A chlorinated non-fullerene acceptor for efficient polymer solar cells
-
* Corresponding author.
E-mail address: yingpingzou@csu.edu.cn (Y. Zou).
Citation:
Luo Mei, Zhu Can, Yuan Jun, Zhou Liuyang, Keshtov M.L., Yu Godovsky D., Zou Yingping. A chlorinated non-fullerene acceptor for efficient polymer solar cells[J]. Chinese Chemical Letters,
;2019, 30(12): 2343-2346.
doi:
10.1016/j.cclet.2019.07.023

The significance of chirality in medicinal chemistry has been widely acknowledged due to the potential dramatic variations in physiological activity and toxicity between stereoisomers [1-3]. This phenomenon has continuously stimulated interest in developing convenient and reliable chiral analysis methods. The commonly used techniques for determining the absolute configuration of chiral molecules include X-ray single-crystal diffraction, nuclear magnetic resonance (NMR), and chiroptical spectroscopy, such as electronic circular dichroism (ECD) and vibrational circular dichroism (VCD) [4-9].
Chiral small molecule drugs account for more than 50% of the top 200 small molecule drugs by sale in 2022. Remote chiral amines, in which the chiral center is separated from the amine group by one or more carbon atoms, frequently appear in small molecule drugs such as lurasidone and rivaroxaban (Fig. 1). Moreover, several drug candidates under development, including KI-301670, also possess remote chiral amine fragments [10].
Chiral transmission often occurs between small molecules at adjacent spaces through intermolecular forces, which results in the generation of new stereocenters in the reaction products. Therefore, remote chirality plays an important role in asymmetric synthesis reactions [11,12]. However, because the chiral center is not directly connected to the functional group, it is challenging to establish the absolute configuration of remote stereocenters. Only a few reports on remote chiral sensing methods, mainly using NMR or ECD as the analysis tools, have been published. Nikolai's team developed a selenium-based chiral chemical probe that showed high efficiency for accessing remote chiral carboxylic acids via 77Se NMR spectroscopy [13]. TBP3 tweezers were suitably introduced to the structure of β-, γ-, or δ-chiral carboxylic acids, yielding typical exciton coupling circular dichroism (ECCD) signals [14]. For α-, β-, and some γ-chiral carboxylic acids, ECCD was also generated by forming a Cu(II)-coordination complex to distort the associated quinolines [15,16]. Additionally, Anslyn's team developed a four-component assembly to perform chiroptical sensing of various β-chiral primary alcohols via ECCD [17].
In addition to being important chiral precursors of drugs, chiral amines are often used as key chiral ligands in asymmetric catalytic synthesis [18-20]. This in turn inspired the development of chiral amine sensing methods. Unfortunately, to our knowledge, specific systematic chiroptical sensing of remote chiral amines has not been reported. Thus, there is an urgent need to develop novel chiral sensing methods for remote chiral amines. Because of its convenience, accuracy and efficiency, ECD spectroscopy has gained popularity in this field. In the past decade, considerable progress has been made in ECD-based chiroptical sensing [21-26]. It is feasible and meaningful to establish a reliable ECD method to analyze the absolute optical purity of remote chiral amines.
In a typical C—H activation reaction, C-X (X = C, N, O, etc.) bond coupling is realized through cleavage of the C—H bond catalyzed by a transition metal complex [27-32]. This class of reactions is atom-economical and has been widely applied to construct various skeleton compounds [33,34]. Here, we report a rapid and accurate chiroptical sensing method for remote chiral amines via a C—H activation reaction using 5-methylisoxazole-3-carboxylic acid (MIA) as the directing group [35,36].
Several β- or γ-chiral primary amines were condensed with MIA to yield bidentate ligands (1-−7-D, Fig. 2). The Pd atom was then introduced by binding the directing group and activating the target C—H bond to form a double five-membered or mixed five-membered or six-membered cyclopalladium complex (Scheme 1). This Pd complex was absorbed in the ultraviolet‒visible range, and Cotton effects (CEs) were induced when this chromophore was disturbed by the chirality of the chiral amines. The ECD spectra of the cyclopalladium complexes were calculated theoretically and were in good agreement with the experimental results. Samples with different enantiomeric excess (ee) values were prepared for the absolute optical purity test, with an absolute error less than 2%. Considering the great progress in the field of Pd-catalyzed C—H activation reactions [37-40], the strategy of using a Pd chromophore to determine the absolute optical purity of chiral molecules is promising.
The sensing reaction was first attempted for (R)−1-D in 1,1,1,3,3,3-hexafluoro-2-propanol (HFIP), which is often used in C—H activation reactions. Two equivalents of Pd(OAc)2 were added, the reaction concentration was 10 mmol/L, and the reaction time was 20 min. Three reaction modes were compared: promotion by 450 W ultrasound, standing or stirring at room temperature (Fig. 3). The difference between these reaction modes was small, indicating that the reaction mode had little effect on this reaction. For the convenience of operation, the reaction mixture was allowed to stand at room temperature.
