New strategy for reversal tolerant anode for automotive polymer electrolyte fuel cell
-
* Corresponding authors.
E-mail addresses: Chanho.pak@gist.ac.kr (C. Pak), eunyoung.you@mobis.co.kr (E. You)
Citation:
Pak Chanho, Woo Lee Seung, Baik Chaekyung, Ho Lee Bong, Jong You Dae, You Eunyoung. New strategy for reversal tolerant anode for automotive polymer electrolyte fuel cell[J]. Chinese Chemical Letters,
;2019, 30(6): 1186-1189.
doi:
10.1016/j.cclet.2019.02.020

Efficient synthesis of novel macrocyclic hosts with unique structures and good host–guest properties is a permanent and challenging topic in the field of supramolecular chemistry [1-7]. During the last decade, considerable effort has been devoted to the development of macrocyclic molecular and a number of new macrocyclic receptors with novel properties have been reported, such as heterocalix[n]aromatics [8-10], pillar[n]arene [11-16], helicarenes [17-19], naphthotubes [20-24], Ex-box and Ex-cage [25-32], and others [33-37]. However, most of the reported macrocyclic hosts showed poor solubility and weak host–guest interactions in water. To develop a new macrocyclic molecule with good water solubility and molecular recognition properties not only helps us to understand and mimic the biological processes, but also enriches the toolbox of supramolecular chemists. Water soluble groups including sulfonate [38-40], carboxylate [41-43] and quaternary amine groups [44, 45] have been modified onto the macrocyclic hosts to increase their water solubility. These approaches often suffer from long synthetic steps and low yields, which restrict their further application in the complicated supramolecular self-assembly. Undoubtedly, it is important to develop novel water-soluble macrocyclic hosts that could be obtained in high yields and show good host–guest properties in water. Recently, Li and coworkers [46, 47] reported the efficient synthesis of water-soluble macrocyclic hosts using dynamic covalent chemistry (DCC) approach. In their method, both macrocycles and [2] catenanes could be obtained in high yields under the thermodynamic control. However, the purification of [2] catenanes need chromatographic. Previously, our group [42, 43] prepared a novel water-soluble cylindrical macrotricyclic host, and found that the host could bind two N-methylquinolinium salts to form 1:2 complexes in water. Inspired by these results, we deduced that whether we could find a new strategy to construct novel water-soluble macrocyclic host with significant host–guest properties in high yields.
So far as we know, 1, 8-bis(4-pyridylethynyl)anthracene, which looks like a molecule "clip", has been used as donor building block to prepare trigonal prisms [48]. Thus, the anthracene-based "clip" could also serve as half part of a macrocycle and a macrocyclic host could be obtained when a suitable building block was introduced to this clip. Herein, we report the efficient synthesis of a novel water soluble macrocyclic host 12+·2Br− and its complexation with neutral guests in water. By the utilization of the anthracene-based "clip" to react with 1, 4-bis(bromomethyl)benzene, host 12+·2Br− can be obtain via simple filtration in a high yield of 82%. Moreover, it was found that host 12+·2Br− could form 1:1 complexes with guests 2 and 3 in water solution (Fig. 1). Interestingly, we discovered that host 12+·2Br− could only selectively accommodate naphthalene among a variety of polycyclic aromatic hydrocarbons in water. This selective host-guest recognition could be employed for the further removal of naphthalene from sewage.
Synthesis of host 12+·2Br− was outlined in Scheme 1. Compound 4 was first prepared according to the literature procedure [48]. By the reaction of 4 and commercially available 1, 4-bis(bromomethyl)benzene in acetonitrile at 90 ℃, host 12+·2Br− could be easily synthesized in 82% yield. Macrocyclic host 12+·2Br− showed moderate solubility in water, and its structure was confirmed by 1H NMR, 13C NMR, HRMS spectra and crystal structure analysis (Supporting information).
Firstly, the binding properties of host 12+·2Br− toward neutral guests 2 and 3 were investigated by 1H NMR spectra in water solution. Unlike the previous results that reported by our group [43], after electron-poor host 12+·2Br− (4.0 mmol/L) and electron-rich guest 2 with 1:1 molar ratio were mixed in water, no obviously color change was observed. The similar phenomenon was also observed for the aqueous solution between host 12+·2Br− and guests 3. These results led us to doubt that whether host 12+·2Br− could form of complexes with guests 2 and 3. Consequently, the 1H NMR experiments were carried out to further investigate the complexation between host 12+·2Br− and guest 2 in water. As shown in Fig. 2, the 1H NMR spectrum of a 1:1 mixture of 12+·2Br− and 2 in D2O showed a great difference with those for free host 12+·2Br− and free guest 2. Upfield shifts of the resonances of protons H1, H2 and H3 corresponding to guest 2 were observed, which indicated that the naphthalene unit of 2 experienced a shielded magnetic environment in the aromatic cavity of 12+·2Br−. Moreover, the signal of protons Hf and Hg in host 12+·2Br− also shifted upfield, implying that the electron-poor pyridine unit of 12+·2Br− was in shielded magnetic environment and a new complex 1·2 could be formed. Meanwhile, by increasing the amount of guest 2, the spectrum of complex 1·2 showed only one set of resonances, which indicated that the complexation and decomplexation between host 12+·2Br− and guest 2 were a fast exchange process on the NMR time scale at room temperature. The formation of complex 1·2 was also supported by 2D NOESY spectral experiment. As shown in the Fig. S4 (Supporting information), the clear correlation signals between protons H1-Hg, H2-Hg and the protons of crown ether units of host 12+·2Br− were observed, which was also consistent with the formation of complex 1·2. Moreover, 1H NMR titrations and nonlinear fitting were then performed to quantitatively estimate the 1:1 binding manner between host 12+·2Br− and guest 2. Consequently, it was found that 1:1 complex 1·2 were formed by the mole ratio plot. The binding constant (K) of the complex 2⊂12+·2Br− was calculated to be 184±4 L/mol by the Scatchard plot [49]. By the counteranion exchange of 12+·2Br−, we also prepared oil-soluble 12+·2PF6− and investigated its complexation with 2 in MeCN. As shown in Fig. S3 (Supporting information), the 1H NMR spectrum of the 1:1 mixture of 12+·2PF6− and 2 was essentially the sum of the two components, indicating that no obvious complexation between 12+·2PF6− and 2 occurred and the major driving force for the formation of 2⊂12+·2Br− in water could be hydrophobic interactions. In addition, the binding constant of the complex 3⊂12+·2Br− was measured to be around 64±2 L/mol in D2O at 298 K. Compared to guest 2 containing a naphthalene unit, guest 3 has a smaller phenyl hydrophobic moiety, which accounted for its lower binding constant within host 12+·2Br−.
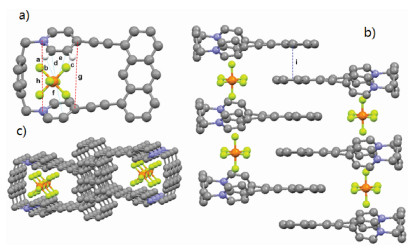
The attempts to obtain the single crystal of 2⊂12+·2Br− and 12+·2Br− were unsuccessful. Fortunately, a yellow single crystal of 12+·2PF6− was obtained by slow vapor diffusion of ether to a solution of 12+·2PF6− in CH3CN, providing unambiguous evidence for the formation of 12+·2Br−. As shown in Fig. 3a, the two pyridinium residues orientate in a face-to-face nanner and the distance between two N and C atoms of pyridinium residues are measured to be 5.812 Å (h) and 5.647 Å (g), indicating the moderate-sized cavity of host 12+·2PF6−. Interestingly, it was found that PF6− ion showed not only multiple CH···F hydrogen bonds but also anion···π interactions with pyridinium rings with the distance of 2.989 (d), 3.062 (e) and 3.159 (f), respectively. Moreover, π···π interaction between two anthracene groups of host 12+·2PF6− with a distance of 3.846 Å (i) was also observed (Fig. 3b). Because of these multiple noncovalent interactions, the macrocyclic molecule 12+·2PF6− could self-assemble to form a 1D tubular channel with PF6− ions inside the channels.
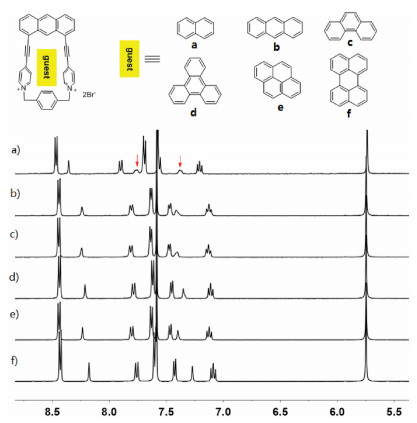
Inspired by the formation of complex 1·2 in water, we further investigated the capability of 12+·2Br− to accommodate a variety of polycyclic aromatic hydrocarbons in water. As shown in Fig. 4a, 1H NMR spectroscopy revealed that 12+·2Br− can encapsulate naphthalene to formation of 1:1 complex. However, the formation of 1:1 complexes between 12+·2Br− and anthracene, phenanthrene, triphenylene, pyrene and perylene were not observed (Figs. 4b–f). These observations could be explained by the fact that host 12+·2Br− had a small cavity and could only recognize small polycyclic aromatic hydrocarbons such as naphthalene. However, because of the extremely poor solubility of naphthalene in water, the corresponding binding constants of host 12+·2Br− and naphthalene could not be determined. The highly selective binding behavior of host 12+·2Br− toward naphthalene could be further used for the separation of naphthalene from a variety of polycyclic aromatic hydrocarbons.
In conclusion, by taking advantage of anthracene-based "clip" structure, we developed an efficient approach to construct a water-soluble macrocycle and studied its binding ability toward neutral guests containing naphthalene or phenyl units in water. The formation of 1:1 complexes between host 12+·2Br− and 2 or 3 were confirmed by the 1H NMR titrations experiment. Additionally, we demonstrated that the major driving force for the formation of 2⊂12+·2Br− or 3⊂12+·2Br− in water might be hydrophobic interactions. We further investigated its ability to host a variety of polycyclic aromatic hydrocarbons in aqueous solution. It was found that host 12+·2Br− could selectively encapsulate of naphthalene to formation of 1:1 complexes over a variety of polycyclic aromatic hydrocarbons. This highly selective accommodation hydrophobic guest in water could be explained by the fact that host 12+·2Br− had a relatively small hydrophobic cavity. The application of this novel water-soluble host for the separation of naphthalene from a variety of polycyclic aromatic hydrocarbons and removal of naphthalene from sewage, are underway in our laboratory.
The authors report no declarations of interest.
The authors are grateful for the financial support from the National Natural Science Foundation of China (Nos. 21602055 and 51772091); Natural Science Foundation of Hunan Province (No. 2017JJ3094) and Research Foundation of Education Bureau of Hunan Province (No. 18C1072).
Supplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.cclet.2020.11.019.
Z.P. Cano, D. Banham, S. Ye, et al., Nature Ener. 3(2018) 279-289.
doi: 10.1038/s41560-018-0108-1
T. Wilberforce, Z. El-Hassan, F.N. Khatib, et al., Int. J. Hydrogen Energy 42(2017) 25695-25734.
doi: 10.1016/j.ijhydene.2017.07.054
A. Alaswad, A. Baroutaji, H. Achour, et al., Int. J. Hydrogen Energy 41(2016) 16499-16508.
doi: 10.1016/j.ijhydene.2016.03.164
B.K. Hong, S.H. Kim, ECS Trans. 86(2018) 3-11.
