Barnes, E.J. Dale, et al., J. Am. Chem. Soc. 135(2013) 12736-12746.
doi: 10.1021/ja4052763
E.J. Dale, N.A. Vermeulen, A.A. Thomas, et al., J. Am. Chem. Soc. 136(2014) 10669-10682.
doi: 10.1021/ja5041557
Y. Chen, C. Qian, Q. Zhao, et al., Chem. Commun. 55(2019) 8072-8075.
doi: 10.1039/C9CC03577J
M. Cheng, J. Zhang, X. Ren, et al., Chem. Commun. 53(2017) 11838-11841.
doi: 10.1039/C7CC07469G
C. Zhang, Z. Wang, L. Tan, et al., Angew. Chem. Int. Ed. 54(2015) 9244-9248.
doi: 10.1002/anie.201502912
Z. Wang, Y. Luo, T.L. Zhai, et al., Org. Lett. 18(2016) 4574-4577.
doi: 10.1021/acs.orglett.6b02219
C. Zhang, Z. Wang, S. Song, et al., J. Org. Chem. 79(2014) 2729-2732.
doi: 10.1021/jo402884a
D.J. Hoffart, J. Tiburcio, A. de la Torre, L.K. Knight, S.J. Loeb, Angew. Chem. Int. Ed. 47(2008) 97-101.
doi: 10.1002/anie.200703019
L. Chen, Y.M. Zhang, Y. Liu, J. Phys. Chem. B 116(2012) 9500-9506.
doi: 10.1021/jp305503e
L. Chen, H.Y. Zhang, Y. Liu, J. Org. Chem. 77(2012) 9766-9773.
doi: 10.1021/jo301911w
X. Ji, M. Zhang, X. Yan, J. Li, Chem. Commun. 49(2013) 1178-1180.
doi: 10.1039/c2cc38472h
F. Zeng, C.F. Chen, Org. Boimol. Chem. 13(2015) 1988-1991.
doi: 10.1039/C4OB02533D
F. Zeng, L.L. Tang, X.M. Chen, et al., J. Org. Chem. 36(2016) 1937-1941.
Y. Ma, X. Ji, F. Xiang, et al., Chem. Commun. 47(2011) 12340-12342.
doi: 10.1039/c1cc15660h
B. Gómez, V. Francisco, F. Fernández-Nieto, et al., Chem. Eur. J. 20(2014) 12123-12132.
doi: 10.1002/chem.201403194
G. Wu, C.Y. Wang, T. Jiao, et al., J. Am. Chem. Soc. 140(2018) 5955-5961.
doi: 10.1021/jacs.8b01651
L. Shen, N. Cao, L. Tong, et al., Angew. Chem. Int. Ed. 57(2018) 16486-16490.
doi: 10.1002/anie.201811025
Y.K. Kryschenko, S.R. Seidel, D.C. Muddiman, et al., J. Am. Chem. Soc. 125(2003) 9647-9652.
doi: 10.1021/ja030209n
K.A. Connors, Binding Constants, J. Wiley and Sons, New York, 1987.

Shuo Li , Qianfa Liu , Lijun Mao , Xin Zhang , Chunju Li , Da Ma . Benzothiadiazole-based water-soluble macrocycle: Synthesis, aggregation-induced emission and selective detection of spermine. Chinese Chemical Letters, 2024, 35(11): 109791-. doi: 10.1016/j.cclet.2024.109791
Qihan Lin , Jiabin Xing , Yue-Yang Liu , Gang Wu , Shi-Jia Liu , Hui Wang , Wei Zhou , Zhan-Ting Li , Dan-Wei Zhang . taBOX: A water-soluble tetraanionic rectangular molecular container for conjugated molecules and taste masking for berberine and palmatine. Chinese Chemical Letters, 2024, 35(5): 109119-. doi: 10.1016/j.cclet.2023.109119
Conghui Wang , Lei Xu , Zhenhua Jia , Teck-Peng Loh . Recent applications of macrocycles in supramolecular catalysis. Chinese Chemical Letters, 2024, 35(4): 109075-. doi: 10.1016/j.cclet.2023.109075
Zhe Li , Ping-Zhao Liang , Li Xu , Fei-Yu Yang , Tian-Bing Ren , Lin Yuan , Xia Yin , Xiao-Bing Zhang . Three positive charge nonapoptotic-induced photosensitizer with excellent water solubility for tumor therapy. Chinese Chemical Letters, 2024, 35(8): 109190-. doi: 10.1016/j.cclet.2023.109190
Cheng-Da Zhao , Huan Yao , Shi-Yao Li , Fangfang Du , Li-Li Wang , Liu-Pan Yang . Amide naphthotubes: Biomimetic macrocycles for selective molecular recognition. Chinese Chemical Letters, 2024, 35(4): 108879-. doi: 10.1016/j.cclet.2023.108879
Zhimin Sun , Xin-Hui Guo , Yue Zhao , Qing-Yu Meng , Li-Juan Xing , He-Lue Sun . Dynamically switchable porphyrin-based molecular tweezer for on−off fullerene recognition. Chinese Chemical Letters, 2024, 35(6): 109162-. doi: 10.1016/j.cclet.2023.109162
Kai An , Qinglong Qiao , Lovelesh , Syed Ali Abbas Abedi , Xiaogang Liu , Zhaochao Xu . "Superimposed" spectral characteristics of fluorophores arising from cross-conjugation hybridization. Chinese Chemical Letters, 2025, 36(1): 109786-. doi: 10.1016/j.cclet.2024.109786