The sensing reaction was then evaluated in eight frequently used solvents for the C—H activation reaction, namely, HFIP, 2,2,2-trifluoroethanol (TFE), acetonitrile (ACN), tert‑butanol (TBA), 1,4-dioxane (Dx), 1,2-dimethoxyethane (DME), 1,2-dichloro-ethane (DCE), and acetic acid (AcOH). Unexpectedly, the solvent affected the position and intensity of the Cotton effect in the ECD spectra (Fig. 4). This fact might be attributed to the solvation effects on the ECD signals as well as the possible participation of the solvent molecule in the cyclopalladium complex. Much more intense ECD responses appeared for HFIP, TFE, ACN, and TBA. In particular, the highest intensity of Cotton effects was observed in TFE, while TBA was not suitable as a reaction solvent because of the poor solubility of Pd(OAc)2 in this solvent.
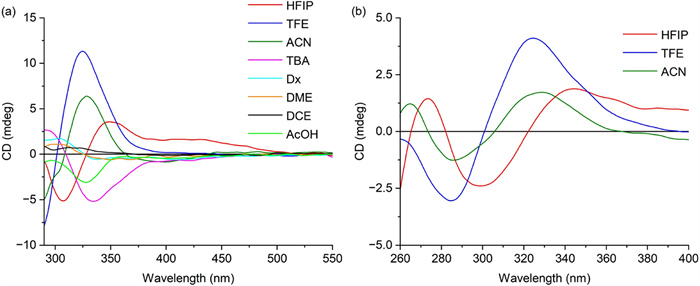
The time required for the reaction to reach a steady state depended on the reactant concentration. Time curves of the ECD signal at 324 nm in TFE were obtained at reactant concentrations of 4, 10, and 20 mmol/L (Fig. 5). When the reactant concentration was set to 4 mmol/L, the reaction did not reach a relatively steady state until 80 min. However, when the reactant concentration increased to 10 mmol/L or 20 mmol/L, the reaction reached a steady state within 20 min. Therefore, the concentration needed to be greater than 10 mmol/L, and the optimal test time was 20 min or longer to ensure that the reaction had completely occurred.
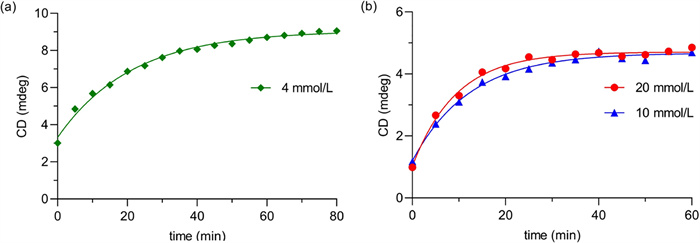
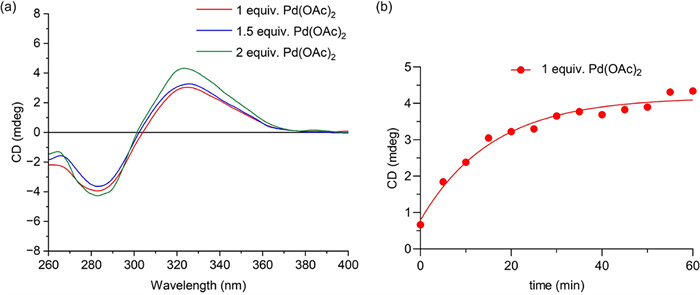
A greater amount of Pd(OAc)2 promoted the chiroptical sensing process; however, excess Pd(OAc)2 exhibited strong UV absorption within the tested wavelength range, reducing the signal-to-noise ratio in the ECD spectra. The molar ratios of Pd(OAc)2 to (R)-1-D were thus set as 1:1, 1.5:1, and 2:1 in TFE when chiroptical sensing occurred at room temperature and was monitored at 20 min. As shown in Fig. 6a, the intensity of the Cotton effect at 324 nm increased slightly with the amount of Pd(OAc)2. However, the reaction did not reach a relatively steady state within 60 min for the reaction mixture with a molar ratio of Pd(OAc)2 to (R)-1-D of 1:1 (Fig. 6b). Thus, 2 equiv. of Pd(OAc)2 were added for subsequent tests, considering the reaction efficiency and the reliability of the ECD signal.
Adopting the established sensing conditions, the substrate range for remote chiroptical sensing was extended to ten commercially available β- or γ-chiral primary amines, including three pairs of enantiomers and four single enantiomers (Fig. 2). As expected, all the sensing mixtures displayed obvious ECD responses over the wavelength range from 250 nm to 550 nm (Fig. 7 and Table S1 in Supporting information).
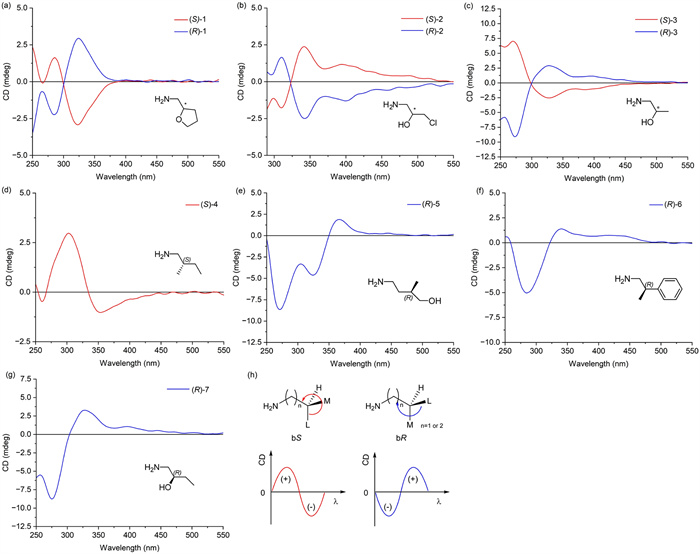
Based on the sensing results, the relationship between the absolute configuration of these β- and γ-chiral amines and the ECD signals was investigated. Similar to the bulkiness rule of the [Rh2(CF3COO)4] complex for chiral alcohols [41], the Cotton effect was found to depend on the group arrangement of these sensing products. First, the amino group fragments were fixed, and then the remaining three groups were arranged in order of decreasing volume. If the order is clockwise, the configuration is bR; if the order is counterclockwise, the configuration is bS. Stereoisomers with a bR configuration showed a positive Cotton effect followed by a negative Cotton effect from long to short wavelengths, and vice versa for a bS isomer. Moreover, the wavelength at which the substituent crossed the zero point varied with the substituent. When a carbon or oxygen atom is connected at the α or β position of the carbon stereocenter ((R/S)-1, (R/S)-3, (S)-4, (R)-5, (R)-6, or (R)-7), the bR/bS configuration corresponds to the R/S configuration. However, in the case of (R/S)-2, the bS configuration corresponds to the R configuration due to the higher priority of the chlorine atom. Chiroptical sensing of the chiral drug fragment atorvastatin intermediate 1 was performed, and the signal of the Cotton effect conformed to the rules we summarized (Fig. S1 in Supporting information). Due to the limited number of compounds attempted, a comprehensive correlation requires further research.
The ECD spectra of several cyclopalladium complexes were theoretically predicted by using time-dependent density functional theory (TDDFT) with a solvation model based on density (SMD) [42]. The calculated ECD spectra of the (S)-1-D-Pd, (R)-2-D-Pd, and (S)-4-D-Pd complexes agreed well with the experimental ECD spectra (Fig. 8a and Fig. S3 in Supporting information). Natural transition orbital (NTO) analysis of the cyclopalladium complex was performed using Multiwfn 3.8 [43]. The results indicated that the d orbitals of the Pd atom participated substantially in the NTO pairs related to the S0→S2 and S0→S6 transitions (Fig. 8b).
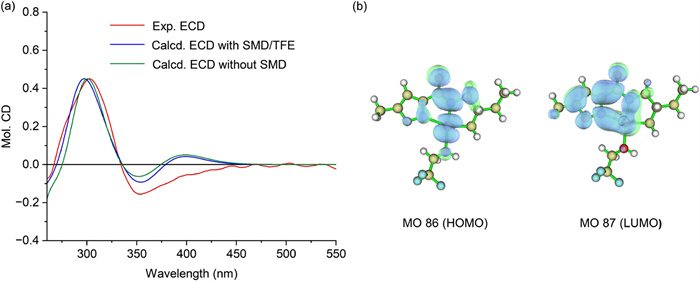
To confirm the structure of the sensing product, single-crystal X-ray diffraction analysis was performed. We obtained a single crystal (CCDC: 2289496), which was proven to be stable dimerization complex 8 (Fig. 9 and Table S2 in Supporting information), without the anticipated C-Pd bond.
Comparing the ECD spectra of 8 and the chiroptical sensing mixture of (S)-4-D, it is obvious that compound 8 displayed different ECD patterns and weaker ECD signals than the chiroptical sensing product, indicating their structural differences. Importantly, the ECD spectrum of compound 8 lacked a negative Cotton effect at approximately 350 nm, which could be attributed to the existence of a C-Pd bond.
Chiral 2-tetrahydrofurfurylamine ((R/S)-1, Fig. 2) is a notable raw material and acts as a chiral source for some drugs or drug candidates (Fig. S5 in Supporting information). For the NUAK1 inhibitor KI-301670 (Fig. 1), which is a promising drug candidate against pancreatic cancer, the S isomer is the eutomer [10]. The stereochemical configuration of the 2-tetrahydrofurfurylamine fragment strongly affected the interaction with the target protein, and the S configuration was necessary for hydrophobic interactions with the Leu185 residue in the protein binding pocket.
Herein, the absolute optical purity of (R/S)-1 was evaluated using the established chiroptical sensing protocol. To avoid the impact of sampling concentration fluctuations, the anisotropy factor (g-factor) was selected as the evaluation index for absolute optical purity. For (R/S)-1-D, a good linear correlation was obtained when the g-factors at 324 nm in TFE were plotted against the ee values (Fig. 10).
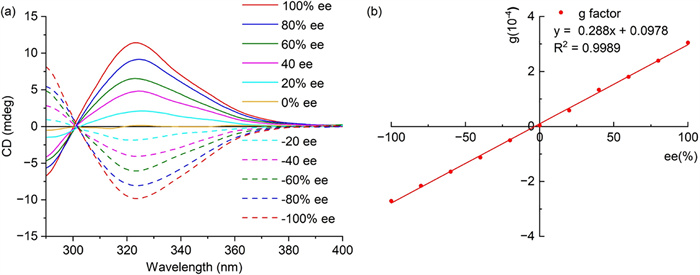
Subsequently, five samples with different ee values were prepared for chiroptical sensing analysis, and the detection error was less than 2% (Table 1). Chiroptical sensing was also tested in HFIP, and the results are listed in Fig. S4 and Table S5 (Supporting information). These results support the feasibility and reliability of the chiroptical sensing method.
In conclusion, a rapid and accurate chiroptical sensing protocol for remote chiral primary amines was established by combining a powerful C—H activation reaction and a specific ECD technique. For these β- or γ-chiral primary amines, the absolute configurations could be unambiguously assigned by constructing cyclopalladium metal complexes. ECD test samples were taken directly from the reaction solution without tedious post-treatment, and a detection error of less than 2% was achieved. This work is an innovative attempt at the application of the C—H activation reaction to determine the absolute optical purity of remote chiral small-molecule compounds. With the continuous development of novel C—H activation reactions, further research is underway for more chiral structures and for monitoring asymmetric synthetic reactions.
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Xin Li: Writing – original draft, Methodology, Investigation, Data curation. Jia-Min Lu: Methodology, Investigation, Data curation. Bo Li: Methodology, Investigation, Data curation, Conceptualization. Chen Zhao: Investigation, Data curation. Bei-Bei Yang: Validation, Resources, Methodology. Li Li: Writing – review & editing, Supervision, Project administration, Funding acquisition, Conceptualization.
All quantum computations were carried out using the Gaussian 16 package supported by the Biomedical High Performance Computing Platform, Chinese Academy of Medical Sciences. This work was financially supported by the CAMS Innovation Fund for Medical Sciences (CIFMS, No. 2023-I2M-2-009).
Supplementary material associated with this article can be found, in the online version, at doi:
Z. Zhang, Z. Zhou, Q. Hu, et al., ACS Appl. Mater. Interfaces 9 (2017) 6213-6219.
Y. Liu, M. Li, X. Zhou, et al., ACS Energy Lett. 3 (2018) 1832-1839.
doi: 10.1021/acsenergylett.8b00928
Q. Fan, Y. Wang, M. Zhang, et al., Adv. Mater. 30 (2018) 1704546.
doi: 10.1002/adma.201704546
L. Gao, Z.G. Zhang, H. Bin, et al., Adv. Mater. 28 (2016) 8288-8295.
doi: 10.1002/adma.201601595
J. Yuan, Y. Zhang, L. Zhou, et al., Joule 3 (2019) 1140-1151.
doi: 10.1016/j.joule.2019.01.004
L.X. Meng, Y.M. Zhang, X.J. Wan, et al., Science 361 (2018) 1094-1098.
doi: 10.1126/science.aat2612
Y. Guo, Y. Li, O. Awartani, et al., Adv. Mater. 29 (2017) 1700309.
doi: 10.1002/adma.201700309
B. Kan, J. Zhang, F. Liu, et al., Adv. Mater. 30 (2018) 1704904.
doi: 10.1002/adma.201704904
Y. Lin, J. Wang, Z.G. Zhang, et al., Adv. Mater. 27 (2015) 1170-1174.
doi: 10.1002/adma.201404317
J. Roncali, P. Leriche, P. Blanchard, Adv. Mater. 26 (2014) 3821-3838.
doi: 10.1002/adma.201305999
W. Ni, X. Wan, M. Li, Y. Wang, Y. Chen, Chem. Commun. (Camb.) 51 (2015) 4936-4950.
doi: 10.1039/C4CC09758K
L. Dou, Y. Liu, Z. Hong, G. Li, Y. Yang, Chem. Rev. 115 (2015) 12633-12665.
doi: 10.1021/acs.chemrev.5b00165
Z. Zhang, J. Yu, X. Yin, et al., Adv. Funct. Mater. 28 (2018) 1705095.
doi: 10.1002/adfm.201705095
B. Huang, L. Chen, X. Jin, et al., Adv. Funct. Mater. 28 (2018) 1800606.
doi: 10.1002/adfm.201800606
J. Wang, W. Gao, Q. An, et al., J. Mater. Chem. A Mater. Energy Sustain. 6 (2018) 11751-11758.
doi: 10.1039/C8TA03453B
Q. An, W. Gao, F. Zhang, et al., J. Mater. Chem. A Mater. Energy Sustain. 6 (2018) 2468-2475.
doi: 10.1039/C7TA10763C
Y. Li, L. Zhong, B. Gautam, et al., Energy Environ. Sci. 10 (2017) 1610-1620.
doi: 10.1039/C7EE00844A
W. Zhao, S. Li, H. Yao, et al., J. Am. Chem. Soc. 139 (2017) 7148-7151.
doi: 10.1021/jacs.7b02677
H. Yao, Y. Cui, R. Yu, et al., Angew. Chem. Int. Ed. 56 (2017) 3045-3049.
doi: 10.1002/anie.201610944
W. Gao, T. Liu, C. Zhong, et al., ACS Energy Lett. 3 (2018) 1760-1768.
doi: 10.1021/acsenergylett.8b00825
X. Wang, Z. Du, K. Dou, et al., Adv. Energy Mater. 9 (2019) 1802530.
C. Li, T. Xia, J. Song, et al., J. Mater. Chem. A Mater. Energy Sustain. 7 (2019) 1435-1441.
doi: 10.1039/C8TA11197A
W. Zhong, J. Cui, B. Fan, et al., Chem. Mater. 29 (2017) 8177-8186.
doi: 10.1021/acs.chemmater.7b02228
B. Kan, H. Feng, H. Yao, et al., Sci. China Chem. 61 (2018) 1307-1313.
doi: 10.1007/s11426-018-9334-9
Y. Cui, H. Yao, B. Gao, et al., J. Am. Chem. Soc. 139 (2017) 7302-7309.
doi: 10.1021/jacs.7b01493
S. Li, L. Ye, W. Zhao, et al., Adv. Energy Mater. 7 (2017) 1700183.
J. Yuan, T. Huang, P. Cheng, et al., Nat. Commun. 10 (2019) 570.
doi: 10.1038/s41467-019-08386-9
J. Yuan, Y. Zhang, L. Zhou, et al., Adv. Mater. 31 (2019) e1807577.
doi: 10.1002/adma.201807577
Y. Cui, C. Yang, H. Yao, et al., Adv. Mater. 29 (2017) 1703080.
doi: 10.1002/adma.201703080
H. Zhang, H. Yao, J. Hou, et al., Adv. Mater. 30 (2018) e1800613.
doi: 10.1002/adma.201800613
S.-S. Wan, X. Xu, J.-L. Wang, et al., J. Mater. Chem. A Mater. Energy Sustain. 7 (2019) 11802-11813.
doi: 10.1039/C9TA03177D
D. Mo, H. Wang, H. Chen, et al., Chem. Mater. 29 (2017) 2819-2830.
doi: 10.1021/acs.chemmater.6b04828
J. Qu, Q. Zhao, J. Zhou, et al., Chem. Mater. 31 (2019) 1664-1671.
doi: 10.1021/acs.chemmater.8b05047
M.L. Tang, J.H. Oh, A.D. Reichardt, et al., J. Am. Chem. Soc. 131 (2009) 3733-3740.
doi: 10.1021/ja809045s
J.H. Oh, S.-L. Suraru, W.-Y. Lee, et al., Adv. Funct. Mater. 20 (2010) 2148-2156.
doi: 10.1002/adfm.201000425
H.H. Gao, Y. Sun, Y. Cai, et al., Adv. Energy Mater. (2019) 1901024.
D. Liu, L. Yang, Y. Wu, et al., Chem. Mater. 30 (2018) 619-628.
doi: 10.1021/acs.chemmater.7b03142
Y. Chen, Y. Geng, A. Tang, et al., Chem. Commun. (Camb.) 55 (2019) 6708-6710.
doi: 10.1039/C9CC02904D
M. Zhang, X. Guo, W. Ma, H. Ade, J. Hou, Adv. Mater. 27 (2015) 4655-4660.
doi: 10.1002/adma.201502110
J. Sun, Z. Zhang, X. Yin, et al., J. Mater. Chem. A Mater. Energy Sustain. 6 (2018) 2549-2554.
doi: 10.1039/C7TA10391C
Z. Zhang, L. Feng, S. Xu, et al., J. Mater. Chem. A Mater. Energy Sustain. 5 (2017) 11286-11293.
doi: 10.1039/C7TA02486J
Y. Gao, Z. Wang, G. Yue, et al., Sol. RRL (2019) 1900012.
X. Li, T. Yan, H. Bin, et al., J. Mater. Chem. A Mater. Energy Sustain. 5 (2017) 22588-22597.
doi: 10.1039/C7TA07049G
T. Li, S. Dai, Z. Ke, et al., Adv. Mater. 30 (2018) 1705969.
doi: 10.1002/adma.201705969
H. Xu, Y. Yang, C. Zhong, et al., J. Mater. Chem. A Mater. Energy Sustain. 6 (2018) 6393-6401.
doi: 10.1039/C8TA00704G
Y. Wu, H. Bai, Z. Wang, et al., Energy Environ. Sci. 8 (2015) 3215-3221.
doi: 10.1039/C5EE02477C
Y. Li, L. Zhong, F.P. Wu, et al., Energy Environ. Sci. 9 (2016) 3429-3435.
doi: 10.1039/C6EE00315J
Z. Zhang, Z. Zhou, Q. Hu, et al., ACS Appl. Mater. Interfaces 9 (2017) 6213-6219.
Y. Liu, M. Li, X. Zhou, et al., ACS Energy Lett. 3 (2018) 1832-1839.
doi: 10.1021/acsenergylett.8b00928
Q. Fan, Y. Wang, M. Zhang, et al., Adv. Mater. 30 (2018) 1704546.
doi: 10.1002/adma.201704546
L. Gao, Z.G. Zhang, H. Bin, et al., Adv. Mater. 28 (2016) 8288-8295.
doi: 10.1002/adma.201601595
J. Yuan, Y. Zhang, L. Zhou, et al., Joule 3 (2019) 1140-1151.
doi: 10.1016/j.joule.2019.01.004
L.X. Meng, Y.M. Zhang, X.J. Wan, et al., Science 361 (2018) 1094-1098.
doi: 10.1126/science.aat2612
Y. Guo, Y. Li, O. Awartani, et al., Adv. Mater. 29 (2017) 1700309.
doi: 10.1002/adma.201700309
B. Kan, J. Zhang, F. Liu, et al., Adv. Mater. 30 (2018) 1704904.
doi: 10.1002/adma.201704904
Y. Lin, J. Wang, Z.G. Zhang, et al., Adv. Mater. 27 (2015) 1170-1174.
doi: 10.1002/adma.201404317
J. Roncali, P. Leriche, P. Blanchard, Adv. Mater. 26 (2014) 3821-3838.
doi: 10.1002/adma.201305999
W. Ni, X. Wan, M. Li, Y. Wang, Y. Chen, Chem. Commun. (Camb.) 51 (2015) 4936-4950.
doi: 10.1039/C4CC09758K
L. Dou, Y. Liu, Z. Hong, G. Li, Y. Yang, Chem. Rev. 115 (2015) 12633-12665.
doi: 10.1021/acs.chemrev.5b00165
Z. Zhang, J. Yu, X. Yin, et al., Adv. Funct. Mater. 28 (2018) 1705095.
doi: 10.1002/adfm.201705095
B. Huang, L. Chen, X. Jin, et al., Adv. Funct. Mater. 28 (2018) 1800606.
doi: 10.1002/adfm.201800606
J. Wang, W. Gao, Q. An, et al., J. Mater. Chem. A Mater. Energy Sustain. 6 (2018) 11751-11758.
doi: 10.1039/C8TA03453B
Q. An, W. Gao, F. Zhang, et al., J. Mater. Chem. A Mater. Energy Sustain. 6 (2018) 2468-2475.
doi: 10.1039/C7TA10763C
Y. Li, L. Zhong, B. Gautam, et al., Energy Environ. Sci. 10 (2017) 1610-1620.
doi: 10.1039/C7EE00844A
W. Zhao, S. Li, H. Yao, et al., J. Am. Chem. Soc. 139 (2017) 7148-7151.
doi: 10.1021/jacs.7b02677
H. Yao, Y. Cui, R. Yu, et al., Angew. Chem. Int. Ed. 56 (2017) 3045-3049.
doi: 10.1002/anie.201610944
W. Gao, T. Liu, C. Zhong, et al., ACS Energy Lett. 3 (2018) 1760-1768.
doi: 10.1021/acsenergylett.8b00825
X. Wang, Z. Du, K. Dou, et al., Adv. Energy Mater. 9 (2019) 1802530.
C. Li, T. Xia, J. Song, et al., J. Mater. Chem. A Mater. Energy Sustain. 7 (2019) 1435-1441.
doi: 10.1039/C8TA11197A
W. Zhong, J. Cui, B. Fan, et al., Chem. Mater. 29 (2017) 8177-8186.
doi: 10.1021/acs.chemmater.7b02228
B. Kan, H. Feng, H. Yao, et al., Sci. China Chem. 61 (2018) 1307-1313.
doi: 10.1007/s11426-018-9334-9
Y. Cui, H. Yao, B. Gao, et al., J. Am. Chem. Soc. 139 (2017) 7302-7309.
doi: 10.1021/jacs.7b01493
S. Li, L. Ye, W. Zhao, et al., Adv. Energy Mater. 7 (2017) 1700183.
J. Yuan, T. Huang, P. Cheng, et al., Nat. Commun. 10 (2019) 570.
doi: 10.1038/s41467-019-08386-9
J. Yuan, Y. Zhang, L. Zhou, et al., Adv. Mater. 31 (2019) e1807577.
doi: 10.1002/adma.201807577
Y. Cui, C. Yang, H. Yao, et al., Adv. Mater. 29 (2017) 1703080.
doi: 10.1002/adma.201703080
H. Zhang, H. Yao, J. Hou, et al., Adv. Mater. 30 (2018) e1800613.
doi: 10.1002/adma.201800613
S.-S. Wan, X. Xu, J.-L. Wang, et al., J. Mater. Chem. A Mater. Energy Sustain. 7 (2019) 11802-11813.
doi: 10.1039/C9TA03177D
D. Mo, H. Wang, H. Chen, et al., Chem. Mater. 29 (2017) 2819-2830.
doi: 10.1021/acs.chemmater.6b04828
J. Qu, Q. Zhao, J. Zhou, et al., Chem. Mater. 31 (2019) 1664-1671.
doi: 10.1021/acs.chemmater.8b05047
M.L. Tang, J.H. Oh, A.D. Reichardt, et al., J. Am. Chem. Soc. 131 (2009) 3733-3740.
doi: 10.1021/ja809045s
J.H. Oh, S.-L. Suraru, W.-Y. Lee, et al., Adv. Funct. Mater. 20 (2010) 2148-2156.
doi: 10.1002/adfm.201000425
H.H. Gao, Y. Sun, Y. Cai, et al., Adv. Energy Mater. (2019) 1901024.
D. Liu, L. Yang, Y. Wu, et al., Chem. Mater. 30 (2018) 619-628.
doi: 10.1021/acs.chemmater.7b03142
Y. Chen, Y. Geng, A. Tang, et al., Chem. Commun. (Camb.) 55 (2019) 6708-6710.
doi: 10.1039/C9CC02904D
M. Zhang, X. Guo, W. Ma, H. Ade, J. Hou, Adv. Mater. 27 (2015) 4655-4660.
doi: 10.1002/adma.201502110
J. Sun, Z. Zhang, X. Yin, et al., J. Mater. Chem. A Mater. Energy Sustain. 6 (2018) 2549-2554.
doi: 10.1039/C7TA10391C
Z. Zhang, L. Feng, S. Xu, et al., J. Mater. Chem. A Mater. Energy Sustain. 5 (2017) 11286-11293.
doi: 10.1039/C7TA02486J
Y. Gao, Z. Wang, G. Yue, et al., Sol. RRL (2019) 1900012.
X. Li, T. Yan, H. Bin, et al., J. Mater. Chem. A Mater. Energy Sustain. 5 (2017) 22588-22597.
doi: 10.1039/C7TA07049G
T. Li, S. Dai, Z. Ke, et al., Adv. Mater. 30 (2018) 1705969.
doi: 10.1002/adma.201705969
H. Xu, Y. Yang, C. Zhong, et al., J. Mater. Chem. A Mater. Energy Sustain. 6 (2018) 6393-6401.
doi: 10.1039/C8TA00704G
Y. Wu, H. Bai, Z. Wang, et al., Energy Environ. Sci. 8 (2015) 3215-3221.
doi: 10.1039/C5EE02477C
Y. Li, L. Zhong, F.P. Wu, et al., Energy Environ. Sci. 9 (2016) 3429-3435.
doi: 10.1039/C6EE00315J

Wenxiang Ma , Xinyu He , Tianyi Chen , De-Li Ma , Hongzheng Chen , Chang-Zhi Li . Near-infrared non-fused electron acceptors for efficient organic photovoltaics. Chinese Chemical Letters, 2024, 35(4): 109099-. doi: 10.1016/j.cclet.2023.109099
Shaonan Liu , Shuixing Dai , Minghua Huang . The impact of ester groups on 1,8-naphthalimide electron transport material in organic solar cells. Chinese Journal of Structural Chemistry, 2024, 43(6): 100277-100277. doi: 10.1016/j.cjsc.2024.100277
Yaohua Li , Qi Cao , Xuanhua Li . Tailoring the configuration of polymer passivators in perovskite solar cells. Chinese Journal of Structural Chemistry, 2025, 44(2): 100413-100413. doi: 10.1016/j.cjsc.2024.100413
Rong-Nan Yi , Wei-Min He . Electron donor-acceptor complex enabled arylation of dithiocarbamate anions with thianthrenium salts under aqueous micellar conditions. Chinese Chemical Letters, 2024, 35(11): 110194-. doi: 10.1016/j.cclet.2024.110194
Long Li , Kang Yang , Chenpeng Xi , Mengchao Li , Borong Li , Gui Xu , Yuanbin Xiao , Xiancai Cui , Zhiliang Liu , Lingyun Li , Yan Yu , Chengkai Yang . Highly-chlorinated inert and robust interphase without mineralization of oxide enhancing high-rate Li metal batteries. Chinese Chemical Letters, 2024, 35(6): 108814-. doi: 10.1016/j.cclet.2023.108814
Rui Cheng , Xin Huang , Tingting Zhang , Jiazhuang Guo , Jian Yu , Su Chen . Solid superacid catalysts promote high-performance carbon dots with narrow-band fluorescence emission for luminescence solar concentrators. Chinese Chemical Letters, 2024, 35(8): 109278-. doi: 10.1016/j.cclet.2023.109278
Jinge Zhu , Ailing Tang , Leyi Tang , Peiqing Cong , Chao Li , Qing Guo , Zongtao Wang , Xiaoru Xu , Jiang Wu , Erjun Zhou . Chlorination of benzyl group on the terminal unit of A2-A1-D-A1-A2 type nonfullerene acceptor for high-voltage organic solar cells. Chinese Chemical Letters, 2025, 36(1): 110233-. doi: 10.1016/j.cclet.2024.110233
Chengcheng Xie , Chengyi Xiao , Hongshuo Niu , Guitao Feng , Weiwei Li . Mesoporous organic solar cells. Chinese Chemical Letters, 2024, 35(11): 109849-. doi: 10.1016/j.cclet.2024.109849
Kangrong Yan , Ziqiu Shen , Yanchun Huang , Benfang Niu , Hongzheng Chen , Chang-Zhi Li . Curing the vulnerable heterointerface via organic-inorganic hybrid hole transporting bilayers for efficient inverted perovskite solar cells. Chinese Chemical Letters, 2024, 35(6): 109516-. doi: 10.1016/j.cclet.2024.109516
Bo Yang , Pu-An Lin , Tingwei Zhou , Xiaojia Zheng , Bing Cai , Wen-Hua Zhang . Facile surface regulation for highly efficient and thermally stable perovskite solar cells via chlormequat chloride. Chinese Chemical Letters, 2024, 35(10): 109425-. doi: 10.1016/j.cclet.2023.109425
Yingfen Li , Zhiqi Wang , Yunhai Zhao , Dajun Luo , Xueliang Zhang , Jun Zhao , Zhenghua Su , Shuo Chen , Guangxing Liang . Potassium doping for grain boundary passivation and defect suppression enables highly-efficient kesterite solar cells. Chinese Chemical Letters, 2024, 35(11): 109468-. doi: 10.1016/j.cclet.2023.109468
Zhiyang Zhang , Yi Chen , Yingnan Zhang , Chuanlang Zhan . Deuterated chloroform replaces ultra-dry chloroform to achieve high-efficient organic solar cells. Chinese Chemical Letters, 2025, 36(1): 110083-. doi: 10.1016/j.cclet.2024.110083
Panke Zhou , Hong Yu , Mun Yin Chee , Tao Zeng , Tianli Jin , Hongling Yu , Shuo Wu , Wen Siang Lew , Xiong Chen . Electron push-pull effects induced performance promotion in covalent organic polymer thin films-based memristor for neuromorphic application. Chinese Chemical Letters, 2024, 35(5): 109279-. doi: 10.1016/j.cclet.2023.109279
Rui Liu , Yue Yu , Lu Deng , Maoxia Xu , Haorong Ren , Wenjie Luo , Xudong Cai , Zhenyu Li , Jingyu Chen , Hua Yu . The synergistic effect of A-site cation engineering and phase regulation enables efficient and stable Ruddlesden-Popper perovskite solar cells. Chinese Chemical Letters, 2024, 35(12): 109545-. doi: 10.1016/j.cclet.2024.109545
Zuyou Song , Yong Jiang , Qiao Gou , Yini Mao , Yimin Jiang , Wei Shen , Ming Li , Rongxing He . Promoting the generation of active sites through "Co-O-Ru" electron transport bridges for efficient water splitting. Chinese Chemical Letters, 2025, 36(4): 109793-. doi: 10.1016/j.cclet.2024.109793
Haowen Shang , Yujie Yang , Bingjie Xue , Yikai Wang , Zhiyi Su , Wenlong Liu , Youzhi Wu , Xinjun Xu . Efficient solution-processed near-infrared organic light-emitting diodes with a binary-mixed electron transport layer. Chinese Chemical Letters, 2025, 36(4): 110511-. doi: 10.1016/j.cclet.2024.110511
Manlin Lu , Sheng Liao , Jiayu Li , Zidong Yu , Ningjiu Zhao , Zuoti Xie , Shunli Chen , Li Dang , Ming-De Li . Face-to-face π-π interactions and electron communication boosting efficient reverse intersystem crossing in through-space charge transfer molecules. Chinese Chemical Letters, 2025, 36(6): 110066-. doi: 10.1016/j.cclet.2024.110066
Boyuan Hu , Jian Zhang , Yulin Yang , Yayu Dong , Jiaqi Wang , Wei Wang , Kaifeng Lin , Debin Xia . Dual-functional POM@IL complex modulate hole transport layer properties and interfacial charge dynamics for highly efficient and stable perovskite solar cells. Chinese Chemical Letters, 2024, 35(7): 108933-. doi: 10.1016/j.cclet.2023.108933
Xinyu Yu , Fei Wu , Xianglang Sun , Linna Zhu , Baoyu Xia , Zhong'an Li . Low-cost dopant-free fluoranthene-based branched hole transporting materials for efficient and stable n-i-p perovskite solar cells. Chinese Chemical Letters, 2024, 35(10): 109821-. doi: 10.1016/j.cclet.2024.109821
Fei Jin , Bolin Yang , Xuanpu Wang , Teng Li , Noritatsu Tsubaki , Zhiliang Jin . Facilitating efficient photocatalytic hydrogen evolution via enhanced carrier migration at MOF-on-MOF S-scheme heterojunction interfaces through a graphdiyne (CnH2n-2) electron transport layer. Chinese Journal of Structural Chemistry, 2023, 42(12): 100198-100198. doi: 10.1016/j.cjsc.2023.100198