Fe doping promoted electrocatalytic N2 reduction reaction of 2H MoS2
-
* Corresponding authors.
E-mail addresses: zaijiantao@sjtu.edu.cn (J. Zai), xfqian@sjtu.edu.cn (X. Qian).
Citation:
Guo Jiaojiao, Tsega Tsegaye Tadesse, Islam Ibrahim Ul, Iqbal Asma, Zai Jiantao, Qian Xuefeng. Fe doping promoted electrocatalytic N2 reduction reaction of 2H MoS2[J]. Chinese Chemical Letters,
;2020, 31(9): 2487-2490.
doi:
10.1016/j.cclet.2020.02.019

Spintronics is a cutting-edge field of developing new electronic devices by manipulating the electron spin and magnetic moment [1]. Traditional spintronic research mainly focuses on transition metals and inorganic semiconductors, while organic molecules have the advantage of being extremely easy to realize efficient spin control by modifying the specific external conditions for desired electronic structures and magnetic characteristics. Corrole, as a ring-contracted porphyrin, is the aromatic analog of the central macrocycle of vitamin B12. Corrole has a squeezed inner cavity and three inner NHs in its free-base form, making it easier to stabilize high-valent metal ions and thus a promising candidate in spintronics.
When coordinated to metal ions such as Cu, Co, and Fe, the electron-rich corrole ligand could be partially oxidized to exhibit radical character, making it difficult to determine the exact oxidation states of central metals and ligands. The most controversial debate was the Cu(Ⅱ)/Cu(Ⅲ) dilemma on copper corrole [2]. The compound was initially thought to be a closed-shell Cu(Ⅲ) complex in 2000, but significant experimental and theoretical evidence over the next twenty years progressively revealed its open-shell singlet state ground state comprised of a Cu(Ⅱ) core and partially oxidized radical ligand (Fig. 1a).
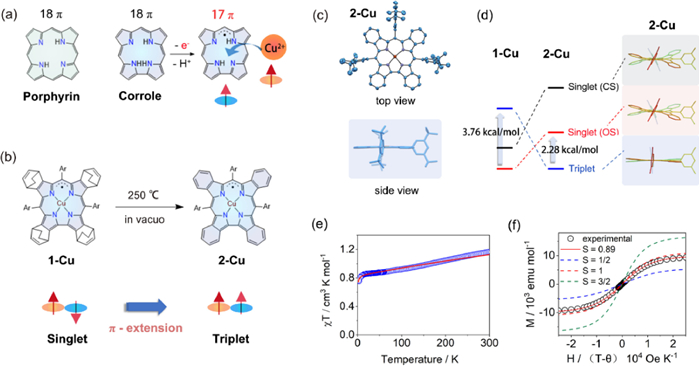
Shen Z. and Wu F. from Nanjing University have developed a series of metallocorroles with extended π-conjugation systems, which not only facilitated the formation and stabilization of radical ligands, but also allowed spin configurations of complexes to be easily controlled [3-5]. Recently, Shen, Wu, and co-workers reported that the unambiguous Cu(Ⅱ) corrole with fully oxidized [4n + 1]π radical ligand was obtained through the benzo-fusion at the β-position of corrole ligand [6]. The ground-state conversion of copper corrole radical from singlet to triplet was achieved via a retro-Diels-Alder reaction (Fig. 1b).
The authors first synthesized a bicyclo[2.2.2]octadiene (BCOD) fused corrole 1-Cu by employing the classic H2O-MeOH approach with starting materials 4, 7-dihydro-4, 7-ethano-2H-isoindole and 3, 5-di-tert-butyl-benzaldehyde. Heating solid 1-Cu at 250 ℃ in vacuo cut the C—C bond in the BCOD bridge, eliminated the ethylene, and quantitatively afforded the benzo-fused 2-Cu. The singlet ground state of 1-Cu was clearly confirmed by the peripheral BCOD protons signals that appeared in the region of 6.60~2.09 ppm, while the signals of benzo protons in 2-Cu were located in a range of −6.2~−25.6 ppm, demonstrating its enhanced paramagnetism.
The conformations of copper corroles were assumed to be "inherently saddle distorted" owing to the strong d-π interactions of antiferromagnetically coupled Cu(Ⅱ) corrole radicals. When compared to other copper corroles, 2-Cu stood out due to its highly planar macrocycle with a mean plane deviation value of only 0.024 Å (Fig. 1c). The planar structure could perfectly sustain the ferromagnetic coupling (S = 1) between Cu(Ⅱ) and corrole radical.
The theoretical analysis of 2-Cu was conducted by the authors for three different states, including a close-shell singlet Cu(Ⅲ) (CS), an open-shell singlet antiferromagnetically coupled Cu(Ⅱ) corrole radical (OS) and a triplet ferromagnetically coupled Cu(Ⅱ) corrole radical (T). A lower T state was discovered for 2-Cu than the CS and OS states with a calculated singlet-triplet energy gap of 2.28 kcal/mol, providing theoretical support for the triplet ground state (Fig. 1d). The strongest support for the triplet ground state came from temperature- and field-dependent superconducting quantum interference device (SQUID) magnetometry. The χT value of 2-Cu in 2 K was 0.77 cm3 K/mol, and it reached approximately 1 cm3 K/mol at 300 K. The singlet-triplet energy gap was estimated to be 1.66 kcal/mol by fitting the χT–T plot (Fig. 1e). The field-dependent magnetization plot of 2-Cu at 2 K was fitted to a Brillouin function with S = 0.89, which was close to the value (S = 1) corresponding to the triplet ground state (Fig. 1f). The magnetic hysteresis of 2-Cu was observed at 2 K. Moreover, 2-Cu exhibits remarkable stability in air despite its radical character. The calculated density plots of spin and SOMO both demonstrate that the density is concentrated mostly in the inner corrole ring, which is nicely protected by fused benzenes with low reactivity.
The research conducted by Shen's group introduces a new approach to the fine-tuning of interactions between metal center and corrole ligand and provides a promising strategy for the creation of stable corrole radical complexes with distinctive high-spin systems. The strategy will further trigger the development of novel functional materials based on corroles and their work will encourage an increasing amount of spintronics research for the use of innovative magnetic and electrical devices.
X. Chen, N. Li, Z. Kong, et al., Mater. Horiz. 5(2018) 9-27.
doi: 10.1039/C7MH00557A
X. Cui, C. Tang, Q. Zhang, Adv. Energy Mater. 8(2018) 1800369.
doi: 10.1002/aenm.201800369
S.L. Foster, S.I.P. Bakovic, R.D. Duda, et al., Nat. Catal. 1(2018) 490-500.
doi: 10.1038/s41929-018-0092-7
K. Ithisuphalap, H. Zhang, L. Guo, et al., Small Method. 3(2018) 1800352.
D. Yan, H. Li, C. Chen, et al., Small Method. 3(2018)1800331.
P. Wang, F. Chang, W. Gao, et al., Nat. Chem. 9(2017) 64-70.
doi: 10.1038/nchem.2595
M. Kitano, Y. Inoue, Y. Yamazaki, et al., Nat. Chem. 4(2012) 934-940.
doi: 10.1038/nchem.1476
D. Bao, Q. Zhang, F.L. Meng, et al., Adv. Mater. 29(2017) 1700001.
doi: 10.1002/adma.201700001
Y. Gong, J. Wu, M. Kitano, et al., Nat. Catal. 1(2018) 178-185.
doi: 10.1038/s41929-017-0022-0
L. Han, X. Liu, J. Chen, et al., Angew. Chem. Int. Ed. 58(2019) 2321-2325.
doi: 10.1002/anie.201811728
B.M. Hoffman, D. Lukoyanov, Z.Y. Yang, et al., Chem. Rev.114(2014) 4041-4062.
doi: 10.1021/cr400641x
J.S. Anderson, J. Rittle, J.C. Peters, Nature 501(2013) 84-87.
doi: 10.1038/nature12435
G. Ung, J.C. Peters, Angew. Chem. Int. Ed. 54(2015) 532-535.
R.B. Yelle, J.L. Crossland, N.K. Szymczak, D.R. Tyler, Inorg. Chem. Front. 48(2008) 861-871.
K.C. MacLeod, P.L. Holland, Nat. Chem. 5(2013) 559-565.
doi: 10.1038/nchem.1620
J. Liu, M.S. Kelley, W. Wu, et al., P. Natl. Acad. Sci. U. S. A. 113(2016) 5530-5535.
doi: 10.1073/pnas.1605512113
A. Banerjee, B.D. Yuhas, E.A. Margulies, et al., J. Am. Chem. Soc. 137(2015) 2030-2034.
doi: 10.1021/ja512491v
K.A. Brown, D.F. Harris, M.B. Wilker, et al., Science 352(2016) 448-450.
doi: 10.1126/science.aaf2091
X. Guo, H. Du, F. Qu, J. Li, J. Mater, Chem. A 7(2019) 3531-3543.
Z. Lei, J. Zhan, L. Tang, et al., Adv. Energy Mater. 8(2018) 1703482.
doi: 10.1002/aenm.201703482
L. Zhang, X. Ji, X. Ren, et al., Adv. Mater. 30(2018) 1800191.
doi: 10.1002/adma.201800191
S. Sun, X. Li, W. Wang, et al., App. Catal. B -Environ. 200(2017) 323-329.
doi: 10.1016/j.apcatb.2016.07.025
L.M. S.C.H. Azofra, L. Cavallo, D.R. Macfarlane, Chem. Eur. J. 23(2017) 8275-8279.
doi: 10.1002/chem.201701113
S. Liu, M. Wang, T. Qian, et al., Nat. Commun. 10(2019) 3898.
doi: 10.1038/s41467-019-11846-x
S.Z. Andersen, V. Colic, S. Yang, et al., Nature 570(2019) 504-508.
doi: 10.1038/s41586-019-1260-x
B. Hu, M. Hu, L. Seefeldt, T.L. Liu, ACS Energy Lett. 4(2019) 1053-1054.
doi: 10.1021/acsenergylett.9b00648
P. Chen, W. Xu, Y. Gao, et al., ACS Appl. Nano Mater. 1(2018) 6976-6988.
doi: 10.1021/acsanm.8b01792
H. Dong, Y. Xu, C. Zhang, et al., Inorg. Chem. Front. 5(2018) 3099-3105.
doi: 10.1039/C8QI00969D
Z. He, R. Zhao, X. Chen, et al., ACS Appl. Mater. Interfaces 10(2018) 42524-42533.
doi: 10.1021/acsami.8b17145
L. Zhang, M. Li, A. Zou, et al., ACS Appl. Energy Mater. 2(2018) 493-502.
W. Jia, X. Zhou, Y. Huang, et al., ChemCatChem 11(2018) 707-714.
X. Chen, N. Li, Z. Kong, et al., Mater. Horiz. 5(2018) 9-27.
doi: 10.1039/C7MH00557A
X. Cui, C. Tang, Q. Zhang, Adv. Energy Mater. 8(2018) 1800369.
doi: 10.1002/aenm.201800369
S.L. Foster, S.I.P. Bakovic, R.D. Duda, et al., Nat. Catal. 1(2018) 490-500.
doi: 10.1038/s41929-018-0092-7
K. Ithisuphalap, H. Zhang, L. Guo, et al., Small Method. 3(2018) 1800352.
D. Yan, H. Li, C. Chen, et al., Small Method. 3(2018)1800331.
P. Wang, F. Chang, W. Gao, et al., Nat. Chem. 9(2017) 64-70.
doi: 10.1038/nchem.2595
M. Kitano, Y. Inoue, Y. Yamazaki, et al., Nat. Chem. 4(2012) 934-940.
doi: 10.1038/nchem.1476
D. Bao, Q. Zhang, F.L. Meng, et al., Adv. Mater. 29(2017) 1700001.
doi: 10.1002/adma.201700001
Y. Gong, J. Wu, M. Kitano, et al., Nat. Catal. 1(2018) 178-185.
doi: 10.1038/s41929-017-0022-0
L. Han, X. Liu, J. Chen, et al., Angew. Chem. Int. Ed. 58(2019) 2321-2325.
doi: 10.1002/anie.201811728
B.M. Hoffman, D. Lukoyanov, Z.Y. Yang, et al., Chem. Rev.114(2014) 4041-4062.
doi: 10.1021/cr400641x
J.S. Anderson, J. Rittle, J.C. Peters, Nature 501(2013) 84-87.
doi: 10.1038/nature12435
G. Ung, J.C. Peters, Angew. Chem. Int. Ed. 54(2015) 532-535.
R.B. Yelle, J.L. Crossland, N.K. Szymczak, D.R. Tyler, Inorg. Chem. Front. 48(2008) 861-871.
K.C. MacLeod, P.L. Holland, Nat. Chem. 5(2013) 559-565.
doi: 10.1038/nchem.1620
J. Liu, M.S. Kelley, W. Wu, et al., P. Natl. Acad. Sci. U. S. A. 113(2016) 5530-5535.
doi: 10.1073/pnas.1605512113
A. Banerjee, B.D. Yuhas, E.A. Margulies, et al., J. Am. Chem. Soc. 137(2015) 2030-2034.
doi: 10.1021/ja512491v
K.A. Brown, D.F. Harris, M.B. Wilker, et al., Science 352(2016) 448-450.
doi: 10.1126/science.aaf2091
X. Guo, H. Du, F. Qu, J. Li, J. Mater, Chem. A 7(2019) 3531-3543.
Z. Lei, J. Zhan, L. Tang, et al., Adv. Energy Mater. 8(2018) 1703482.
doi: 10.1002/aenm.201703482
L. Zhang, X. Ji, X. Ren, et al., Adv. Mater. 30(2018) 1800191.
doi: 10.1002/adma.201800191
S. Sun, X. Li, W. Wang, et al., App. Catal. B -Environ. 200(2017) 323-329.
doi: 10.1016/j.apcatb.2016.07.025
L.M. S.C.H. Azofra, L. Cavallo, D.R. Macfarlane, Chem. Eur. J. 23(2017) 8275-8279.
doi: 10.1002/chem.201701113
S. Liu, M. Wang, T. Qian, et al., Nat. Commun. 10(2019) 3898.
doi: 10.1038/s41467-019-11846-x
S.Z. Andersen, V. Colic, S. Yang, et al., Nature 570(2019) 504-508.
doi: 10.1038/s41586-019-1260-x
B. Hu, M. Hu, L. Seefeldt, T.L. Liu, ACS Energy Lett. 4(2019) 1053-1054.
doi: 10.1021/acsenergylett.9b00648
P. Chen, W. Xu, Y. Gao, et al., ACS Appl. Nano Mater. 1(2018) 6976-6988.
doi: 10.1021/acsanm.8b01792
H. Dong, Y. Xu, C. Zhang, et al., Inorg. Chem. Front. 5(2018) 3099-3105.
doi: 10.1039/C8QI00969D
Z. He, R. Zhao, X. Chen, et al., ACS Appl. Mater. Interfaces 10(2018) 42524-42533.
doi: 10.1021/acsami.8b17145
L. Zhang, M. Li, A. Zou, et al., ACS Appl. Energy Mater. 2(2018) 493-502.
W. Jia, X. Zhou, Y. Huang, et al., ChemCatChem 11(2018) 707-714.

Sajid Mahmood , Haiyan Wang , Fang Chen , Yijun Zhong , Yong Hu . Recent progress and prospects of electrolytes for electrocatalytic nitrogen reduction toward ammonia. Chinese Chemical Letters, 2024, 35(4): 108550-. doi: 10.1016/j.cclet.2023.108550
Hong-Rui Li , Xia Kang , Rui Gao , Miao-Miao Shi , Bo Bi , Ze-Yu Chen , Jun-Min Yan . Interfacial interactions of Cu/MnOOH enhance ammonia synthesis from electrochemical nitrate reduction. Chinese Chemical Letters, 2025, 36(2): 109958-. doi: 10.1016/j.cclet.2024.109958
Zhenfei Tang , Yunwu Zhang , Zhiyuan Yang , Haifeng Yuan , Tong Wu , Yue Li , Guixiang Zhang , Xingzhi Wang , Bin Chang , Dehui Sun , Hong Liu , Lili Zhao , Weijia Zhou . Iron-doping regulated light absorption and active sites in LiTaO3 single crystal for photocatalytic nitrogen reduction. Chinese Chemical Letters, 2025, 36(3): 110107-. doi: 10.1016/j.cclet.2024.110107
Ping Wang , Ting Wang , Ming Xu , Ze Gao , Hongyu Li , Bowen Li , Yuqi Wang , Chaoqun Qu , Ming Feng . Keplerate polyoxomolybdate nanoball mediated controllable preparation of metal-doped molybdenum disulfide for electrocatalytic hydrogen evolution in acidic and alkaline media. Chinese Chemical Letters, 2024, 35(7): 108930-. doi: 10.1016/j.cclet.2023.108930
Ruiheng Liang , Huizhong Wu , Zhongzheng Hu , Ge Song , Xuyang Zhang , Omotayo A. Arotiba , Minghua Zhou . Hierarchical Fe-Bi/Bi7O9I3/OVs microspheres coupled with natural air diffusion electrode to achieve efficient heterogeneous visible-light-driven photoelectro-Fenton degradation of tetracycline without aeration. Chinese Chemical Letters, 2025, 36(4): 110136-. doi: 10.1016/j.cclet.2024.110136
Liwei Hou , Xianyun Peng , Siliu Lyu , Zhongjian Li , Bin Yang , Qinghua Zhang , Qinggang He , Lecheng Lei , Yang Hou . Advancements in MXene-based nanohybrids for electrochemical water splitting. Chinese Chemical Letters, 2025, 36(6): 110392-. doi: 10.1016/j.cclet.2024.110392
Haijiao Liu , Qiao Feng , Yu Huang , Feng Wu , Yali Liu , Minxia Shen , Xiao Guo , Wenting Dai , Weining Qi , Yifan Zhang , Lu Li , Qiyuan Wang , Bianhong Zhou , Jianjun Li . Composition and size distribution of wintertime inorganic aerosols at ground and alpine regions of northwest China. Chinese Chemical Letters, 2024, 35(11): 109636-. doi: 10.1016/j.cclet.2024.109636
Ting Xie , Xun He , Lang He , Kai Dong , Yongchao Yao , Zhengwei Cai , Xuwei Liu , Xiaoya Fan , Tengyue Li , Dongdong Zheng , Shengjun Sun , Luming Li , Wei Chu , Asmaa Farouk , Mohamed S. Hamdy , Chenggang Xu , Qingquan Kong , Xuping Sun . CoSe2 nanowire array enabled highly efficient electrocatalytic reduction of nitrate for ammonia synthesis. Chinese Chemical Letters, 2024, 35(11): 110005-. doi: 10.1016/j.cclet.2024.110005
Junan Pan , Xinyi Liu , Huachao Ji , Yanwei Zhu , Yanling Zhuang , Kang Chen , Ning Sun , Yongqi Liu , Yunchao Lei , Kun Wang , Bao Zang , Longlu Wang . The strategies to improve TMDs represented by MoS2 electrocatalytic oxygen evolution reaction. Chinese Chemical Letters, 2024, 35(11): 109515-. doi: 10.1016/j.cclet.2024.109515
Sanmei Wang , Yong Zhou , Hengxin Fang , Chunyang Nie , Chang Q Sun , Biao Wang . Constant-potential simulation of electrocatalytic N2 reduction over atomic metal-N-graphene catalysts. Chinese Chemical Letters, 2025, 36(3): 110476-. doi: 10.1016/j.cclet.2024.110476
Ying Chen , Xingyuan Xia , Lei Tian , Mengying Yin , Ling-Ling Zheng , Qian Fu , Daishe Wu , Jian-Ping Zou . Constructing built-in electric field via CuO/NiO heterojunction for electrocatalytic reduction of nitrate at low concentrations to ammonia. Chinese Chemical Letters, 2024, 35(12): 109789-. doi: 10.1016/j.cclet.2024.109789
Yiwen Xu , Chaozheng He , Chenxu Zhao , Ling Fu . Single-atom Ti doping on S-vacancy two-dimensional CrS2 as a catalyst for ammonia synthesis: A DFT study. Chinese Chemical Letters, 2025, 36(4): 109797-. doi: 10.1016/j.cclet.2024.109797
Jiayu Huang , Kuan Chang , Qi Liu , Yameng Xie , Zhijia Song , Zhiping Zheng , Qin Kuang . Fe-N-C nanostick derived from 1D Fe-ZIFs for Electrocatalytic oxygen reduction. Chinese Journal of Structural Chemistry, 2023, 42(10): 100097-100097. doi: 10.1016/j.cjsc.2023.100097
Shuqi Yu , Yu Yang , Keisuke Kuroda , Jian Pu , Rui Guo , Li-An Hou . Selective removal of Cr(Ⅵ) using polyvinylpyrrolidone and polyacrylamide co-modified MoS2 composites by adsorption combined with reduction. Chinese Chemical Letters, 2024, 35(6): 109130-. doi: 10.1016/j.cclet.2023.109130
Xiangyu Chen , Aihao Xu , Dong Wei , Fang Huang , Junjie Ma , Huibing He , Jing Xu . Atomic cerium-doped CuOx catalysts for efficient electrocatalytic CO2 reduction to CH4. Chinese Chemical Letters, 2025, 36(1): 110175-. doi: 10.1016/j.cclet.2024.110175
Maomao Liu , Guizeng Liang , Ningce Zhang , Tao Li , Lipeng Diao , Ping Lu , Xiaoliang Zhao , Daohao Li , Dongjiang Yang . Electron-rich Ni2+ in Ni3S2 boosting electrocatalytic CO2 reduction to formate and syngas. Chinese Journal of Structural Chemistry, 2024, 43(8): 100359-100359. doi: 10.1016/j.cjsc.2024.100359
Yuwei Liu , Yihui Zhu , Weijian Duan , Yizhuo Yang , Haorui Tuo , Chunhua Feng . Electrocatalytic nitrate reduction on Fe, Fe3O4, and Fe@Fe3O4 cathodes: Elucidating structure-sensitive mechanisms of direct electron versus hydrogen atom transfer. Chinese Chemical Letters, 2025, 36(6): 110347-. doi: 10.1016/j.cclet.2024.110347
Pingping HAO , Fangfang LI , Yawen WANG , Houfen LI , Xiao ZHANG , Rui LI , Lei WANG , Jianxin LIU . Hydrogen production performance of the non-platinum-based MoS2/CuS cathode in microbial electrolytic cells. Chinese Journal of Inorganic Chemistry, 2024, 40(9): 1811-1824. doi: 10.11862/CJIC.20240054
Xian-Rui Meng , Qian Chen , Mei-Feng Wu , Qiang Wu , Su-Qin Wang , Li-Ping Jin , Fan Zhou , Ren-Li Ma , Jian-Ping Zou . Nano-flowers FeS/MoS2 composites as a peroxymonosulfate activator for efficient p-chlorophenol degradation. Chinese Journal of Structural Chemistry, 2025, 44(3): 100543-100543. doi: 10.1016/j.cjsc.2025.100543
Qingwang LIU . MoS2/Ag/g-C3N4 Z-scheme heterojunction: Preparation and photocatalytic performance. Chinese Journal of Inorganic Chemistry, 2025, 41(4): 821-832. doi: 10.11862/CJIC.20240148