Rui Wang , Yang Liang , Julius Rebek Jr. , Yang Yu . Stabilization and detection of labile reaction intermediates in supramolecular containers. Chinese Chemical Letters, 2024, 35(6): 109228-. doi: 10.1016/j.cclet.2023.109228
Jie Yang , Xin-Yue Lou , Dihua Dai , Jingwei Shi , Ying-Wei Yang . Desymmetrized pillar[8]arenes: High-yield synthesis, functionalization, and host-guest chemistry. Chinese Chemical Letters, 2025, 36(1): 109818-. doi: 10.1016/j.cclet.2024.109818
Chao Zhang , Ai-Feng Liu , Shihui Li , Fang-Yuan Chen , Jun-Tao Zhang , Fang-Xing Zeng , Hui-Chuan Feng , Ping Wang , Wen-Chao Geng , Chuan-Rui Ma , Dong-Sheng Guo . A supramolecular formulation of icariin@sulfonatoazocalixarene for hypoxia-targeted osteoarthritis therapy. Chinese Chemical Letters, 2025, 36(1): 109752-. doi: 10.1016/j.cclet.2024.109752
Zhenzhu Wang , Chenglong Liu , Yunpeng Ge , Wencan Li , Chenyang Zhang , Bing Yang , Shizhong Mao , Zeyuan Dong . Differentiated self-assembly through orthogonal noncovalent interactions towards the synthesis of two-dimensional woven supramolecular polymers. Chinese Chemical Letters, 2024, 35(5): 109127-. doi: 10.1016/j.cclet.2023.109127
Xuanyu Wang , Zhao Gao , Wei Tian . Supramolecular confinement effect enabling light-harvesting system for photocatalytic α-oxyamination reaction. Chinese Chemical Letters, 2024, 35(11): 109757-. doi: 10.1016/j.cclet.2024.109757
Kun Zhang , Xin-Yue Lou , Yan Wang , Weiwei Huan , Ying-Wei Yang . Emission enhancement induced by the supramolecular assembly of leggero pillar[5]arenes for the detection and separation of silver ions. Chinese Chemical Letters, 2025, 36(6): 110464-. doi: 10.1016/j.cclet.2024.110464
Shengyong Liu , Hui Li , Wei Zhang , Yan Zhang , Yan Dong , Wei Tian . Multiple host-guest and metal coordination interactions induce supramolecular assembly and structural transition. Chinese Chemical Letters, 2025, 36(6): 110465-. doi: 10.1016/j.cclet.2024.110465
Ying-Mei Zhong , Zi-Jun Xia , Yu-Hang Hu , Li-Peng Zhou , Li-Xuan Cai , Qing-Fu Sun . Effective separation of phenanthrene from isomeric anthracene using a water-soluble macrocycle-based cage. Chinese Chemical Letters, 2025, 36(4): 110164-. doi: 10.1016/j.cclet.2024.110164
Kang Wei , Jiayu Li , Wen Zhang , Bing Yuan , Ming-De Li , Pingwu Du . A strained π-extended [10]cycloparaphenylene carbon nanoring. Chinese Chemical Letters, 2024, 35(5): 109055-. doi: 10.1016/j.cclet.2023.109055
Junying Zhang , Ruochen Li , Haihua Wang , Wenbing Kang , Xing-Dong Xu . Photo-induced tunable luminescence from an aggregated amphiphilic ethylene-pyrene derivative in aqueous media. Chinese Chemical Letters, 2024, 35(6): 109216-. doi: 10.1016/j.cclet.2023.109216
Zixi Zou , Jingyuan Wang , Yian Sun , Qian Wang , Da-Hui Qu . Controlling molecular assembly on time scale: Time-dependent multicolor fluorescence for information encryption. Chinese Chemical Letters, 2024, 35(7): 108972-. doi: 10.1016/j.cclet.2023.108972
Zhengzhong Zhu , Shaojun Hu , Zhi Liu , Lipeng Zhou , Chongbin Tian , Qingfu Sun . A cationic radical lanthanide organic tetrahedron with remarkable coordination enhanced radical stability. Chinese Chemical Letters, 2025, 36(2): 109641-. doi: 10.1016/j.cclet.2024.109641
Jingyu Chen , Sha Wu , Yuhao Wang , Jiong Zhou . Near-perfect separation of alicyclic ketones and alicyclic alcohols by nonporous adaptive crystals of perethylated pillar[5]arene and pillar[6]arene. Chinese Chemical Letters, 2025, 36(4): 110102-. doi: 10.1016/j.cclet.2024.110102