Pyridine-Si-xanthene: A novel near-infrared fluorescent platform for biological imaging
-
* Corresponding authors.
E-mail addresses: kli@scu.edu.cn (K. Li), xqyu@scu.edu.cn (X.-Q. Yu)
Citation:
Hong Zhang, Kun Li, Li Ling-Ling, Yu Kang-Kang, Liu Xin-Yao, Li Meng-Yang, Wang Nan, Liu Yan-Hong, Yu Xiao-Qi. Pyridine-Si-xanthene: A novel near-infrared fluorescent platform for biological imaging[J]. Chinese Chemical Letters,
;2019, 30(5): 1063-1066.
doi:
10.1016/j.cclet.2019.03.017

Fluorescent imaging is a powerful technique in monitoring biological analytes and processes in living systems [1]. The developed fluorescent probes have made great contribution to the significant advances in cell biology and medical diagnostic imaging [2]. Near infrared (NIR) fluorescent probes are particularly favorable due to the distinct advantages of NIR light including deep tissue penetration, low background noise and minimum photodamage to biological samples [3]. However, the construction of small-molecule based NIR fluorescent probes is highly challenging and vigorously pursued.
Rhodamine derivatives have attracted extensive attention because of their high quantum yield, large extinction coefficients and good biocompatibility [4]. But the absorption and emission spectra of the rhodamine derivatives are not in the near infrared region, which is unfavorable for bioimaging in vivo. Therefore, in 2008, Qian reported the first Si-substituted xanthene derivatives as far-red to NIR fluorescent dyes [5]. Afterwards, Nagano extended this concept and developed various Si-rhodamines based on the Sixanthene platform [6-10]. Although the family of Si-rhodamine derivatives has been widely employed for functional fluorescent probes, there is still much room for improvement. For example, the developed Si-substituted xanthene derivatives usually have poor water solubility and are hardly modifiable, both of which will greatly hinder their further biological applications. Therefore, construction of novel water-soluble and easily modified skeleton is necessary and urgent.
To address above mentioned issues, in this communication, we changed the phenyl group to pyridine expecting to achieve better solubility. Pyridine differs from benzene markedly in the electronic properties and water solubility. We hope that by the introduction of pyridine the hydrophilicity and modifiability of Si-xanthene will be greatly improved. As expected, the innovatively designed pyridine-Si-xanthene (Py-SiRh) shows red-shifted absorption and emission wavelength and much better water solubility. Such properties endow the fluorescent compound with elevated potential in biological applications. Furthermore, Py-SiRh shows excellent intrinsic targeting ability for lysosomes. As we know that lysosomes have played an important role in a series of cellular processes, such as intracellular transportation, metabolism, cell membrane recycling, pathogen defence and apoptosis [11-14]. Lysosome-targeted probes provide a potent tool for the diagnosis of lysosomal associated diseases. More importantly, the nitrogen atom of pyridine offers possibilities for further modification. That is to say Py-SiRh can be exploited for development of NIR fluorescent probe for lysosome-targeted biological imaging applications. To demonstrate the advantage of Py-SiRh, we further synthesized a novel NIR fluorescent probe for the detection of cysteine in cells and living animals (Scheme 1).
We started with the synthesis of the easily modifiable NIR platform Py-SiRh. Based on the traditional synthetic method, Py-SiRh could be achieved in 45% yield with the starting materials of 4-pyridinecarboxaldehyde and diaryl silyl ether (Scheme S1 in Supporting information) and were characterized by 1H NMR, 13C NMR and HRMS (Supporting information). Unlike reported Sirhodamines that need DMSO or CH3CN as co-solvent to improve their solubility [15], Py-SiRh exhibited good water solubility in PBS buffer solution (Fig. S1 in Supporting information). In PBS buffer solution, its absorption and emission wavelength were found at 655 nm and 680 nm respectively (5 μmol/L), both of which in the near infrared region. Increasing its concentration, the fluorescence of Py-SiRh would be quenched with emission red-shifted (larger than 30 μmol/L). The absolute quantum yield was measured to be 0.12. Before explore its application in cells and tissues, we tested the biocompatibility of Py-SiRh. An MTS assay was carried out and the result revealed that the viabilities of HeLa cells were not noticeably affected by incubation with different concentration of Py-SiRh (Fig. S2 in Supporting information), suggesting the low cytotoxicity of the present fluorescent platform.
It was reported that Si-rhodamines could selectively localize in lysosomes. Then, we performed the co-staining assays in HeLa cells to test the subcellular localization of Py-SiRh. As shown in Fig. 1, when HeLa cells were co-stained with the Py-SiRh and commercially available Lyso-Tracker green, we observed excellent overlapping images along with high Pearson coefficient (0.91), which indicated that Py-SiRh could specifically localize in lysosomes. The outstanding intrinsic lysosome-targeting ability of Py-SiRh could greatly facilitate the study of lysosome-related pathological and physiological effects.
Photostability is another important factor for bioimaging. Hence, we tested the photodegradation of Py-SiRh and commercially available Lyso-Traker deep red in HeLa cells. After irradiated by 5-milliwatt 633 nm laser of the confocal fluorescence microscopy for 50 s, the fluorescence intensity of the Py-SiRh retained ~58% of its initial fluorescence intensity and the commercial dye were photobleached to 53% (Fig. 2). In other words, the photostability of Py-SiRh is comparable to commercial dyes and conventional Si-rhodamine [15]. Moreover, to exhibit deep tissue imaging capability of the present NIR platform, we tested the tissue penetration ability of Py-SiRh using the 80 μm thick frozen kidney tissue slice of rat. The fluorescence signal in different tissue depths were measured through the Z-scan mode of confocal fluorescence microscopy. The results showed that the imaging depths of Py-SiRh were 70 μm (Fig. S3 in Supporting information), indicating the similar ability of the reported Si-rhodamines [15-17].
As we know, intracellular biothiols including cysteine (Cys), homocysteine (Hcy) and glutathione (GSH) are involved in many physiological and pathological processes like redox homeostasis, cardiovascular and Alzheimer's diseases [18, 19]. So, the detection and discrimination for these biothiols are of great importance, and many probes have been reported [20-27]. With the excellent optical properties, biocompatibility, intrinsic lysosome-targeting ability and imaging ability of Py-SiRh mentioned above, to further demonstrate the easy modifiability of Py-SiRh, we connected the platform with Cys-responsive moiety to build Py-SiRh-Cys for the detection of Cys. The 4-(bromomethyl)phenyl acrylate could be easily linked to the platform through formation of the benzyl pyridinium salt to construct Py-SiRh-Cys (Scheme 1 and Scheme S1 in Supporting information). We hypothesized that the transformation of benzyl pyridinium salt group would quench the fluorescence of Py-SiRh due to the photo-induced electron transfer (PET) effect. After triggered with Cys, acrylate leaved by formation with cyclizing, followed by the methyl phenyl group selfiμmolation to form Py-SiRh with near infrared fluorescence emission turned on.
We initially investigated whether Py-SiRh-Cys can selectively respond to Cys among different biothiols. As shown in Fig. 3, in the absence of Cys, no fluorescence was found in the solution of Py-SiRh-Cys (10 μmol/L) in PBS (pH 7.4, 10 μmol/L) buffer solution; while 80 μmol/L Cys was added at 37 ℃, fluorescence was enhanced obviously (70-fold), and an absorption peak at 650 nm appeared, accompanied by a color change from colorless to light blue, which indicated the transformation from Py-SiRh-Cys to Py-SiRh. When other thiols added to the solution of Py-SiRh-Cys, no obvious fluorescence change was observed, except Hcy caused slight fluorescence enhancement. The kinetics study indicated that fluorescence emission reached maximum intensity within 180 min (Fig. 3B and Fig. S4 in Supporting information). Then, the titration experiment was explored (Fig. 3D and Figs. S5 and S6 in Supporting information). Fluorescence of Py-SiRh-Cys enhanced gradually upon the addition of Cys, and a linearly proportional increment of emission intensity to the concentration of the range of 0–20 μmol/L was disclosed. According to the titration profiles, the detection limit (S/N = 3) of Py-SiRh-Cys toward Cys was calculated to be 32 nmol/L. Meanwhile, the developing of test paper for Cys was also tried. We dipped filter papers into the water solution of Py-SiRh-Cys, after drying up, it showed colorless under daylight and blue emission under 365 nm. Obviously different color towards Cys, Hcy and GSH were found under daylight and 365 nm UV light. These results proved that Py-SiRh-Cys could be served as a dye of test-paper for discrimination of thiol-containing amino acids. Additionally, high resolution mass spectrum was used to prove the response mechanism of Py-SiRh-Cys to Cys (Fig. S7 in Supporting information). A peak at m/z 386.2053 assigned to Py-SiRh was observed; meanwhile, the cyclic addition product of acrylic and Cys (calcd. 174.0230) was found (174.0225) in the mass spectra, which in agreement with the reported mechanism.
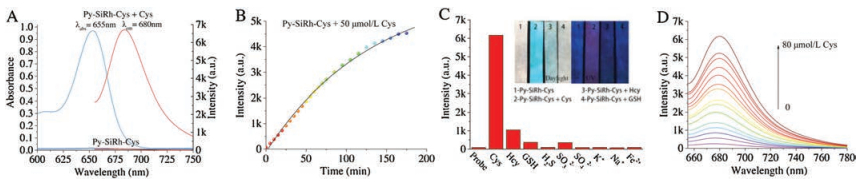
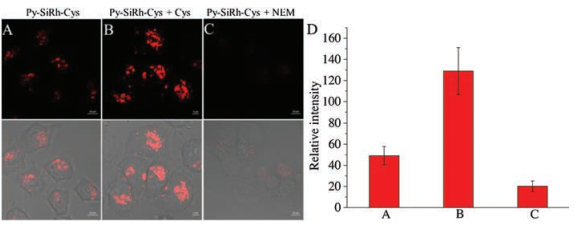
The desirable fluorescence properties of Py-SiRh-Cys for Cys prompted us to study its intracellular application. The imaging experiment of Py-SiRh-Cys for detection of cysteine in living cells was conducted. As shown in Fig. 4, the HeLa cells showed a weak red fluorescence when only treated with the probe, due to the intracellular Cys existence. A stronger red fluorescence was observed after treated with extra cysteine (100 μmol/L). When the HeLa cells were pre-treated with N-ethylmaleimide (NEM) (100 μmol/L), a well-known thiol-blocking reagent, no fluorescence signal is detected. Thus, Py-SiRh-Cys displays an excellent ability for monitoring the cysteine in HeLa cells. Meanwhile, the MTS assay and subcellular localization experiment were carried out and the result revealed that Py-SiRh-Cys exhibited low cytotoxicity as well as good lysosome-targeting ability (Pearson coefficient 0.91).
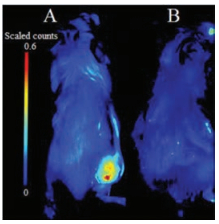
Encouraged by the imaging results in cells, we attempted to examine whether the Py-SiRh-Cys could perform well in B16 tumor-bearing Balb/c mouse. Tumor-bearing Balb/c mice were prepared by subcutaneous injection of B16 cells into the right rear buttock of the Balb/c mice. As shown in Fig. 5, when the Py-SiRhCys was injected into the tumour region, a strong NIR fluorescent signal could be detected, and when the tumor-bearing Balb/c mouse was pretreated with NEM, a well-known inhibitor of cysteine in the tumor region, no obvious fluorescence signal was detected suggesting this NIR fluorescent probe performed well for the detection of Cys in vivo. Especially, it demonstrated the potential application of Py-SiRh as a novel NIR fluorescent probe can be modified flexibly and widely used in medical diagnostic in vivo.
In summary, we have developed a novel Pyridine-Si-Xanthene Py-SiRh as a near-infrared fluorescent platform, which shows good solubility and intrinsic targeting ability for lysosomes. Especially, the platform was extended to construct a lysosomes-targeting probe for Cys, which capable of imaging cysteine in cells and in vivo. Py-SiRh showed good modifiability and red-shifted emission wavelength compared to traditional Si-rhodamines. The present study indicated that the Py-SiRh is an excellent fluorescent platform to study lysosomal cell death related the pathological process of cancers and early diagnosis of cancers, more application are in progress.
This work was financially supported by the National Natural Science Foundation of China (Nos. 21572147 and 21877082). We also thank the Comprehensive Training Platform of Specialized Laboratory, College of Chemistry, Sichuan University for sample analysis.
Supplementary data associated with this article can be found, in the online version, at https://doi.org/10.1016/j.cclet.2019.03.017.
(a) R.N. Germain, E.A. Robey, M.D. Cahalan, Science 336 (2012) 1676-1681;
(b) R. Weissleder, M.J. Pittet, Nature 452 (2008) 580-589.
J.B. Grimm, A.K. Muthusamy, Y. Liang, T.A. Brown, L.D. Lavis, Nat. Methods 14(2017) 987-994.
doi: 10.1038/nmeth.4403
(a) L.D. Lavis, Annu. Rev. Biochem. 86 (2017) 825-843;
(b) H. Zhou, Y. Xiao, X. Hong, Chin. Chem. Lett. 29 (2018) 1425-1428;
(c) L. Yuan, W. Lin, Y. Yang, H. Chen, J. Am. Chem. Soc. 134 (2012) 1200-1211;
(d) W. Chen, Y. Pan, J. Chen, et al., Chin. Chem. Lett. 29 (2018) 1429-1435;
(e) S.A. Hilderbrand, R. Weissleder, Curr. Opin. Chem. Biol. 14 (2010) 71-79;
(f) J. Xu, L. Shang, Chin. Chem. Lett. 29 (2018) 1436-1444;
(g) M.Y. Wu, K. Li, C.Y. Li, J.T. Hou, X.Q. Yu, Chem. Commun. 50 (2014) 183-185;
(h) D. Wu, Y. Shen, J. Chen, et al., Chin. Chem. Lett. 28 (2017) 1979-1982;
(i) L. Yuan, W. Lin, K. Zheng, et al., Chem. Soc. Rev. 42 (2013) 622-661;
(j) L. Yuan, W. Lin, K. Zheng, et al., Acc. Chem. Res. 46 (2013) 1462-1473.
(a) Y. Sun, J. Liu, X. Lv, et al., Angew. Chem. Int. Ed. 51 (2012) 7634-7636;
(b) F. Deng, Z.C. Xu, Chin. Chem. Lett. (2018), doi: 10.1016/j.cclet.2018.12.012.
M. Fu, Y. Xiao, X. Qian, D. Zhao, Y. Xu, Chem. Commun. 15(2008) 1780-17824.
Y. Koide, Y. Urano, K. Hanaoka, T. Terai, T. Nagano, ACS Chem. Biol. 6(2011) 600-608.
doi: 10.1021/cb1002416
T. Egawa, K. Hanaoka, Y. Koide, et al., J. Am. Chem. Soc.133(2011) 14157-14159.
doi: 10.1021/ja205809h
T.E. McCann, N. Kosaka, Y. Koide, et al., Bioconjugate Chem. 22(2011) 2531-2538.
doi: 10.1021/bc2003617
Y. Koide, Y. Urano, K. Hanaoka, et al., J. Am. Chem. Soc. 134(2012) 5029-5031.
doi: 10.1021/ja210375e
Y. Koide, Y. Urano, K. Hanaoka, T. Terai, T. Nagano, J. Am. Chem. Soc. 133(2011) 5680-5682.
doi: 10.1021/ja111470n
(a) S. Goldstein, J. Lind, G. Merényi, Chem. Rev. 105 (2005) 2457-2470;
(b) F.Q. Schafer, G.R. Buettner, Free Radic. Biol. Med. 30 (2001) 1191-1212.
R. Radi, J. Biol. Chem. 288(2013) 26464-26472.
doi: 10.1074/jbc.R113.472936
(a) J.P. Luzio, P.R. Pryor, N.A. Bright, Nat. Rev. Mol. Cell Biol. 8 (2007) 622-632;
(b) P. Ning, W. Wang, M. Chen, Y. Feng, X. Meng, Chin. Chem. Lett. 28 (2017) 1943-1951.
(a) P. Saftig, J. Klumperman, Nat. Rev. Mol. Cell Biol. 10 (2009) 623-635;
(b) T. Kallunki, O.D. Olsen, M. Jaattela, Oncogene 32 (2013) 1995-2004.
(a) Y. Huo, J. Miao, L. Han, et al., Chem. Sci. 8 (2017) 6857-6864;
(b) K. Umezawa, M. Kamiya, Y. Urano, Angew. Chem. Int. Ed. 57 (2018) 9346-9350.
H. Zhang, J. Liu, L. Wang, et al., Biomaterials 158(2018) 10-22.
doi: 10.1016/j.biomaterials.2017.12.013
H. Zhang, J. Liu, C. Liu, et al., Biomaterials 133(2017) 60-69.
doi: 10.1016/j.biomaterials.2017.04.023
(a) M. Kim, S.K. Ko, H. Kim, I. Shin, J. Tae, Chem. Commun. 49 (2013) 7959-7961;
(b) T. Peng, X. Chen, L. Gao, et al., Chem. Sci. 7 (2016) 5407-5413.
(a) J. Hu, N. Wong, M. Lu, et al., Chem. Sci. 7 (2016) 2094-2099;
(b) M.Y. Wu, T. He, K. Li, et al., Analyst 138 (2013) 3018-3025;
(c) Y. Liu, K. Li, M.Y. Wu, et al., Chem. Commun. 51 (2015) 10236-10239.
(a) W. Lin, L. Long, L. Yuan, et al., Org. Lett. 10 (2008) 5577-5580;
(b) J. Liu, Y. Sun, H. Zhang, et al., Chem. Sci. 5 (2014) 3183-3188;
(c) L. Song, L.M. Ma, Q. Sun, et al., Chin. Chem. Lett. 27 (2016) 330-334;
(d) Y. Liu, K. Li, K.X. Xie, Chem. Commun. 52 (2016) 3430-3433.
(a) K.S. Lee, T.K. Kim, J.H. Lee, H.J. Kim, J.I. Hong, Chem. Commun. 46 (2008) 6173-6175;
(b) P. Zhang, Z. Guo, C. Yan, W. Zhu, Chin. Chem. Lett. 28 (2017) 1952-1956.
(a) Y. Yue, F. Huo, P. Ning, et al., J. Am. Chem. Soc. 139 (2017) 3181-3185;
(b) Y. Yang, H. Wang, Y. Wei, et al., Chin. Chem. Lett. 28 (2017) 2023-2026;
(c) J. Yang, K. Li, J.T. Hou, et al., Sci. China Chem. 60 (2017) 793-798.
(a) J.T. Hou, J. Yang, K. Li, K.K. Yu, X.Q. Yu, Sens. Actuators B: Chem. 214 (2015) 92-100;
(b) M. Li, P. Cui, K. Li, et al., Chin. Chem. Lett. 29 (2018) 992-994;
(c) J.Y. Xie, C.Y. Li, Y.F. Li, et al., Anal. Chem. 88 (2016) 9746-9752.
(a) J. Liu, Y.Q. Sun, Y. Huo, et al., J. Am. Chem. Soc. 136 (2014) 574-577;
(b) Y. Liu, X. Lv, M. Hou, Y. Shi, W. Guo, Anal. Chem. 87 (2015) 11475-11483;
(c) Y. He, Z. Li, Q. Jia, et al., Chin. Chem. Lett. 28 (2017) 1969-1974.
(a) Y. Yue, F. Huo, P. Ning, et al., J. Am. Chem. Soc. 139 (2017) 3181-3185;
(b) J. Yang, K. Li, J.T. Hou, et al., ACS Sen. 1 (2016) 166-172.
(a) K. Dou, Q. Fu, G. Chen, et al., Biomaterials 133 (2017) 82-93;
(b) M.Y. Wu, K. Li, J.T. Hou, Z. Huang, X.Q. Yu, Org. Biomol. Chem. 10 (2012) 8342-8347.
(a) Y. Chen, T. Zhang, X. Gao, et al., Chin. Chem. Lett. 28 (2017) 1983-1986;
(b) Z. Lei, Z. Zeng, X. Qian, Y. Yang, Chin. Chem. Lett. 28 (2017) 2001-2004.
(a) R.N. Germain, E.A. Robey, M.D. Cahalan, Science 336 (2012) 1676-1681;
(b) R. Weissleder, M.J. Pittet, Nature 452 (2008) 580-589.
J.B. Grimm, A.K. Muthusamy, Y. Liang, T.A. Brown, L.D. Lavis, Nat. Methods 14(2017) 987-994.
doi: 10.1038/nmeth.4403
(a) L.D. Lavis, Annu. Rev. Biochem. 86 (2017) 825-843;
(b) H. Zhou, Y. Xiao, X. Hong, Chin. Chem. Lett. 29 (2018) 1425-1428;
(c) L. Yuan, W. Lin, Y. Yang, H. Chen, J. Am. Chem. Soc. 134 (2012) 1200-1211;
(d) W. Chen, Y. Pan, J. Chen, et al., Chin. Chem. Lett. 29 (2018) 1429-1435;
(e) S.A. Hilderbrand, R. Weissleder, Curr. Opin. Chem. Biol. 14 (2010) 71-79;
(f) J. Xu, L. Shang, Chin. Chem. Lett. 29 (2018) 1436-1444;
(g) M.Y. Wu, K. Li, C.Y. Li, J.T. Hou, X.Q. Yu, Chem. Commun. 50 (2014) 183-185;
(h) D. Wu, Y. Shen, J. Chen, et al., Chin. Chem. Lett. 28 (2017) 1979-1982;
(i) L. Yuan, W. Lin, K. Zheng, et al., Chem. Soc. Rev. 42 (2013) 622-661;
(j) L. Yuan, W. Lin, K. Zheng, et al., Acc. Chem. Res. 46 (2013) 1462-1473.
(a) Y. Sun, J. Liu, X. Lv, et al., Angew. Chem. Int. Ed. 51 (2012) 7634-7636;
(b) F. Deng, Z.C. Xu, Chin. Chem. Lett. (2018), doi: 10.1016/j.cclet.2018.12.012.
M. Fu, Y. Xiao, X. Qian, D. Zhao, Y. Xu, Chem. Commun. 15(2008) 1780-17824.
Y. Koide, Y. Urano, K. Hanaoka, T. Terai, T. Nagano, ACS Chem. Biol. 6(2011) 600-608.
doi: 10.1021/cb1002416
T. Egawa, K. Hanaoka, Y. Koide, et al., J. Am. Chem. Soc.133(2011) 14157-14159.
doi: 10.1021/ja205809h
T.E. McCann, N. Kosaka, Y. Koide, et al., Bioconjugate Chem. 22(2011) 2531-2538.
doi: 10.1021/bc2003617
Y. Koide, Y. Urano, K. Hanaoka, et al., J. Am. Chem. Soc. 134(2012) 5029-5031.
doi: 10.1021/ja210375e
Y. Koide, Y. Urano, K. Hanaoka, T. Terai, T. Nagano, J. Am. Chem. Soc. 133(2011) 5680-5682.
doi: 10.1021/ja111470n
(a) S. Goldstein, J. Lind, G. Merényi, Chem. Rev. 105 (2005) 2457-2470;
(b) F.Q. Schafer, G.R. Buettner, Free Radic. Biol. Med. 30 (2001) 1191-1212.
R. Radi, J. Biol. Chem. 288(2013) 26464-26472.
doi: 10.1074/jbc.R113.472936
(a) J.P. Luzio, P.R. Pryor, N.A. Bright, Nat. Rev. Mol. Cell Biol. 8 (2007) 622-632;
(b) P. Ning, W. Wang, M. Chen, Y. Feng, X. Meng, Chin. Chem. Lett. 28 (2017) 1943-1951.
(a) P. Saftig, J. Klumperman, Nat. Rev. Mol. Cell Biol. 10 (2009) 623-635;
(b) T. Kallunki, O.D. Olsen, M. Jaattela, Oncogene 32 (2013) 1995-2004.
(a) Y. Huo, J. Miao, L. Han, et al., Chem. Sci. 8 (2017) 6857-6864;
(b) K. Umezawa, M. Kamiya, Y. Urano, Angew. Chem. Int. Ed. 57 (2018) 9346-9350.
H. Zhang, J. Liu, L. Wang, et al., Biomaterials 158(2018) 10-22.
doi: 10.1016/j.biomaterials.2017.12.013
H. Zhang, J. Liu, C. Liu, et al., Biomaterials 133(2017) 60-69.
doi: 10.1016/j.biomaterials.2017.04.023
(a) M. Kim, S.K. Ko, H. Kim, I. Shin, J. Tae, Chem. Commun. 49 (2013) 7959-7961;
(b) T. Peng, X. Chen, L. Gao, et al., Chem. Sci. 7 (2016) 5407-5413.
(a) J. Hu, N. Wong, M. Lu, et al., Chem. Sci. 7 (2016) 2094-2099;
(b) M.Y. Wu, T. He, K. Li, et al., Analyst 138 (2013) 3018-3025;
(c) Y. Liu, K. Li, M.Y. Wu, et al., Chem. Commun. 51 (2015) 10236-10239.
(a) W. Lin, L. Long, L. Yuan, et al., Org. Lett. 10 (2008) 5577-5580;
(b) J. Liu, Y. Sun, H. Zhang, et al., Chem. Sci. 5 (2014) 3183-3188;
(c) L. Song, L.M. Ma, Q. Sun, et al., Chin. Chem. Lett. 27 (2016) 330-334;
(d) Y. Liu, K. Li, K.X. Xie, Chem. Commun. 52 (2016) 3430-3433.
(a) K.S. Lee, T.K. Kim, J.H. Lee, H.J. Kim, J.I. Hong, Chem. Commun. 46 (2008) 6173-6175;
(b) P. Zhang, Z. Guo, C. Yan, W. Zhu, Chin. Chem. Lett. 28 (2017) 1952-1956.
(a) Y. Yue, F. Huo, P. Ning, et al., J. Am. Chem. Soc. 139 (2017) 3181-3185;
(b) Y. Yang, H. Wang, Y. Wei, et al., Chin. Chem. Lett. 28 (2017) 2023-2026;
(c) J. Yang, K. Li, J.T. Hou, et al., Sci. China Chem. 60 (2017) 793-798.
(a) J.T. Hou, J. Yang, K. Li, K.K. Yu, X.Q. Yu, Sens. Actuators B: Chem. 214 (2015) 92-100;
(b) M. Li, P. Cui, K. Li, et al., Chin. Chem. Lett. 29 (2018) 992-994;
(c) J.Y. Xie, C.Y. Li, Y.F. Li, et al., Anal. Chem. 88 (2016) 9746-9752.
(a) J. Liu, Y.Q. Sun, Y. Huo, et al., J. Am. Chem. Soc. 136 (2014) 574-577;
(b) Y. Liu, X. Lv, M. Hou, Y. Shi, W. Guo, Anal. Chem. 87 (2015) 11475-11483;
(c) Y. He, Z. Li, Q. Jia, et al., Chin. Chem. Lett. 28 (2017) 1969-1974.
(a) Y. Yue, F. Huo, P. Ning, et al., J. Am. Chem. Soc. 139 (2017) 3181-3185;
(b) J. Yang, K. Li, J.T. Hou, et al., ACS Sen. 1 (2016) 166-172.
(a) K. Dou, Q. Fu, G. Chen, et al., Biomaterials 133 (2017) 82-93;
(b) M.Y. Wu, K. Li, J.T. Hou, Z. Huang, X.Q. Yu, Org. Biomol. Chem. 10 (2012) 8342-8347.
(a) Y. Chen, T. Zhang, X. Gao, et al., Chin. Chem. Lett. 28 (2017) 1983-1986;
(b) Z. Lei, Z. Zeng, X. Qian, Y. Yang, Chin. Chem. Lett. 28 (2017) 2001-2004.

Yuyang Zhou , Ziwang Mao , Jing-Juan Xu . Recent advances in near infrared (NIR) electrochemiluminescence luminophores. Chinese Chemical Letters, 2024, 35(11): 109622-. doi: 10.1016/j.cclet.2024.109622
Pei Huang , Weijie Zhang , Junping Wang , Fangjun Huo , Caixia Yin . Rapid and specific fluorescent probe visualizes dynamic correlation of Cys and HClO in OGD/R. Chinese Chemical Letters, 2025, 36(1): 109778-. doi: 10.1016/j.cclet.2024.109778
Yudi Cheng , Xiao Wang , Jiao Chen , Zihan Zhang , Jiadong Ou , Mengyao She , Fulin Chen , Jianli Li . A near-infrared fluorescent probe for visualizing transformation pathway of Cys/Hcy and H2S and its applications in living system. Chinese Chemical Letters, 2024, 35(5): 109156-. doi: 10.1016/j.cclet.2023.109156
Rakesh Kumar Gupta , Zhi Wang , Di Sun . Shining bright: Revolutionary near-unity NIR phosphorescent metal nanoclusters. Chinese Journal of Structural Chemistry, 2024, 43(11): 100417-100417. doi: 10.1016/j.cjsc.2024.100417
Hui-Juan Wang , Wen-Wen Xing , Zhen-Hai Yu , Yong-Xue Li , Heng-Yi Zhang , Qilin Yu , Hongjie Zhu , Yao-Yao Wang , Yu Liu . Cucurbit[7]uril confined phenothiazine bridged bis(bromophenyl pyridine) activated NIR luminescence for lysosome imaging. Chinese Chemical Letters, 2024, 35(6): 109183-. doi: 10.1016/j.cclet.2023.109183
Ji Liu , Dongsheng He , Tianjiao Hao , Yumin Hu , Yan Zhao , Zhen Li , Chang Liu , Daquan Chen , Qiyue Wang , Xiaofei Xin , Yan Shen . Gold mineralized "hybrid nanozyme bomb" for NIR-II triggered tumor effective permeation and cocktail therapy. Chinese Chemical Letters, 2024, 35(9): 109296-. doi: 10.1016/j.cclet.2023.109296
Biao Huang , Tao Tang , Fushou Liu , Shi-Hui Chen , Zhi-Ling Zhang , Mingxi Zhang , Ran Cui . Quantum dots boost large-view NIR-Ⅱ imaging with high fidelity for fluorescence-guided tumor surgery. Chinese Chemical Letters, 2024, 35(12): 109694-. doi: 10.1016/j.cclet.2024.109694
Lulu Cao , Yikun Li , Dongxiang Zhang , Shuai Yue , Rong Shang , Xin-Dong Jiang , Jianjun Du . Engineering aggregates of julolidine-substituted aza-BODIPY nanoparticles for NIR-II photothermal therapy. Chinese Chemical Letters, 2024, 35(12): 109735-. doi: 10.1016/j.cclet.2024.109735
Xiaoshuai Wu , Bailei Wang , Yichen Li , Xiaoxuan Guan , Mingjing Yin , Wenquan Lv , Yin Chen , Fei Lu , Tao Qin , Huyang Gao , Weiqian Jin , Yifu Huang , Cuiping Li , Ming Gao , Junyu Lu . NIR driven catalytic enhanced acute lung injury therapy by using polydopamine@Co nanozyme via scavenging ROS. Chinese Chemical Letters, 2025, 36(2): 110211-. doi: 10.1016/j.cclet.2024.110211
Du Liu , Yuyan Li , Hankun Zhang , Benhua Wang , Chaoyi Yao , Minhuan Lan , Zhanhong Yang , Xiangzhi Song . Three-in-one erlotinib-modified NIR photosensitizer for fluorescence imaging and synergistic chemo-photodynamic therapy. Chinese Chemical Letters, 2025, 36(2): 109910-. doi: 10.1016/j.cclet.2024.109910
Shu Tian , Wenxin Huang , Junrui Hu , Huiling Wang , Zhipeng Zhang , Liying Xu , Junrong Li , Yao Sun . Exploring the frontiers of plant health: Harnessing NIR fluorescence and surface-enhanced Raman scattering modalities for innovative detection. Chinese Chemical Letters, 2025, 36(3): 110336-. doi: 10.1016/j.cclet.2024.110336
Xiao-Fang Lv , Xiao-Yun Ran , Yu Zhao , Rui-Rui Zhang , Li-Na Zhang , Jing Shi , Ji-Xuan Xu , Qing-Quan Kong , Xiao-Qi Yu , Kun Li . Combing NIR-Ⅱ molecular dye with magnetic nanoparticles for enhanced photothermal theranostics with a 95.6% photothermal conversion efficiency. Chinese Chemical Letters, 2025, 36(4): 110027-. doi: 10.1016/j.cclet.2024.110027
Mengjie Gao , Zhiqiang Cui , Yue Shen , Yikun Li , Dongxiang Zhang , Xiaoyan Gao , Yaguang Sun , Xin-Dong Jiang , Jianjun Du , Xiaohong Sun . NIR-II emissive aza-BODIPY-based nanoparticles for triggering glioblastoma apoptosis in brain. Chinese Chemical Letters, 2025, 36(5): 110098-. doi: 10.1016/j.cclet.2024.110098
Baoli Yin , Xinlin Liu , Zhe Li , Zhifei Ye , Youjuan Wang , Xia Yin , Sulai Liu , Guosheng Song , Shuangyan Huan , Xiao-Bing Zhang . Ratiometric NIR-Ⅱ fluorescent organic nanoprobe for imaging and monitoring tumor-activated photodynamic therapy. Chinese Chemical Letters, 2025, 36(5): 110119-. doi: 10.1016/j.cclet.2024.110119
Songtao Cai , Liuying Wu , Yuan Li , Soham Samanta , Jinying Wang , Bing Liu , Feihu Wu , Kaitao Lai , Yingchao Liu , Junle Qu , Zhigang Yang . Intermolecular hydrogen-bonding as a robust tool toward significantly improving the photothermal conversion efficiency of a NIR-II squaraine dye. Chinese Chemical Letters, 2024, 35(4): 108599-. doi: 10.1016/j.cclet.2023.108599
Jianqiu Li , Yi Zhang , Songen Liu , Jie Niu , Rong Zhang , Yong Chen , Yu Liu . Cucurbit[8]uril-based non-covalent heterodimer realized NIR cell imaging through topological transformation from nanowire to nanorod. Chinese Chemical Letters, 2024, 35(10): 109645-. doi: 10.1016/j.cclet.2024.109645
Jieqiong Xu , Wenbin Chen , Shengkai Li , Qian Chen , Tao Wang , Yadong Shi , Shengyong Deng , Mingde Li , Peifa Wei , Zhuo Chen . Organic stoichiometric cocrystals with a subtle balance of charge-transfer degree and molecular stacking towards high-efficiency NIR photothermal conversion. Chinese Chemical Letters, 2024, 35(10): 109808-. doi: 10.1016/j.cclet.2024.109808
Zhaorui Song , Qiulian Hao , Bing Li , Yuwei Yuan , Shanshan Zhang , Yongkuan Suo , Hai-Hao Han , Zhen Cheng . NIR-Ⅱ fluorescence lateral flow immunosensor based on efficient energy transfer probe for point-of-care testing of tumor biomarkers. Chinese Chemical Letters, 2025, 36(1): 109834-. doi: 10.1016/j.cclet.2024.109834
Shangqian Zhang , Jiaxuan Li , Xuan Hu , Zelong Chen , Junliang Dong , Chenhao Hu , Shuang Chao , Yinghua Lv , Yuxin Pei , Zhichao Pei . H2S and NIR light-driven nanomotors induce disulfidptosis for targeted anticancer therapy by enhancing disruption of tumor metabolic symbiosis. Chinese Chemical Letters, 2025, 36(1): 110314-. doi: 10.1016/j.cclet.2024.110314
Fuzheng Zhang , Chao Shi , Jiale Li , Fulin Jia , Xinyu Liu , Feiyang Li , Xinyu Bai , Qiuxia Li , Aihua Yuan , Guohua Xie . B-embedded narrowband pure near-infrared (NIR) phosphorescent iridium(Ⅲ) complexes and solution-processed OLED application. Chinese Chemical Letters, 2025, 36(1): 109596-. doi: 10.1016/j.cclet.2024.109596