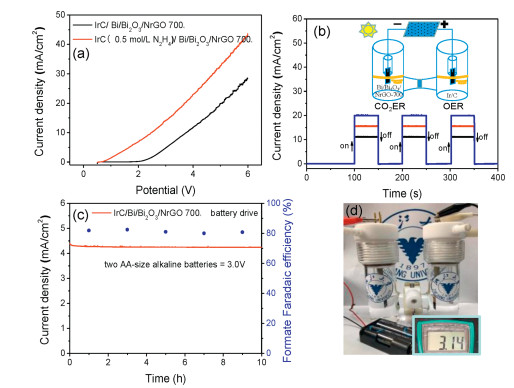
-
[1]
(a) F. Li, L. Chen, G.P. Knowles, D.R. MacFarlane, J. Zhang, Angew. Chem. Int. Ed. 56 (2017) 505-509;
(b) C. Lu, J. Yang, S. Wei, et al., Adv. Funct. Mater. 29 (2019) 1806884;
(c) T. Wang, Q. Zhao, Y. Fu, et al., Small Methods 3 (2019) 1900210;
(d) W. Xiong, J. Yang, L. Shuai, et al., ChemElectroChem 6 (2019) 5951-5957;
(e) W. Zheng, J. Yang, H. Chen, et al., Adv. Funct. Mater. 30 (2019) 1907658;
(f) W. Zheng, C. Guo, J. Yang, et al., Carbon 150 (2019) 52-59;
(g) C. Wang, Y. Zhao, H. Xu, et al., Appl. Catal. B 263 (2020) 118314;
(h) X. Wu, C. Wang, Y. Wei, et al., J. Catal. 377 (2019) 309-321;
(i) Y. Zhao, Y. Wei, X. Wu, et al., Appl. Catal. B 226 (2018) 360-372.
-
[2]
L. Sun, V. Reddu, A.C. Fisher, X. Wang, Energy. Environ. Sci. 13(2020) 374-403.
doi: 10.1039/C9EE03660A
-
[3]
E. Irtem, T. Andreu, A. Parra, et al., J. Mater. Chem. A 4(2016) 13582-13588.
doi: 10.1039/C6TA04432H
-
[4]
Z.B. Hoffman, T.S. Gray, K.B. Moraveck, T.B. Gunnoe, G. Zangari, ACS Catal. 7(2017) 5381-5390.
doi: 10.1021/acscatal.7b01161
-
[5]
J. Schneider, H. Jia, K. Kobiro, et al., Energy. Environ. Sci. 5(2012) 9502-9510.
doi: 10.1039/c2ee22528j
-
[6]
C. Yan, H. Li, Y. Ye, et al., Energy. Environ. Sci. 11(2018) 1204-1210.
doi: 10.1039/C8EE00133B
-
[7]
N. Han, Y. Wang, H. Yang, et al., Nat. Commun. 9(2018) 1320.
doi: 10.1038/s41467-018-03712-z
-
[8]
(a) S. Gao, Y. Lin, X. Jiao, et al., Nature 529 (2016) 68-71;
(b) H. Mistry, A.S. Varela, C.S. Bonifacio, et al., Nat. Commun. 7 (2016) 12123;
(c) Y. Chen, M.W. Kanan, J. Am. Chem. Soc. 134 (2012) 1986-1989.
-
[9]
(a) S. Liu, X.F. Lu, J. Xiao, X. Wang, X.W.D. Lou, Angew. Chem. Int. Ed. 58 (2019) 13828-13833;
(b) Q. Gong, P. Ding, M. Xu, et al., Nat. Commun. 10 (2019) 13828-13833;
(c) Y. Zhang, F. Li, X. Zhang, et al., J. Mater. Chem. A 6 (2018) 4714-4720;
(d) C.C. Miao, G.Q. Yuan, ChemElectroChem 5 (2018) 3741-3747.
-
[10]
(a) H. Zhong, Y. Qiu, T. Zhang, et al., J. Mater. Chem. A 4 (2016) 13746-13753;
(b) J.H. Koh, D.H. Won, T. Eom, et al., ACS Catal. 7 (2017) 5071-5077.
-
[11]
H. Huang, Y. He, X. Li, et al., J. Mater. Chem. A 3(2015) 24547-24556.
doi: 10.1039/C5TA07655B
-
[12]
C. Liu, X. Huang, J. Liu, et al., Adv. Sci. 7(2020) 1901480.
doi: 10.1002/advs.201901480
-
[13]
W. Zhang, Y. Hu, L. Ma, et al., Nano Energy 53(2018) 808-816.
doi: 10.1016/j.nanoen.2018.09.053
-
[14]
M. Ahila, M. Malligavathy, E. Subramanian, D.P. Padiyan, Solid State Ionics 298(2016) 23-34.
doi: 10.1016/j.ssi.2016.10.017
-
[15]
J. Hou, C. Yang, Z. Wang, et al., Appl. Catal. B 142-143(2013) 504-511.
-
[16]
Y. Wang, J. Zhao, Y. Zhu, et al., Colloids Surf. A 434(2013) 296-302.
doi: 10.1016/j.colsurfa.2013.05.078
-
[17]
Y. Yu, C. Cao, H. Liu, et al., J. Mater. Chem. A 2(2014) 1677-1681.
doi: 10.1039/C3TA14494A
-
[18]
P.T. Babar, A.C. Lokhande, B.S. Pawar, et al., Appl. Surf. Sci. 427(2018) 253-259.
-
[19]
G.H. Jiang, X. Li, Z. Wei, et al., Acta Metall. Sin. 28(2015) 460-466.
doi: 10.1007/s40195-015-0220-1
-
[20]
W. Tian, H. Zhang, H. Sun, et al., Adv. Funct. Mater. 26(2016) 8651-8661.
doi: 10.1002/adfm.201603937
-
[21]
Q. Lai, N. Yang, G. Yuan, Electrochem. Commun. 83(2017) 24-27.
doi: 10.1016/j.elecom.2017.08.015
-
[22]
C.W. Li, J. Ciston, M.W. Kanan, Nature 508(2014) 504-507.
doi: 10.1038/nature13249
-
[23]
X. Li, W. Bi, M. Chen, et al., J. Am. Chem. Soc. 139(2017) 14889-14892.
doi: 10.1021/jacs.7b09074

 Login In
Login In

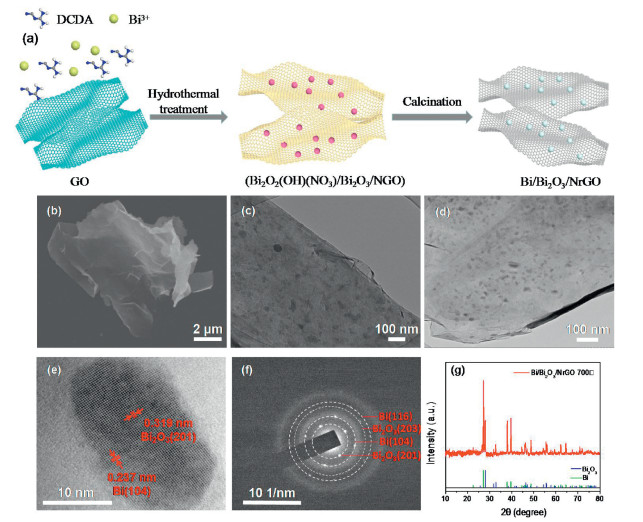





 DownLoad:
DownLoad: