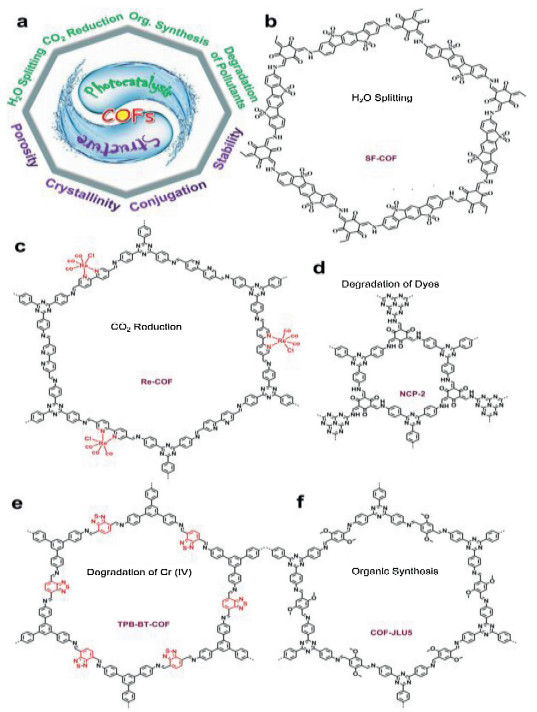
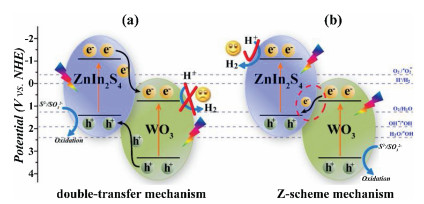
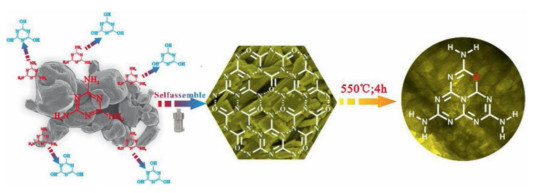
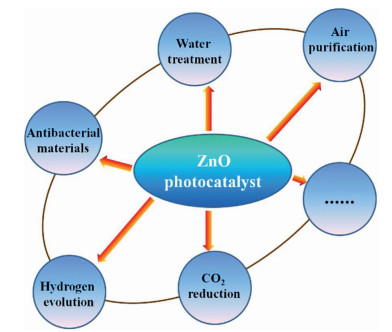
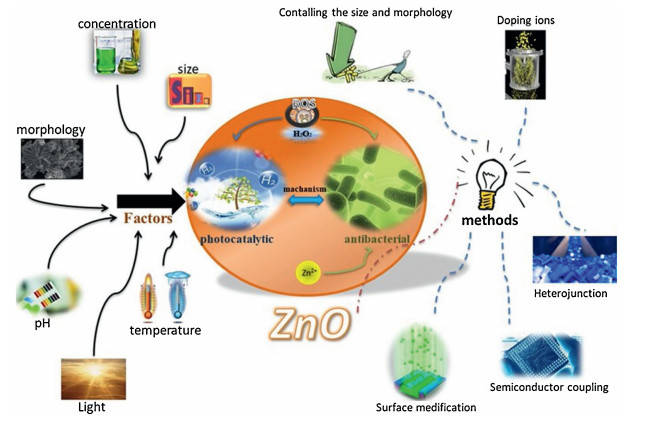
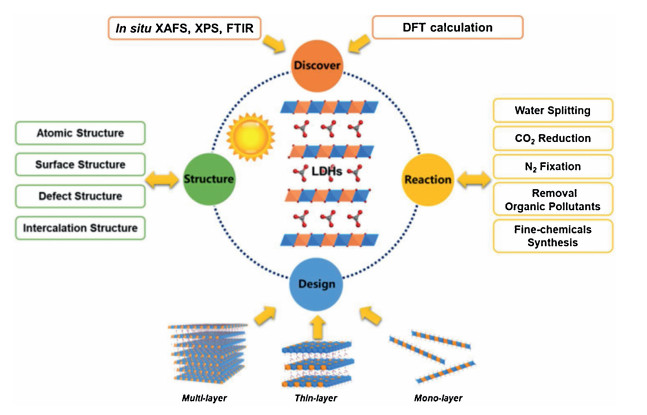
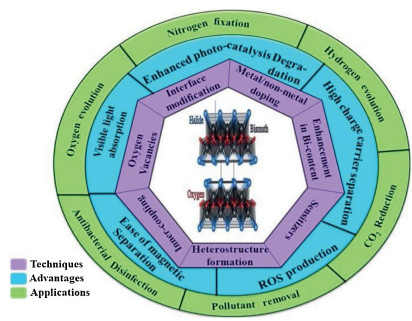
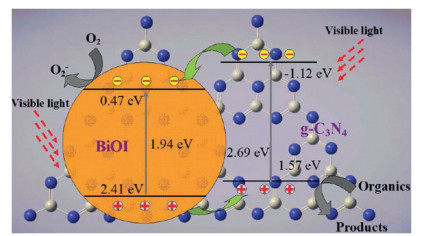
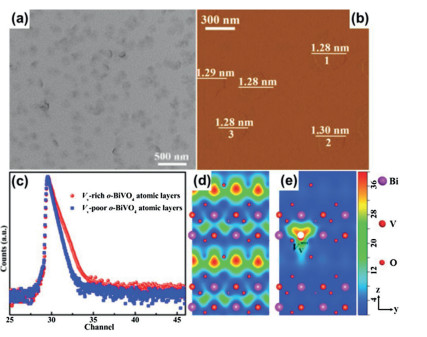
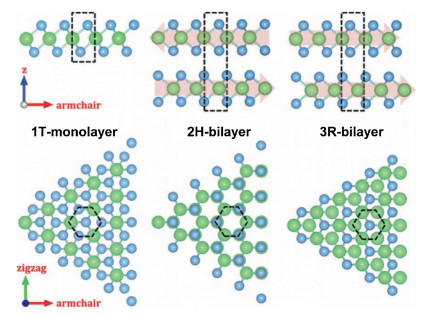
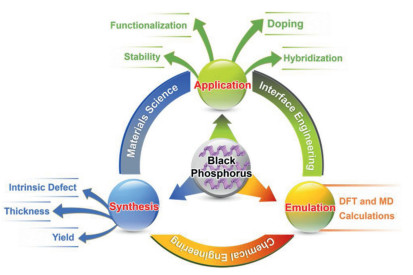
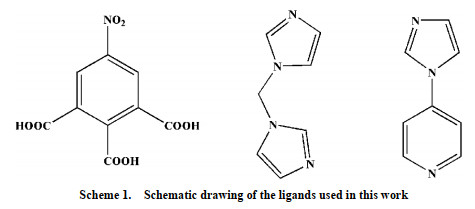
Recently, the design and assembly of Zn(Ⅱ) coordination polymers (CPs) have received remarkable attention because of their intriguing topological structures and potential applications in many fields[1-4]. In order to generate novel CPs, the important step is to choose suitable organic ligands. As we know, bidentate μ2-N, N΄ ligands bearing an appropriate auxiliary group, such as 4, 4΄-bipyridine, 1, 2-bis(4-pyridyl)-ethylene, 1, 3-bis(4-pyridyl)propane, 4-bis(2-methylimidazolyl)butane and related species, have been widely used as bridging ligands with carboxylic acids in crystal engineering[5-9]. However, the presently known cases of CPs with carboxylic acids and bis(imidazol-1-yl)methane (bimm) or N-(4-pyridylmethyl)imidazole (pyim) organic ligand are still very rare[10-13]. In the crystal self-assembly of CPs, these μ2-bridging bridging ligands are expected to construct multi-dimensional compounds. Among them, the flexible bimm ligand with an alkyl spacer between two imidazole unities may coordinate with central metal ions via two imidazole-type nitrogen atoms (Scheme 1). Compared with bimm ligand, the rigid pyim ligand may coordinate with central metal ions using pyridine-type and imidazole-type nitrogen atoms, yielding intriguing structures.
All analytical grade chemicals and solvents were purchased and used as received without further purification. The IR spectra were recorded as KBr pellets on a Nicolet Avatar-360 spectrometer in the range of 4000~400 cm−1. Elemental analyses for C, H and N were carried out on a Flash 2000 elemental analyzer. Thermogravimetric analyses (TGA) were carried out on a SDTQ600 thermogravimetric analyzer. A platinum pan was used for heating the sample at a heating rate of 10 ℃/min under air atmosphere. Fluorescence measurements were recorded with a Hitachi F4500 fluorescence spectrophotometer.
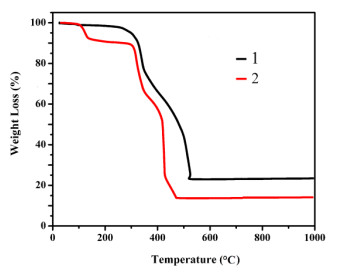
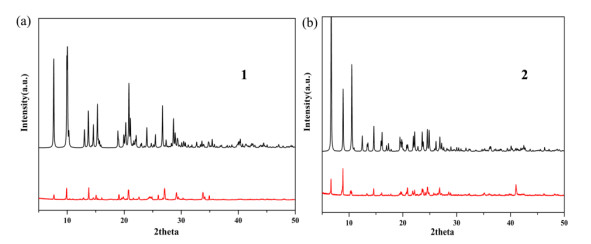
To confirm the phase purity of bulk samples, the X-ray powder diffraction pattern was recorded. As seen in Fig. 3, the peak positions of experimental and simulated patterns are in good agreement with each other, demonstrating the phase purity of 1 and 2. The dissimilarities in intensity may be owing to the preferred orientation of the samples. In addition, thermal behaviors of 1 and 2 were examined by thermal gravimetric analysis (TGA) in a dry air atmosphere from 30 to 700 ℃. As shown in Fig. 4, compound 1 undergoes two steps of weight loss, with the first one of 9.66% corresponding to the removal of water molecules in the temperature range of 97~107 ℃ (calcd. 9.59%). From then on, almost no weight loss is observed until 253 ℃, beyond which the intense weight loss is attributed to the decomposition and collapse of the structure. Compound 2 also undergoes two steps of weight loss. The weight loss of 9.79% from 97 to 116 ℃ corresponds to the release of water molecules (calcd. 9.83%) and that from 273 ºC results from the decomposition and collapse of the structure.
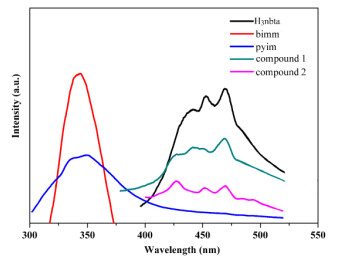
CPs with Zn lefts usually present photoluminescent properties with potential applications such as chemical sensors and photochemistry fields[20, 21]. Here, the solid-state emission spectra of 1, 2 and free ligands were explored at room temperature. As shown in Fig. 5, compound 1 shows a main peak at 471 nm with two shoulders at 453, 440 nm upon excitation at 290 nm and compound 2 shows a main peak at 420 nm with two shoulders at 452 and 469 nm under 374 nm excitation. The free H3nbta shows a main peak at 469 nm with a shoulder at 453 nm upon excitation at 250 nm and the free bimm and pyim ligands were observed with wavelengths at 344 and 350 nm, respectively. Considering the Zn2+ ion is difficultly oxidized or reduced, the peaks of compounds 1 and 2 should be attributed to the transitions of Hnbta2−/nbta3− anions because similar peaks also appear for the free H3nbta ligand. The peaks for 1 and 2 exhibit a blue-shift with respect to the free H3nbta, which may be tentatively assigned to the intraligand charge transfer of Hnbta2−/nbta3− anions and/or metal-ligand coordination interactions.
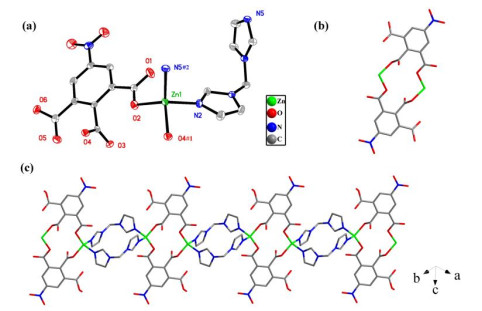
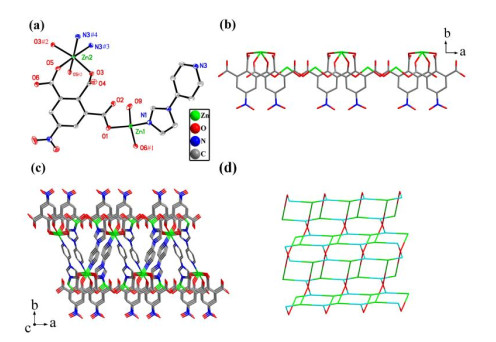
In summary, we have successfully synthesized and characterized two new CPs based on 5-nitro-1, 2, 3-benzenetricarboxylic acid and bimm/pyim N-donor auxiliary ligands. Compound 1 is a 1D chain structure and compound 2 features a 2D network with a 4-connected sql topology. The results indicate the bimm and pyim ligands may act as additional metal linkers to mediate the structures of CPs with 5-nitro-1, 2, 3-benzenetricarboxylic acid in crystal engineering. What is more, both compounds show photoluminescence and could be good candidates for potential luminescence materials.
-
[1]
W. Lei, G. Liu, J. Zhang, M. Liu, Chem. Soc. Rev. 46 (2017) 3492-3509.
doi: 10.1039/C7CS00021A
-
[2]
H. Liu, A.T. Neal, Z. Zhu, et al., ACS Nano 8 (2014) 4033-4041.
doi: 10.1021/nn501226z
-
[3]
A.H. Woomer, T.W. Farnsworth, J. Hu, et al., ACS Nano 9 (2015) 8869-8884.
doi: 10.1021/acsnano.5b02599
-
[4]
D. Hanlon, C. Backes, E. Doherty, et al., Nat. Commun. 6 (2015) 8563.
doi: 10.1038/ncomms9563
-
[5]
Y. Zhao, H. Wang, H. Huang, et al., Angew. Chem. Int. Ed. 55 (2016) 5003-5007.
doi: 10.1002/anie.201512038
-
[6]
L. Bai, X. Wang, S. Tang, et al., Adv. Mater. 30 (2018) 1803641.
doi: 10.1002/adma.201803641
-
[7]
M.S. Zhu, S. Kim, L. Mao, et al., J. Am. Chem. Soc. 139 (2017) 13234-13242.
doi: 10.1021/jacs.7b08416
-
[8]
X. Zhu, J. Yang, X. She, et al., J. Mater. Chem. A 7 (2019) 5209-5213.
doi: 10.1039/C8TA11497H
-
[9]
A.P. Cote, A.I. Benin, N.W. Ockwig, et al., Science 310 (2005) 1166-1170.
doi: 10.1126/science.1120411
-
[10]
N. Huang, P. Wang, D. Jiang, Nat. Rev. Mater. 1 (2016) 16068.
doi: 10.1038/natrevmats.2016.68
-
[11]
X. Wang, L. Chen, S.Y. Chong, et al., Nat. Chem. 10 (2018) 1180-1189.
doi: 10.1038/s41557-018-0141-5
-
[12]
J. Pan, L. Guo, S. Zhang, et al., Chem. Asian J. 13 (2018) 1674-1677.
doi: 10.1002/asia.201800506
-
[13]
W. Chen, Z. Yang, Z. Xie, et al., J. Mater. Chem. A 7 (2019) 998-1004.
doi: 10.1039/C8TA10046B
-
[14]
Y. Zhi, Z. Li, X. Feng, et al., J. Mater. Chem. A 5 (2017) 22933-22938.
doi: 10.1039/C7TA07691F
-
[15]
V.S. Vyas, F. Haase, L. Stegbauer, et al., Nat. Commun. 6 (2015) 8508.
doi: 10.1038/ncomms9508
-
[16]
S. Yang, W. Hu, X. Zhang, et al., J. Am. Chem. Soc. 140 (2018) 14614-14618.
doi: 10.1021/jacs.8b09705
-
[17]
Y. Fu, X. Zhu, L. Huang, et al., Appl. Catal. B:Environ. 239 (2018) 46-51.
doi: 10.1016/j.apcatb.2018.08.004
-
[18]
Z. Li, Y. Zhi, P. Shao, et al., Appl. Catal. B:Environ. 245 (2019) 334-342.
doi: 10.1016/j.apcatb.2018.12.065
-
[19]
J. Qi, W. Zhang, R. Cao, Adv. Energy Mater. 8 (2018) 16.
-
[20]
A. Fujishima, K. Honda, Nature 238 (1972) 37.
-
[21]
L. Cheng, Q. Xiang, Y. Liao, H. Zhang, Energy Environ. Sci.11 (2018) 1362-1391.
doi: 10.1039/C7EE03640J
-
[22]
Z.R. Tang, B. Han, C. Han, Y.J. Xu, J. Mater. Chem. A 5 (2017) 2387-2410.
doi: 10.1039/C6TA06373J
-
[23]
J. Zhang, Z. Yu, Z. Gao, et al., Angew. Chem. Int. Ed. 56 (2017) 816-820.
doi: 10.1002/anie.201611137
-
[24]
H. Li, W. Tu, Y. Zhou, Z. Zou, Adv. Sci. 3 (2016) 1500389.
doi: 10.1002/advs.201500389
-
[25]
W. Jiang, X. Zong, L. An, et al., ACS Catal. 8 (2018) 2209-2217.
doi: 10.1021/acscatal.7b04323
-
[26]
P. Tan, A. Zhu, L. Qiao, et al., Inorg. Chem. Front. 6 (2019) 929-939.
doi: 10.1039/C8QI01359D
-
[27]
P. Xia, B. Cheng, J. Jiang, H. Tang, Appl. Surf. Sci. 487 (2019) 335-342.
doi: 10.1016/j.apsusc.2019.05.064
-
[28]
X. Wang, K. Maeda, A. Thomas, et al., Nat. Mater. 8 (2009) 76.
doi: 10.1038/nmat2317
-
[29]
Y. Li, P. Li, J. Wang, et al., Appl. Catal. B:Environ. 225 (2018) 519-529.
doi: 10.1016/j.apcatb.2017.12.017
-
[30]
N. Tian, H. Huang, X. Du, F. Dong, Y. Zhang, J. Mater. Chem. A 7 (2019) 11584-11612.
doi: 10.1039/C9TA01819K
-
[31]
J.Y. Tang, X.Y. Kong, B.J. Ng, et al., Catal. Sci. Technol. 9 (2019) 2335-2343.
doi: 10.1039/C9CY00449A
-
[32]
B.J. Ng, L.K. Putri, X.Y. Kong, et al., Appl. Catal. B:Environ. 224 (2018) 360-367.
doi: 10.1016/j.apcatb.2017.10.005
-
[33]
M. Shalom, M. Guttentag, C. Fettkenhauer, et al., Chem. Mater. 26 (2014) 5812-5818.
doi: 10.1021/cm503258z
-
[34]
J.W. Zhang, S. Gong, N. Mahmood, et al., Appl. Catal. B:Environ. 221 (2018) 9-16.
doi: 10.1016/j.apcatb.2017.09.003
-
[35]
B. Antil, L. Kumar, K.P. Reddy, C.S. Gopinath, S. Deka, ACS Sustain. Chem. Eng. 7 (2019) 9428-9438.
doi: 10.1021/acssuschemeng.9b00626
-
[36]
A. Alkauskas, M.D. McCluskey, C.G. Van de Walle, J. Appl. Phys. 119 (2016) 181101.
doi: 10.1063/1.4948245
-
[37]
H. Kisch, Semiconductor Photocatalysis: Principles and Applications, John Wiley & Sons, 2015.
-
[38]
V. Augugliaro, V. Loddo, M. Pagliaro, G. Palmisano, L. Palmisano, Clean by Light Irradiation: Practical Applications of Supported TiO2, Royal Society of Chemistry, 2010.
-
[39]
M. Lu, Photocatalysis and Water Purification: From Fundamentals to Recent Applications, John Wiley & Sons, 2013.
-
[40]
R. Fagan, D.E. McCormack, D.D. Dionysiou, S.C. Pillai, Mat. Sci. Semicon. Proc. 42 (2016) 2-14.
doi: 10.1016/j.mssp.2015.07.052
-
[41]
F. Parrino, M. Bellardita, E.I. García-López, et al., ACS Catal. 8 (2018) 11191-11225.
doi: 10.1021/acscatal.8b03093
-
[42]
D. Friedmann, A. Hakki, H. Kim, W. Choi, D. Bahnemann, Green Chem. 18 (2016) 5391-5411.
doi: 10.1039/C6GC01582D
-
[43]
X. Lang, X. Chen, J. Zhao, Chem. Soc. Rev. 43 (2014) 473-486.
doi: 10.1039/C3CS60188A
-
[44]
D. Heggo, S. Ookawara, Chem. Eng. Sci. 169 (2017) 67-77.
doi: 10.1016/j.ces.2017.01.019
-
[45]
R. Molinari, C. Lavorato, P. Argurio, et al., Catalysts 9 (2019) 239.
doi: 10.3390/catal9030239
-
[46]
V. Vaiano, M. Matarangolo, J.J. Murcia, et al., Appl. Catal. B:Environ. 225 (2018) 197-206.
doi: 10.1016/j.apcatb.2017.11.075
-
[47]
B. Yang, Y. Chen, J. Shi, Chem. Rev. 119 (2019) 4881-4985.
doi: 10.1021/acs.chemrev.8b00626
-
[48]
R. Ahmad, S.M. Majhi, X. Zhang, T.M. Swager, K.N. Salama, Adv. Colloid Interface Sci. 270 (2019) 1-27.
doi: 10.1016/j.cis.2019.05.006
-
[49]
K. Wenderich, G. Mul, Chem. Rev. 116 (2016) 14587-14619.
doi: 10.1021/acs.chemrev.6b00327
-
[50]
W. Yu, J. Zhang, T. Peng, Appl. Catal. B:Environ. 181 (2016) 220-227.
doi: 10.1016/j.apcatb.2015.07.031
-
[51]
K. Qi, B. Cheng, J. Yu, W. Ho, J. Alloys. Compd. 727 (2017) 792-820.
doi: 10.1016/j.jallcom.2017.08.142
-
[52]
J. Liu, Y. Wang, J. Ma, Y. Peng, A. Wang, J. Alloys. Compd. 783 (2019) 898-918.
doi: 10.1016/j.jallcom.2018.12.330
-
[53]
J. Liu, M.D. Rojas-Andrade, G. Chata, et al., Nanoscale 10 (2017) 158-166.
-
[54]
A. Hui, J. Ma, J. Liu, Y. Bao, J. Zhang, J. Alloys. Compd. 696 (2017) 639-647.
doi: 10.1016/j.jallcom.2016.10.319
-
[55]
M. Jianzhong, J. Liu, Y. Bao, Z. Zhu, H. Liu, Cryst. Res. Technol. 48 (2013) 251-260.
doi: 10.1002/crat.201300026
-
[56]
C. Tan, X. Cao, X.J. Wu, et al., Chem. Rev. 117 (2017) 6225-6331.
doi: 10.1021/acs.chemrev.6b00558
-
[57]
J. Yu, Q. Wang, D. O'Hare, L. Sun, Chem. Soc. Rev. 46 (2017) 5950-5974.
doi: 10.1039/C7CS00318H
-
[58]
G. Fan, F. Li, D.G. Evans, X. Duan, Chem. Soc. Rev. 43 (2014) 7040-7066.
doi: 10.1039/C4CS00160E
-
[59]
Y. Xu, Z. Wang, L. Tan, et al., Ind. Eng. Chem. Res. 57 (2018) 5259-5267.
doi: 10.1021/acs.iecr.8b00170
-
[60]
Y. Zhao, X. Jia, G.I.N. Waterhouse, et al., Adv. Energy Mater. 6 (2016)1501974.
-
[61]
Y.F. Song, L. Tan, S.M. Xu, et al., Angew. Chem. Int. Ed.131 (2019) 11986-11993.
doi: 10.1002/ange.201904246
-
[62]
H. Yin, Z. Tang, Chem. Soc. Rev. 45 (2016) 4873-4891.
doi: 10.1039/C6CS00343E
-
[63]
Z. Wang, S.M. Xu, Y. Xu, et al., Chem. Sci. 10 (2019) 378-384.
doi: 10.1039/C8SC04480E
-
[64]
M. Xu, M. Wei, Adv. Fun. Mater. 28 (2018) 1802943.
-
[65]
Y. Zhao, G.I.N. Waterhouse, G. Chen, et al., Chem. Soc. Rev. 48 (2019) 1972-2010.
doi: 10.1039/C8CS00607E
-
[66]
J. Di, J. Xia, H. Li, S. Guo, S.J.N.E. Dai, Nano Energy 41 (2017) 172-192.
doi: 10.1016/j.nanoen.2017.09.008
-
[67]
K. Sharma, V. Dutta, S. Sharma, et al., J. Ind. Eng. Chem. 78 (2019) 1-20.
doi: 10.1016/j.jiec.2019.06.022
-
[68]
J. Li, Y. Yu, L. Zhang, Nanoscale 6 (2014) 8473-8488.
doi: 10.1039/C4NR02553A
-
[69]
L. Ye, Y. Su, X. Jin, H. Xie, C. Zhang, Environ. Sci-Nano 1 (2014) 90-112.
doi: 10.1039/c3en00098b
-
[70]
X. Meng, Z. Zhang, J. Mol. Catal. A:Chem. 423 (2016) 533-549.
doi: 10.1016/j.molcata.2016.07.030
-
[71]
P. Raizada, P. Singh, A. Kumar, B. Pare, S.B. Jonnalagadda, Sep. Purif. Technol. 133 (2014) 429-437.
doi: 10.1016/j.seppur.2014.07.012
-
[72]
Y. Yang, C. Zhang, C. Lai, et al., Adv. Colloid Interface Sci. 254 (2018) 76-93.
doi: 10.1016/j.cis.2018.03.004
-
[73]
H. Cheng, B. Huang, P. Wang, et al., Chem. Commun. (Camb.) 47 (2011) 7054-7056.
doi: 10.1039/c1cc11525a
-
[74]
J. Di, J. Xia, Y. Ge, et al., J. Mater. Chem. A 2 (2014) 15864-15874.
doi: 10.1039/C4TA02400A
-
[75]
H. Wang, X. Zhang, Y. Xie, Mat. Sci. Eng. R. 130 (2018) 1-39.
doi: 10.1016/j.mser.2018.04.002
-
[76]
Z. Chen, K. Mou, X. Wang, L. Liu, Angew. Chem. Int. Ed. 57 (2018) 12790-12794.
doi: 10.1002/anie.201807643
-
[77]
C. Dong, S. Lu, S. Yao, et al., ACS Catal. 8 (2018) 8649-8658.
doi: 10.1021/acscatal.8b01645
-
[78]
D. Jiang, W. Ma, P. Xiao, et al., J. Colloid Interf. Sci. 512 (2018) 693-700.
doi: 10.1016/j.jcis.2017.10.074
-
[79]
J. Di, J. Xia, H. Li, Z. Liu, Nano Energy 35 (2017) 79-91.
doi: 10.1016/j.nanoen.2017.03.030
-
[80]
S. Bai, N. Zhang, C. Gao, Y. Xiong, Nano Energy 53 (2018) 296-336.
doi: 10.1016/j.nanoen.2018.08.058
-
[81]
Y. Zhou, Y. Zhang, M. Lin, et al., Nat. Commun. 6 (2015) 8340.
doi: 10.1038/ncomms9340
-
[82]
S. Gao, B. Gu, X. Jiao, et al., J. Am. Chem. Soc. 139 (2017) 3438-3445.
doi: 10.1021/jacs.6b11263
-
[83]
J. Hu, D. Chen, Z. Mo, et al., Angew. Chem. 131 (2019) 2095-2099.
doi: 10.1002/ange.201813417
-
[84]
L. Yuan, B. Weng, J.C. Colmenares, Y. Sun, Y.J. Xu, Small 13 (2017) 1702253.
doi: 10.1002/smll.201702253
-
[85]
X. Li, H. Zhu, J. Mater. 1 (2015) 33-44.
-
[86]
M. Javaid, D.W. Drumm, S.P. Russo, A.D. Greentree, Sci. Rep. 7 (2017) 9775.
doi: 10.1038/s41598-017-09305-y
-
[87]
A.M. Appel, D.L. DuBois, M. Rakowski DuBois, J. Am. Chem. Soc. 127 (2005) 12717-12726.
doi: 10.1021/ja054034o
-
[88]
J. Yang, H.S. Shin, J. Mater. Chem. A 2 (2014) 5979-5985.
doi: 10.1039/C3TA14151A
-
[89]
J. Shi, P. Yu, F. Liu, et al., Adv. Mater. 29 (2017) 1701486.
doi: 10.1002/adma.201701486
-
[90]
Y. Liu, Y. Cao, H. Lv, S. Li, H. Zhang, Mater. Lett. 188 (2017) 99-102.
doi: 10.1016/j.matlet.2016.11.060
-
[91]
E. Benavente, F. Durán, C. Sotomayor-Torres, G. González, J. Phys. Chem. Solids 113 (2018) 119-124.
doi: 10.1016/j.jpcs.2017.10.027
-
[92]
P.Y. Jia, R.T. Guo, W.G. Pan, et al., Colloid. Surface. A 570 (2019) 306-316.
doi: 10.1016/j.colsurfa.2019.03.045
-
[93]
C. Liu, L. Wang, Y. Tang, et al., Appl. Catal. B:Environ. 164 (2015) 1-9.
doi: 10.1016/j.apcatb.2014.08.046
-
[94]
R.A. Geioushy, S.M. El-Sheikh, I.M. Hegazy, et al., Mater. Res. Bull. 118 (2019) 100499.
-
[95]
F. Xu, B. Zhu, B. Cheng, J. Yu, J. Xu, Adv. Opt. Mater. 6 (2018) 1800911.
-
[96]
H. Qin, R.T. Guo, X.Y. Liu, et al., Dalton Trans. 47 (2018) 15155-15163.
doi: 10.1039/C8DT02901F
-
[97]
S. Kamila, B. Mohanty, A.K. Samantara, et al., Sci. Rep. 7 (2017) 8378.
doi: 10.1038/s41598-017-08677-5
-
[98]
J. Zhang, Z. Xia, L. Dai, Sci. Adv. 1 (2015) e1500564.
doi: 10.1126/sciadv.1500564
-
[99]
K. Gong, F. Du, Z. Xia, M. Durstock, L. Dai, Science 323 (2009) 760-764.
doi: 10.1126/science.1168049
-
[100]
J. Zhang, L. Dai, ACS Catal. 5 (2015) 7244-7253.
doi: 10.1021/acscatal.5b01563
-
[101]
J. Zhang, H. Li, P. Guo, H. Ma, X.S. Zhao, J. Mater. Chem. A 4 (2016) 8497-8511.
doi: 10.1039/C6TA01657J
-
[102]
L. Lin, B. Deng, J. Sun, H. Peng, Z. Liu, Chem. Rev. 118 (2018) 9281-9343.
doi: 10.1021/acs.chemrev.8b00325
-
[103]
L. Ma, W. Ren, Z. Dong, L. Liu, H. Cheng, Chin. Sci. Bull. 57 (2012) 2995-2999.
doi: 10.1007/s11434-012-5335-4
-
[104]
B. Deng, Z. Liu, H. Peng, Adv. Mater. 31 (2019) 1800996.
-
[105]
Z. Lei, J. Zhang, L.L. Zhang, N.A. Kumar, X. Zhao, Energy Environ. Sci. 9 (2016) 1891-1930.
doi: 10.1039/C6EE00158K
-
[106]
K. Zhang, Chemically Derived Graphene: Functionalization, Properties and Applications, Royal Society of Chemistry, 2018.
-
[107]
D. Guo, R. Shibuya, C. Akiba, et al., Science 351 (2016) 361-365.
doi: 10.1126/science.aad0832
-
[108]
Y. Jia, L. Zhang, L. Zhuang, et al., Nature Catal. 2 (2019) 688-695.
doi: 10.1038/s41929-019-0297-4
-
[109]
J. Zhang, Z. Zhao, Z. Xia, L. Dai, Nat. Nanotechnol. 10 (2015) 444-452.
doi: 10.1038/nnano.2015.48
-
[110]
J. Zhou, X. Gao, R. Liu, et al., J. Am. Chem. Soc. 137 (2015) 7596-7599.
doi: 10.1021/jacs.5b04057
-
[111]
Z. Zuo, D. Wang, J. Zhang, F. Lu, Y. Li, Adv. Mater. 31 (2019)1803762.
-
[112]
Y. Zhao, J. Wan, H. Yao, et al., Nat. Chem. 10 (2018) 924-931.
doi: 10.1038/s41557-018-0100-1
-
[113]
R. Feng, W. Lei, G. Liu, M. Liu, Adv. Mater. 30 (2018) 1804770.
doi: 10.1002/adma.201804770
-
[114]
H. Liu, K. Hu, D. Yan, et al., Adv. Mater. 30 (2018) 1800295.
doi: 10.1002/adma.201800295
-
[115]
Z. Sofer, D. Sedmidubský, Š. Huber, et al., Angew. Chem. Int. Ed. 55 (2016) 3382-3386.
doi: 10.1002/anie.201511309
-
[116]
X. Ren, J. Zhou, X. Qi, et al., Adv. Energy Mater. 7 (2017) 1700396.
-
[117]
Q. Jiang, L. Xu, N. Chen, et al., Angew. Chem. Int. Ed. 55 (2016) 13849-13853.
doi: 10.1002/anie.201607393
-
[118]
J. Wang, D. Liu, H. Huang, et al., Angew. Chem. Int. Ed. 57 (2018) 2600-2604.
doi: 10.1002/anie.201710859
-
[119]
Z. Zhang, M. Khurram, Z. Sun, Q. Yan, Inorg. Chem. 57 (2018) 4098-4103.
doi: 10.1021/acs.inorgchem.8b00278
-
[120]
R. Prasannachandran, T.V. Vineesh, A. Anil, B.M. Krishna, M.M. Shaijumon, ACS Nano 12 (2018) 11511-11519.
doi: 10.1021/acsnano.8b06671
-
[121]
L. Shao, H. Sun, L. Miao, et al., J. Mater. Chem. A 6 (2018) 2494-2499.
doi: 10.1039/C7TA10884B
-
[122]
M.F. Shao, R.K. Zhang, Z.H. Li, et al., Chem. Commun. (Camb.) 51 (2015) 15880-15893.
doi: 10.1039/C5CC07296D
-
[123]
H. Yang, Z. Li, B. Lu, et al., ACS Nano 12 (2018) 11407-11416.
doi: 10.1021/acsnano.8b06380
-
[124]
F.Y. Ning, M.F. Shao, S.M. Xu, et al., Energy Environ. Sci. 9 (2016) 2633-2643.
doi: 10.1039/C6EE01092J
-
[125]
Z.H. Li, M.F. Shao, L. Zhou, et al., Adv. Mater. 28 (2016) 2337-2344.
doi: 10.1002/adma.201505086
-
[126]
Z.H. Li, M.F. Shao, H.L. An, et al., Chem. Sci. 6 (2015) 6624-6631.
doi: 10.1039/C5SC02417J
-
[127]
P.S. Li, X.X. Duan, Y. Kuang, et al., Adv. Energy Mater. 8 (2018) 8.
-
[128]
L. Zhou, M. Shao, M. Wei, X. Duan, J. Energ. Chem. 26 (2017) 1094-1106.
doi: 10.1016/j.jechem.2017.09.015
-
[129]
C. Tang, H.S. Wang, H.F. Wang, et al., Adv. Mater. 27 (2015) 4516-4522.
doi: 10.1002/adma.201501901
-
[130]
Z. Li, X. Zhang, H. Cheng, et al., Adv. Energy Mater. (2019) 1900486.
doi: 10.1002/aenm.201900486
-
[131]
N. Hussain, W. Yang, J. Dou, et al., J. Mater. Chem. A 7 (2019) 9656-9664.
doi: 10.1039/C9TA01017C
-
[132]
M. Naguib, M. Kurtoglu, V. Presser, et al., Adv. Mater. 23 (2011) 4248-4253.
doi: 10.1002/adma.201102306
-
[133]
M. Ghidiu, M.R. Lukatskaya, M.Q. Zhao, Y. Gogotsi, M.W. Barsoum, Nature 516 (2014) 78.
doi: 10.1038/nature13970
-
[134]
J. Ran, G. Gao, F.T. Li, et al., Nat. Commun. 8 (2017) 13907.
doi: 10.1038/ncomms13907
-
[135]
J. Peng, X. Chen, W.J. Ong, X. Zhao, N. Li, Chem. 5 (2019) 18-50.
doi: 10.1016/j.chempr.2018.08.037
-
[136]
L.M. Azofra, N. Li, D.R. MacFarlane, C. Sun, Energy Environ. Sci. 9 (2016) 2545-2549.