A. Kongkanad, M.F. Mathias, J. Phys. Chem. Lett. 7(2016) 1127-1137.
doi: 10.1021/acs.jpclett.6b00216
T. Zhang, P. Wang, H. Chen, P. Pei, Acs Appl. Energy Mater. 223(2018) 249-262.
T. Mittermeier, A. Weiß, F. Hasché, G. Hübner, H.A. Gasteiger, J. Electrochem. Soc. 164(2017) F127-F137.
doi: 10.1149/2.1061702jes
G.S. Harzer, J.N. Schwämmlein, et al., J. Electrochem. Soc. 165(2018) F3118-F3131.
doi: 10.1149/2.0161806jes
H. Zhang, H. Haas, J. Hu, et al., J. Electrochem. Soc. 160(2013) F840-F847.
doi: 10.1149/2.083308jes
N. Macauley, D.D. Papadias, J. Fairweather, et al., J. Electrochem. Soc.165(2018) F3148-F3160.
doi: 10.1149/2.0061806jes
F. Forouzandeh, X. Li, D.W. Banham, et al., J. Electrochem. Soc. 165(2018) F3230-F3240.
doi: 10.1149/2.0261806jes
C. Qin, J. Wang, D. Yang, B. Li, C. Zhang, Catalysts 6(2016) 197-217.
doi: 10.3390/catal6120197
Ž. Penga, G. Radica, F. Barbir, P. Eckert, Degradation mechanisms in automotive fuel cell systems, FCHJU deliverable D1.3, (2018).
A. Taniguchi, T. Akita, K. Yasuda, Y. Miyazaki, Int. J. Hydrogen Energy 33(2008) 2323-2329.
doi: 10.1016/j.ijhydene.2008.02.049
A. Taniguchi, T. Akita, K. Yasuda, Y. Miyazaki, J. Power Sources 130(2004) 42-49.
doi: 10.1016/j.jpowsour.2003.12.035
T.R. Ralph, M.P. Hogath, Platinum Metals Rev. 46(2002) 117-135.
B.K. Hong, P. Mandal, J.G. Oh, S. Lister, J. Power Sources 328(2016) 280-288.
doi: 10.1016/j.jpowsour.2016.07.002
W.R.W. Daud, R.E. Rosli, E.H. Majlan, et al., Renew. Energy 113(2017) 620-638.
doi: 10.1016/j.renene.2017.06.027
J. Thangavelautham, Degradation in PEM fuel cells and mitigation strategies using system design and control, in: T. Taner (Ed.), Proton Exchange Membrane Fuel Cell, Intech Open Ltd, London, 2018, pp. 63-95.
Y.B. Park, E. You, C. Pak, M. Min, Electrochim. Acta 284(2018) 242-252.
doi: 10.1016/j.electacta.2018.07.171
W.S. Jung, B.N. Popov, Carbon 122(2017) 746-755.
doi: 10.1016/j.carbon.2017.07.028
Y.J. Wang, B. Fang, H. Li, X. Bi, H. Wang, Prog. Mater. Sci. 82(2016) 445-498.
doi: 10.1016/j.pmatsci.2016.06.002
Y. Jeon, Y. Ji, Y.I. Cho, et al., ACS Nano 12(2018) 6819-6829.
doi: 10.1021/acsnano.8b02040
O. Lori, L. Elbaz, Catalysts 5(2015) 1445-1464.
doi: 10.3390/catal5031445
P. Mandal, B.K. Hong, J.G. Oh, S. Litster, J. Power Sources 397(2018) 397-404.
doi: 10.1016/j.jpowsour.2018.06.083
K.H. Lim, W.H. Lee, Y. Jeong, H. Kim, J. Electrochem. Soc. 164(2017) F1580-F1586.
doi: 10.1149/2.0731714jes
T.R. Ralph, S. Hudson, D.P. Wilkinson, ECS Trans. 1(2006) 67-84.
Z. Zhu, X. Yan, H. Tang, et al., J. Power Sources 351(2017) 138-144.
doi: 10.1016/j.jpowsour.2017.03.076
E. You, M. Min, S.A. Jin, T. Kim, C. Pak, J. Electrochem. Soc. 165(2018) F3094-F3099.
doi: 10.1149/2.0121806jes
S.D. Knights, D.P. Wilkinson, S.A. Campbell, et al., Patent PCT WO 01/15247 A2, (2001).
J.L. Taylor, D.P. Wilkinson, D.S. Wainwright, T.R. Ralph, S.D. Knights, Patent PCT WO 01/15249 A2, (2001).
S. Ye, P. Beattie, S. A. Campbell, et al., Patent, US 2004/0013935 A1, 2004.
S. Ye, Reversal-tolerant catalyst layers, in: J. Zhang (Ed.), PEM Fuel Cell Electrocatalysts and Catalyst Layers, Fundamentals and Applications, SpringerVerlag Ltd., London, 2008835-680.
T. Yasuda, A. Ogawa, M. Kanno, et al., Chem. Lett. 38(2009) 692-693.
doi: 10.1246/cl.2009.692
G.G. Eshetu, M. Armand, H. Ohno, B. Scrosati, S. Passerini, Energy Environ. Sci. 9(2016) 49-61.
doi: 10.1039/C5EE02284C
A.R. Neale, P. Li, J. Jacquemin, et al., Phys. Chem. Chem. Phys. 18(2016) 11251-11262.
doi: 10.1039/C5CP07160G
C. Pozo-Gonzalo, M. Kar, E. Jónsson, et al., Electrochim. Acta 196(2016) 727-734.
doi: 10.1016/j.electacta.2016.02.208
E. You, S.W. Lee, C. Pak, Development and application of cell reversal durable, carbon supported anode catalysts for membrane electrode assembly of automotive PEMFC, International Symposium on Advancement and Prospect of Catalysis Science (2018) 25-27.
D.A. Stevens, R.J. Sanderson, T.D. Hatchard, et al., ECS Trans. 33(2010) 419-423.
D.A. Cullen, K.L. More, K.S. Reeves, et al., ECS Trans. 41(2011) 1099-1103.
J. Durst, A. Orfanidi, P.J. Rheinländer, et al., ECS Trans. 69(2015) 67-76.
S.A. Jin, C. Pak, D. J. Yoo, K. H. Lee, Patent, US 2013/0137009 A1, 2013.
D.J. You, D.H. Kim, J.R. De Lile, et al., Appl. Catal. A:General 562(2018) 250-257.
doi: 10.1016/j.apcata.2018.06.018
Z.P. Cano, D. Banham, S. Ye, et al., Nature Ener. 3(2018) 279-289.
doi: 10.1038/s41560-018-0108-1
T. Wilberforce, Z. El-Hassan, F.N. Khatib, et al., Int. J. Hydrogen Energy 42(2017) 25695-25734.
doi: 10.1016/j.ijhydene.2017.07.054
A. Alaswad, A. Baroutaji, H. Achour, et al., Int. J. Hydrogen Energy 41(2016) 16499-16508.
doi: 10.1016/j.ijhydene.2016.03.164
B.K. Hong, S.H. Kim, ECS Trans. 86(2018) 3-11.
A. Kongkanad, M.F. Mathias, J. Phys. Chem. Lett. 7(2016) 1127-1137.
doi: 10.1021/acs.jpclett.6b00216
T. Zhang, P. Wang, H. Chen, P. Pei, Acs Appl. Energy Mater. 223(2018) 249-262.
T. Mittermeier, A. Weiß, F. Hasché, G. Hübner, H.A. Gasteiger, J. Electrochem. Soc. 164(2017) F127-F137.
doi: 10.1149/2.1061702jes
G.S. Harzer, J.N. Schwämmlein, et al., J. Electrochem. Soc. 165(2018) F3118-F3131.
doi: 10.1149/2.0161806jes
H. Zhang, H. Haas, J. Hu, et al., J. Electrochem. Soc. 160(2013) F840-F847.
doi: 10.1149/2.083308jes
N. Macauley, D.D. Papadias, J. Fairweather, et al., J. Electrochem. Soc.165(2018) F3148-F3160.
doi: 10.1149/2.0061806jes
F. Forouzandeh, X. Li, D.W. Banham, et al., J. Electrochem. Soc. 165(2018) F3230-F3240.
doi: 10.1149/2.0261806jes
C. Qin, J. Wang, D. Yang, B. Li, C. Zhang, Catalysts 6(2016) 197-217.
doi: 10.3390/catal6120197
Ž. Penga, G. Radica, F. Barbir, P. Eckert, Degradation mechanisms in automotive fuel cell systems, FCHJU deliverable D1.3, (2018).
A. Taniguchi, T. Akita, K. Yasuda, Y. Miyazaki, Int. J. Hydrogen Energy 33(2008) 2323-2329.
doi: 10.1016/j.ijhydene.2008.02.049
A. Taniguchi, T. Akita, K. Yasuda, Y. Miyazaki, J. Power Sources 130(2004) 42-49.
doi: 10.1016/j.jpowsour.2003.12.035
T.R. Ralph, M.P. Hogath, Platinum Metals Rev. 46(2002) 117-135.
B.K. Hong, P. Mandal, J.G. Oh, S. Lister, J. Power Sources 328(2016) 280-288.
doi: 10.1016/j.jpowsour.2016.07.002
W.R.W. Daud, R.E. Rosli, E.H. Majlan, et al., Renew. Energy 113(2017) 620-638.
doi: 10.1016/j.renene.2017.06.027
J. Thangavelautham, Degradation in PEM fuel cells and mitigation strategies using system design and control, in: T. Taner (Ed.), Proton Exchange Membrane Fuel Cell, Intech Open Ltd, London, 2018, pp. 63-95.
Y.B. Park, E. You, C. Pak, M. Min, Electrochim. Acta 284(2018) 242-252.
doi: 10.1016/j.electacta.2018.07.171
W.S. Jung, B.N. Popov, Carbon 122(2017) 746-755.
doi: 10.1016/j.carbon.2017.07.028
Y.J. Wang, B. Fang, H. Li, X. Bi, H. Wang, Prog. Mater. Sci. 82(2016) 445-498.
doi: 10.1016/j.pmatsci.2016.06.002
Y. Jeon, Y. Ji, Y.I. Cho, et al., ACS Nano 12(2018) 6819-6829.
doi: 10.1021/acsnano.8b02040
O. Lori, L. Elbaz, Catalysts 5(2015) 1445-1464.
doi: 10.3390/catal5031445
P. Mandal, B.K. Hong, J.G. Oh, S. Litster, J. Power Sources 397(2018) 397-404.
doi: 10.1016/j.jpowsour.2018.06.083
K.H. Lim, W.H. Lee, Y. Jeong, H. Kim, J. Electrochem. Soc. 164(2017) F1580-F1586.
doi: 10.1149/2.0731714jes
T.R. Ralph, S. Hudson, D.P. Wilkinson, ECS Trans. 1(2006) 67-84.
Z. Zhu, X. Yan, H. Tang, et al., J. Power Sources 351(2017) 138-144.
doi: 10.1016/j.jpowsour.2017.03.076
E. You, M. Min, S.A. Jin, T. Kim, C. Pak, J. Electrochem. Soc. 165(2018) F3094-F3099.
doi: 10.1149/2.0121806jes
S.D. Knights, D.P. Wilkinson, S.A. Campbell, et al., Patent PCT WO 01/15247 A2, (2001).
J.L. Taylor, D.P. Wilkinson, D.S. Wainwright, T.R. Ralph, S.D. Knights, Patent PCT WO 01/15249 A2, (2001).
S. Ye, P. Beattie, S. A. Campbell, et al., Patent, US 2004/0013935 A1, 2004.
S. Ye, Reversal-tolerant catalyst layers, in: J. Zhang (Ed.), PEM Fuel Cell Electrocatalysts and Catalyst Layers, Fundamentals and Applications, SpringerVerlag Ltd., London, 2008835-680.
T. Yasuda, A. Ogawa, M. Kanno, et al., Chem. Lett. 38(2009) 692-693.
doi: 10.1246/cl.2009.692
G.G. Eshetu, M. Armand, H. Ohno, B. Scrosati, S. Passerini, Energy Environ. Sci. 9(2016) 49-61.
doi: 10.1039/C5EE02284C
A.R. Neale, P. Li, J. Jacquemin, et al., Phys. Chem. Chem. Phys. 18(2016) 11251-11262.
doi: 10.1039/C5CP07160G
C. Pozo-Gonzalo, M. Kar, E. Jónsson, et al., Electrochim. Acta 196(2016) 727-734.
doi: 10.1016/j.electacta.2016.02.208
E. You, S.W. Lee, C. Pak, Development and application of cell reversal durable, carbon supported anode catalysts for membrane electrode assembly of automotive PEMFC, International Symposium on Advancement and Prospect of Catalysis Science (2018) 25-27.
D.A. Stevens, R.J. Sanderson, T.D. Hatchard, et al., ECS Trans. 33(2010) 419-423.
D.A. Cullen, K.L. More, K.S. Reeves, et al., ECS Trans. 41(2011) 1099-1103.
J. Durst, A. Orfanidi, P.J. Rheinländer, et al., ECS Trans. 69(2015) 67-76.
S.A. Jin, C. Pak, D. J. Yoo, K. H. Lee, Patent, US 2013/0137009 A1, 2013.
D.J. You, D.H. Kim, J.R. De Lile, et al., Appl. Catal. A:General 562(2018) 250-257.
doi: 10.1016/j.apcata.2018.06.018

Baokang Geng , Xiang Chu , Li Liu , Lingling Zhang , Shuaishuai Zhang , Xiao Wang , Shuyan Song , Hongjie Zhang . High-efficiency PdNi single-atom alloy catalyst toward cross-coupling reaction. Chinese Chemical Letters, 2024, 35(7): 108924-. doi: 10.1016/j.cclet.2023.108924
Peng Wang , Daijie Deng , Suqin Wu , Li Xu . Cobalt-based deep eutectic solvent modified nitrogen-doped carbon catalyst for boosting oxygen reduction reaction in zinc-air batteries. Chinese Journal of Structural Chemistry, 2024, 43(1): 100199-100199. doi: 10.1016/j.cjsc.2023.100199
Yanling Yang , Zhenfa Ding , Huimin Wang , Jianhui Li , Yanping Zheng , Hongquan Guo , Li Zhang , Bing Yang , Qingqing Gu , Haifeng Xiong , Yifei Sun . Dynamic tracking of exsolved PdPt alloy/perovskite catalyst for efficient lean methane oxidation. Chinese Chemical Letters, 2024, 35(4): 108585-. doi: 10.1016/j.cclet.2023.108585
Mengxiang Zhu , Tao Ding , Yunzhang Li , Yuanjie Peng , Ruiping Liu , Quan Zou , Leilei Yang , Shenglei Sun , Pin Zhou , Guosheng Shi , Dongting Yue . Graphene controlled solid-state growth of oxygen vacancies riched V2O5 catalyst to highly activate Fenton-like reaction. Chinese Chemical Letters, 2024, 35(12): 109833-. doi: 10.1016/j.cclet.2024.109833
Hong Yin , Zhipeng Yu . Hexavalent iridium catalyst enhances efficiency of hydrogen production. Chinese Journal of Structural Chemistry, 2025, 44(1): 100382-100382. doi: 10.1016/j.cjsc.2024.100382
Jiawei Ge , Xian Wang , Heyuan Tian , Hao Wan , Wei Ma , Jiangying Qu , Junjie Ge . Iridium-based catalysts for oxygen evolution reaction in proton exchange membrane water electrolysis. Chinese Chemical Letters, 2025, 36(5): 109906-. doi: 10.1016/j.cclet.2024.109906
Yizhe Chen , Yuzhou Jiao , Liangyu Sun , Cheng Yuan , Qian Shen , Peng Li , Shiming Zhang , Jiujun Zhang . Nonmetallic phosphorus alloying to regulate the oxygen reduction mechanisms of platinum catalyst. Chinese Chemical Letters, 2025, 36(4): 110789-. doi: 10.1016/j.cclet.2024.110789
Jing Cao , Dezheng Zhang , Bianqing Ren , Ping Song , Weilin Xu . Mn incorporated RuO2 nanocrystals as an efficient and stable bifunctional electrocatalyst for oxygen evolution reaction and hydrogen evolution reaction in acid and alkaline. Chinese Chemical Letters, 2024, 35(10): 109863-. doi: 10.1016/j.cclet.2024.109863
Xiao Li , Wanqiang Yu , Yujie Wang , Ruiying Liu , Qingquan Yu , Riming Hu , Xuchuan Jiang , Qingsheng Gao , Hong Liu , Jiayuan Yu , Weijia Zhou . Metal-encapsulated nitrogen-doped carbon nanotube arrays electrode for enhancing sulfion oxidation reaction and hydrogen evolution reaction by regulating of intermediate adsorption. Chinese Chemical Letters, 2024, 35(8): 109166-. doi: 10.1016/j.cclet.2023.109166
Fenglin Wang , Chengwei Kuang , Zhicheng Zheng , Dan Wu , Hao Wan , Gen Chen , Ning Zhang , Xiaohe Liu , Renzhi Ma . Noble metal clusters substitution in porous Ni substrate renders high mass-specific activities toward oxygen evolution reaction and methanol oxidation reaction. Chinese Chemical Letters, 2025, 36(6): 109989-. doi: 10.1016/j.cclet.2024.109989
Gang Hu , Chun Wang , Qinqin Wang , Mingyuan Zhu , Lihua Kang . The controlled oxidation states of the H4PMo11VO40 catalyst induced by plasma for the selective oxidation of methacrolein. Chinese Chemical Letters, 2025, 36(2): 110298-. doi: 10.1016/j.cclet.2024.110298
Hao WANG , Kun TANG , Jiangyang SHAO , Kezhi WANG , Yuwu ZHONG . Electro-copolymerized film of ruthenium catalyst and redox mediator for electrocatalytic water oxidation. Chinese Journal of Inorganic Chemistry, 2024, 40(11): 2193-2202. doi: 10.11862/CJIC.20240176
Kexin Yin , Jingren Yang , Yanwei Li , Qian Li , Xing Xu . Metal-free diatomaceous carbon-based catalyst for ultrafast and anti-interference Fenton-like oxidation. Chinese Chemical Letters, 2024, 35(12): 109847-. doi: 10.1016/j.cclet.2024.109847
Meng Wang , Yan Zhang , Yunbo Yu , Wenpo Shan , Hong He . High-temperature calcination dramatically promotes the activity of Cs/Co/Ce-Sn catalyst for soot oxidation. Chinese Chemical Letters, 2025, 36(1): 109928-. doi: 10.1016/j.cclet.2024.109928
Fangwen Peng , Zhen Luo , Yingjin Ma , Haibo Ma . Theoretical study of aromaticity reversal in dimethyldihydropyrene derivatives. Chinese Journal of Structural Chemistry, 2024, 43(5): 100273-100273. doi: 10.1016/j.cjsc.2024.100273
Ling Tang , Yan Wan , Yangming Lin . Lowering the kinetic barrier via enhancing electrophilicity of surface oxygen to boost acidic oxygen evolution reaction. Chinese Journal of Structural Chemistry, 2024, 43(11): 100345-100345. doi: 10.1016/j.cjsc.2024.100345
Xiaoya Cui , Yanchang Liu , Qiang Li , He Zhu , Shibo Xi , Jianrong Zeng . Ultrafast crystallinity engineering of PtCo3 alloy for enhanced oxygen reduction reaction. Chinese Chemical Letters, 2025, 36(5): 110069-. doi: 10.1016/j.cclet.2024.110069
Jiayu Xu , Meng Li , Baoxia Dong , Ligang Feng . Fully fluorinated hybrid zeolite imidazole/Prussian blue analogs with combined advantages for efficient oxygen evolution reaction. Chinese Chemical Letters, 2024, 35(6): 108798-. doi: 10.1016/j.cclet.2023.108798
Qiyan Wu , Ruixin Zhou , Zhangyi Yao , Tanyuan Wang , Qing Li . Effective approaches for enhancing the stability of ruthenium-based electrocatalysts towards acidic oxygen evolution reaction. Chinese Chemical Letters, 2024, 35(10): 109416-. doi: 10.1016/j.cclet.2023.109416
Junan Pan , Xinyi Liu , Huachao Ji , Yanwei Zhu , Yanling Zhuang , Kang Chen , Ning Sun , Yongqi Liu , Yunchao Lei , Kun Wang , Bao Zang , Longlu Wang . The strategies to improve TMDs represented by MoS2 electrocatalytic oxygen evolution reaction. Chinese Chemical Letters, 2024, 35(11): 109515-. doi: 10.1016/j.cclet.2024.109515