doi: 10.1039/C6EE01800A
-
[137]
N. Li, X. Chen, W.J. Ong, et al., ACS Nano 11 (2017) 10825-10833.
doi: 10.1021/acsnano.7b03738
-
[138]
S. Cao, B. Shen, T. Tong, J. Fu, J. Yu, Adv. Funct. Mater. 28 (2018) 1800136.
doi: 10.1002/adfm.201800136
-
[139]
Y. Luo, G.F. Chen, L. Ding, et al., Joule 3 (2019) 279-289.
doi: 10.1016/j.joule.2018.09.011
-
[140]
T. Hu, M. Hu, B. Gao, W. Li, X. Wang, J. Phys. Chem. C 122 (2018) 18501-18509.
doi: 10.1021/acs.jpcc.8b04427
-
[141]
D. Magne, V. Mauchamp, S. Célérier, P. Chartier, T. Cabioc'h, Phys. Chem. Chem. Phys. 18 (2016) 30946-30953.
doi: 10.1039/C6CP05985F
-
[142]
Y. Xie, M. Naguib, V.N. Mochalin, et al., J. Am. Chem. Soc. 136 (2014) 6385-6394.
doi: 10.1021/ja501520b
-
[143]
C. Ling, L. Shi, Y. Ouyang, Q. Chen, J. Wang, Adv. Sci. 3 (2016) 1600180.
doi: 10.1002/advs.201600180
-
[144]
J. Yan, C.E. Ren, K. Maleski, et al., Adv. Funct. Mater. 27 (2017) 1701264.
doi: 10.1002/adfm.201701264
-
[145]
C. Xu, L. Wang, Z. Liu, et al., Nat. Mater. 14 (2015) 1135.
doi: 10.1038/nmat4374
-
[146]
L. Verger, C. Xu, V. Natu, et al., Curr. Opin. Solid State Mater. Sci. 23 (2019) 149-163.
doi: 10.1016/j.cossms.2019.02.001
-
[147]
X. Xie, S. Chen, W. Ding, Y. Nie, Z. Wei, Chem. Commun. (Camb.) 49 (2013) 10112-10114.
doi: 10.1039/c3cc44428g
-
[148]
O. Mashtalir, K.M. Cook, V.N. Mochalin, et al., J. Mater. Chem. A 2 (2014) 14334-14338.
doi: 10.1039/C4TA02638A
-
[149]
T.Y. Ma, J.L. Cao, M. Jaroniec, S.Z. Qiao, Angew. Chem. Int. Ed. 55 (2016) 1138-1142.
doi: 10.1002/anie.201509758
-
[150]
Z.W. Seh, K.D. Fredrickson, B. Anasori, et al., ACS Energy Lett. 1 (2016) 589-594.
doi: 10.1021/acsenergylett.6b00247
-
[151]
P. Li, J. Zhu, A.D. Handoko, et al., J. Mater. Chem. A 6 (2018) 4271-4278.
doi: 10.1039/C8TA00173A
-
[152]
A. Schoedel, Z. Ji, O.M. Yaghi, Nat. Energy 1 (2016) 16034.
doi: 10.1038/nenergy.2016.34
-
[153]
M. Zhao, Y. Huang, Y. Peng, et al., Chem. Soc. Rev. 47 (2018) 6267-6295.
doi: 10.1039/C8CS00268A
-
[154]
N. Heidary, T.G.A.A. Harris, K.H. Ly, N. Kornienko, Phys. Plant.166 (2019) 460-471.
doi: 10.1111/ppl.12935
-
[155]
A.J. Clough, J.W. Yoo, M.H. Mecklenburg, S.C. Marinescu, J. Am. Chem. Soc.137 (2015) 118-121.
doi: 10.1021/ja5116937
-
[156]
N. Kornienko, Y. Zhao, C.S. Kley, et al., J. Am. Chem. Soc. 137 (2015) 14129-14135.
doi: 10.1021/jacs.5b08212
-
[157]
J. Duan, S. Chen, C. Zhao, Nat. Commun. 8 (2017) 15341.
-
[158]
E.M. Miner, S. Gul, N.D. Ricke, et al., ACS Catal. 7 (2017) 7726-7731.
doi: 10.1021/acscatal.7b02647
-
[159]
E.M. Miner, L. Wang, M. Dinca, Chem. Sci. 9 (2018) 6286-6291.
doi: 10.1039/C8SC02049C
-
[160]
W. Cheng, X. Zhao, H. Su, et al., Nat. Energy 4 (2019) 115-122.
doi: 10.1038/s41560-018-0308-8
-
[161]
N. Heidary, K.H. Ly, N. Kornienko, Nano Lett. 19 (2019) 4817-4826.
doi: 10.1021/acs.nanolett.9b01582
-
[162]
D. Voiry, J. Yang, M. Chhowalla, Adv. Mater. 28 (2016) 6197-6206.
doi: 10.1002/adma.201505597
-
[163]
X. Ding, F. Peng, J. Zhou, et al., Nat. Commun. 10 (2019) 41.
doi: 10.1038/s41467-018-07835-1
-
[164]
Z. Gholamvand, D. McAteer, C. Backes, et al., Nanoscale 8 (2016) 5737-5749.
doi: 10.1039/C5NR08553E
-
[165]
A.Y. Lu, H. Zhu, J. Xiao, et al., Nat. Nanotechnol. 12 (2017) 744.
doi: 10.1038/nnano.2017.100
-
[166]
G. Singh, K. Ramadass, J.M. Lee, et al., Microporous Mesoporous Mater. 287 (2019) 1-8.
doi: 10.1016/j.micromeso.2019.05.042
-
[167]
C. Tan, Z. Luo, A. Chaturvedi, et al., Adv. Mater. 30 (2018) 1705509.
doi: 10.1002/adma.201705509
-
[168]
L. Zhang, X. Ji, X. Ren, et al., Adv. Mater. 30 (2018) 1800191.
doi: 10.1002/adma.201800191
-
[169]
G. Babu, N. Masurkar, H. Al Salem, L.M. Arava, J. Am. Chem. Soc. 139 (2017) 171-178.
doi: 10.1021/jacs.6b08681

 Login In
Login In





 DownLoad:
DownLoad:

 DownLoad:
DownLoad: