Layered MoS2@graphene functionalized with nitrogen-doped graphene quantum dots as an enhanced electrochemical hydrogen evolution catalyst
-
* Corresponding author.
E-mail address: zhouxb@nwnu.edu.cn (X. Zhou)
Citation:
Guo Tong, Wang Lina, Sun Sen, Wang Yan, Chen Xiaoling, Zhang Kangning, Zhang Dongxia, Xue Zhonghua, Zhou Xibin. Layered MoS2@graphene functionalized with nitrogen-doped graphene quantum dots as an enhanced electrochemical hydrogen evolution catalyst[J]. Chinese Chemical Letters,
;2019, 30(6): 1253-1260.
doi:
10.1016/j.cclet.2019.02.009

Cancers still remain the first and second leading causes of death in economically developed and developing countries, respectively [1, 2]. It is expected that annual cancer cases will rise from 14 million in 2012 to 22 million within the next two decades [3]. Encouragingly, substantial progress has been made to develop more effective and safer strategies for treating cancers, and many classes of antitumor agents with diverse novel structures have been introduced in the market or under development in the past decades [4]. However, there continues to be a need for new antitumor drugs from either novel scaffolds or further structural modifications of existing drugs.
Quinolones represent an extremely successful family of antibiotics that have a broad-spectrum antibacterial activity and relatively few side effects [5]. Among of them, ciprofloxacin, ofloxacin and levofloxacin are also used as second-line drugs to treat tuberculosis [6]. Recently, quinolones as "privileged building blocks" have been reported to display many "nonclassical" biological profiles, such as antitumor, anti-HIV-1 integrase, anti-HCV-NS3 helicase and -NS5B-polymerase activities [7, 8]. It's exciting that Voreloxin (Fig. 1) with a broad-spectrum antitumor activity [9] the first quinolone antitumor drug, was approved as an orphan drug for the treatment of acute myeloid leukemia by the US FDA in 2009, and phase I-III clinical trials in patients with various tumors are currently ongoing [10].
In the course of our search for more potent antitumor agents, we focused our interest on structural modifications of Voreloxin. Given that 3-aminopyrrolidine, but not (3S, 4S)-3-methoxy-4-(methylamino) pyrrolidine, is commercially available, compound 1a (Fig. 1) possessing the in vitro antitumor activity comparable to Voreloxin [11] was chosen as the lead compound in our work. First, the thiazoly-2-yl group at the N-1 position of 1a was replaced with its isosteres (1H-imidazol-2-yl and oxazol-2-yl) to examine whether it is the optimal group of this position. On the other hand, our previous works have emphasized the importance of an oxime functional moiety of the C-7 side chain with respect to biological activities of quinolones [12-15]. Thus, a four, five-or sixmembered nitrogen heterocycle containing an alkoxyimino group was introduced at the C-7 position instead of the 3-aminopyrrolidin-1-yl one of 1a, to identify if a nitrogen heterocycle with an oxime moiety is permitted at this position. Our primary objective was to optimize the potency of these compounds against human cancer cell lines. A simple structure-activity relationship (SAR) study was also explored to facilitate the further development of the naphthyridinone derivatives.
To study the effect of various substituents at the N-1 position of the 7-(3-aminopyrrolidin-1-yl) naphthyridinone core, eight analogs of 1a were designed. A synthetic route to these compounds is illustrated in Scheme 1. The key intermediates 6a-i were readily prepared from 2, 6-dichloronicotinic acid (2) via a five-step procedure [14, 16]. Hydrolysis of the core esters 6a-d in HCl-HOAc followed by condensation with 3-N-Boc-aminopyrrolidine in the presence of triethylamine gave 8a-d. However, the esters 6e-i were not converted to the corresponding acids in a similar manner as for preparation of 8a-d. After various attempts, 8e-i were successfully obtained from 6e-i by condensation with the side chain compounds and then hydrolysis of the resulted esters 7e-i. The Boc-protecting group of 8a-i was removed by hydrogen chloride gas in dichloromethane to yield the desired naphthyridinone derivatives 1a-i as hydrochloric acid salts.
The synthetic route of novel 1-(thiazol-2-yl) naphthyridinones 10a-n containing nitrogen heterocycles with an alkoxyimino group was depicted in Scheme 2. Condensation of 6a with various side chain compounds [12, 17-19] and then hydrolysis of the resulted esters 9a-n yielded 10a-n.
Since the oxime group can exist in the E or Z configuration, it was necessary to determine the geometries of all the oxime target compounds 10a-n. Preparing X-ray quality single crystals of any oxime intermediate or product met with no success in this study, but the oxime geometry would be expected to have the E-configuration according to the data in published papers [20, 21]. The concrete synthetic procedures, the physical characteristics, and 1H NMR for all the synthesized compounds are listed in Supporting information.
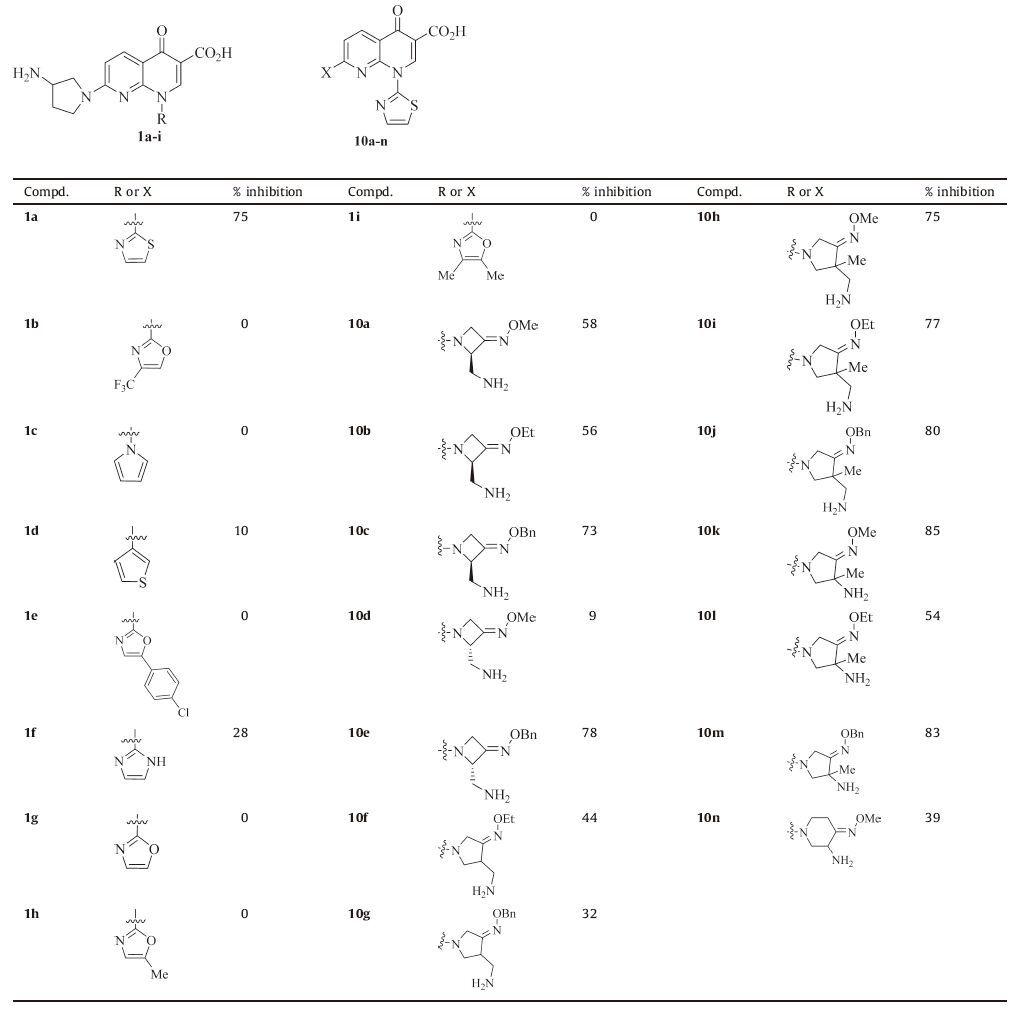
All the synthesized target compounds were preliminarily investigated for their in vitro activity against HL60 (leukemia) at the concentration of 30 μmol/L by SRB (Sulforhodamine B) assay [22]. And the compounds having >70% inhibition were subjected to IC50 (50% inhibition concentrations) determination against ten human cancer cell lines, including HL60, HepG2 (liver carcinoma), HCT-116 (colon cancer), A549 (lung adenocarcinoma), PANC-1 (pancreatic carcinoma), Hela (cervical cancer), DU145 (prostatic cancer), SKOV3 (ovarian carcinoma), MCF-7 (breast cancer) and MCF-7/DOX (Doxorubicin-resistant MCF-7) by SRB assay.
The inhibitory effects of the 7-(3-aminopyrrolidin-1-yl) naphthyridinone derivatives 1a-i and 1-(thiazol-2-yl) naphthyridinone derivatives 10a-n were evaluated and listed in Tables 1 and 2. Unfortunately, contrary to our predictions, the results indicated that all the newly synthesized 7-(3-aminopyrrolidin-1-yl) naphthyridinone derivatives 1b-i (inhibition rates: 0-28%) were much less active than the reference 1a (75%, Table 1). Therefore, our modifications are not effective, and the thiazol-2-yl group is optimal for the N-1 position.
 |
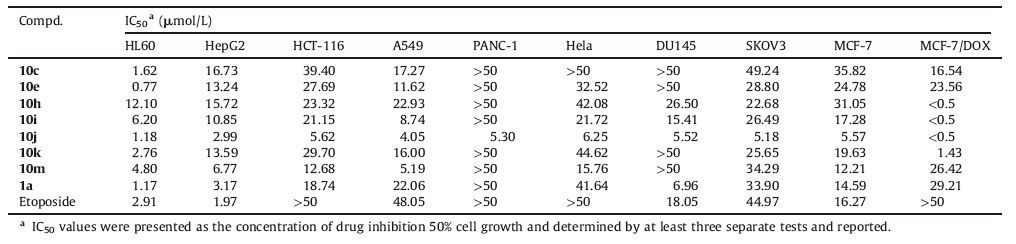 |
The IC50 values were compared with those of Etoposide and 1a (Table 2). The selected 1-(thiazol-2-yl) naphthyridinones have potent activity against these tested human cancer cell lines. All of them (IC50: < 0.5-49.23 μmol/L) are more active or comparable to Etoposide and 1a (IC50: 1.17 to >50 μmol/L) against HCT-116, A549, SKOV3 and MCF-7/DOX. Contrary to 1a, compounds 10h-j show significantly better activity against MCF-7/DOX (IC50: < 0.5 μmol/L) than MCF-7 (IC50: 5.57-31.04 μmol/L). Moreover, 10j was found to have a broad-spectrum activity (IC50: < 0.5-6.25 μmol/L) against all of the tested cell lines including Etoposide-and/or 1a -resistant ones, and is 1.3 to >100 fold more potent than those of the two references against these cell lines, except HL60 and HepG2.
According to the biological evaluation results shown in Tables 1 and 2, the antitumor activity of the naphthyridinone derivatives in this study depends on both of the groups at the N-1 and C-7 positions. The relative contribution of the heteroaromatic ring at the N-1 position to activity is as follows: thiazol-2-yl (1a) >> 1H-imidazol-2-yl (1f) >thiophen-3-yl (1d), and the others (1b, 1c, 1e, 1g-i) have no activity (Table 1).
On the other hand, 1-(thiazol-2-yl) naphthyridinones 10a-n generally exhibit in vitro activity (Table 1), which indicates that a nitrogen heterocycle with an oxime group is permitted at the C-7 position. Although a simple SAR is difficult to explore, the sizes of the heterocycle and the oxime group are important for the activity. For example, the activity imparted to the 7-(3-aminomethyl-3-methylpyrrolidin-1-yl) naphthyridinone ring by the alkyl group of the oxime moiety is as follows: benzyl > ethyl > methyl (10j vs.10i vs.10h), which suggests that simply increasing the lipophilicity could improve the activity (Table 2). Above all, thiazol-2-yl and 3-aminomethyl-4-benzyl oxyimino-3-methylpyrrolidin-1-yl groups are the most active at the N-1 and C-7 positions of naphthyridinone core, respectively.
In summary, structural modifications of compound 1a (a precursor of Voreloxin) at the N-1 position (various heteroaromatic rings instead of the thiazol-2-yl) and C-7 position (four-/ five-/six-membered nitrogen heterocyclic amine moieties with an oxime group instead of 3-aminopyrrolidin-1-yl one), respectively, were made in this study. Our results reveal that thiazol-2-yl and 3-aminomethyl-4-benzyloxyimino-3-methylpyrrolidin-1-yl groups are optimal at the N-1 and C-7 positions of naphthyridinone core, respectively. The most active compound 10j shows considerable broad-spectrum antitumor activity (IC50: < 0.5-6.25 μmol/L) against all of the tested cell lines including Etoposide-and/or 1a-resistant ones and is 1.3 to >100 fold more potent than those of the two references against these cell lines, except HL60 and HepG2.
This work was supported by National Key Research and Development Program (No. 2016YFA0201500), the National S & T Major Special Project on Major New Drug Innovations (Nos. 2014ZX09507009-003, 2015ZX09102007) and NSFC (Nos. 81373267, 21502237).
Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.cclet.2016.07.024.
J.A. Turner, Science 305(2004) 972-974.
doi: 10.1126/science.1103197
J.K. Norskov, C.H. Christensen, Science 312(2006) 1322-1323.
doi: 10.1126/science.1127180
F.E. Osterloh, Chem. Soc. Rev. 42(2013) 2294-2320.
doi: 10.1039/C2CS35266D
J.X. Feng, H. Xu, Y.T. Dong, et al., Angew. Chem. Int. Ed. 56(2017) 2960-2964.
doi: 10.1002/anie.201611767
J.X. Feng, J.Q. Wu, Y.X. Tong, et al., J. Am. Chem. Soc. 140(2018) 610-617.
doi: 10.1021/jacs.7b08521
J.X. Feng, S.Y. Tong, Y.X. Tong, et al., J. Am. Chem. Soc. 140(2018) 5118-5126.
doi: 10.1021/jacs.7b12968
M.A. Abbas, J.H. Bang, Chem. Mater. 27(2015) 7218-7235.
doi: 10.1021/acs.chemmater.5b03331
M.S. Faber, S. Jin, Energy Environ. Sci. 7(2014) 3519-3542.
doi: 10.1039/C4EE01760A
N.P. Sweeny, C.S. Rohrer, O.W. Brown, J. Am. Chem. Soc. 80(1958) 799-800.
doi: 10.1021/ja01537a012
J. Wang, F. Xu, H. Jin, et al., Adv. Mater. 29(2017) 1605838.
doi: 10.1002/adma.v29.14
H. Vrubel, X. Hu, Angew. Chem. 124(2012) 12875-12878.
doi: 10.1002/ange.v124.51
W.F. Chen, J.T. Muckerman, E. Fujita, Chem. Commun. 49(2013) 8896-8909.
doi: 10.1039/c3cc44076a
Z. Chen, D. Higgins, A. Yu, et al., Energy Environ. Sci. 4(2011) 3167-3192.
doi: 10.1039/c0ee00558d
X. Zou, Y. Zhang, Chem. Soc. Rev. 44(2015) 5148-5180.
doi: 10.1039/C4CS00448E
J. Xie, H. Zhang, S. Li, et al., Adv. Mater. 25(2013) 5807-5813.
doi: 10.1002/adma.v25.40
A.B. Laursen, S. Kegnæs, S. Dahl, et al., Energy Environ. Sci. 5(2012) 5577-5591.
doi: 10.1039/c2ee02618j
L. Ma, Y. Hu, G. Zhu, et al., Chem. Mater. 28(2016) 5733-5742.
doi: 10.1021/acs.chemmater.6b01980
X. Xu, Y. Sun, W. Qiao, et al., Appl. Surf. Sci. 396(2017) 1520-1527.
doi: 10.1016/j.apsusc.2016.11.201
S. Reddy, R. Du, L. Kang, et al., Appl. Catal. B: Environ. 194(2016) 16-21.
doi: 10.1016/j.apcatb.2016.04.007
H. Zhu, M.L. Du, M. Zhang, et al., Chem. Commun. 50(2014) 015435-015438.
doi: 10.1039/C4CC06480A
B. Guo, K. Yu, H. Li, et al., ACS Appl. Mater. Interfaces 9(2017) 3653-3660.
doi: 10.1021/acsami.6b14035
Z.C. Xiang, Z. Zhang, X.J. Xu, et al., Carbon 98(2016) 84-89.
doi: 10.1016/j.carbon.2015.10.071
I.S. Amiinu, Z. Pu, X. Liu, et al., Adv. Func. Mater. 27(2017) 1702300.
doi: 10.1002/adfm.v27.44
D. He, Y. Xiong, J. Yang, et al., J. Mater. Chem. A 5(2017) 1930-1934.
doi: 10.1039/C5TA09232A
V.N. Patel, N. Tandon, R.K. Pandey, Procedia Technol. 14(2014) 312-319.
doi: 10.1016/j.protcy.2014.08.041
D.J. Li, U.N. Maiti, J. Lim, et al., Nano Lett. 14(2014) 1228-1233.
doi: 10.1021/nl404108a
K.J.Huang, L.Wang, Y.J.Liu, et al., Inter. J. Hydrogen Energy 38(2013) 14027-14034.
doi: 10.1016/j.ijhydene.2013.08.112
J. Zhang, L. Zhao, A. Liu, et al., Electrochim. Acta 182(2015) 652-658.
doi: 10.1016/j.electacta.2015.09.147
W. Zhou, K. Zhou, D. Hou, et al., ACS Appl. Mater. Interfaces 6(2014) 21534-21540.
doi: 10.1021/am506545g
L. Zhao, C. Hong, L. Lin, et al., Carbon 116(2017) 223-231.
doi: 10.1016/j.carbon.2017.02.010
J. Sun, L. Li, X. Zhang, et al., RSC Adv. 5(2015) 11925-11932.
doi: 10.1039/C4RA13857K
Q. Lian, Z. He, Q. He, et al., Anal. Chim. Acta 823(2014) 32-39.
doi: 10.1016/j.aca.2014.03.032
X. Zhou, Z. He, Q. Lian, et al., Sensor. Actuat. B-Chem. 193(2014) 198-204.
doi: 10.1016/j.snb.2013.11.085
Y. Duan, T. Zeng, T. Sun, et al., Nano 13(2018) 1830003.
doi: 10.1142/S1793292018300037
L.A.Ponomarenko, F.Schedin, M.I.Katsnelson, et al., Science 320(2008) 356-358.
doi: 10.1126/science.1154663
S. Zhu, Y. Song, J. Wang, et al., Nano Today 13(2017) 10-14.
doi: 10.1016/j.nantod.2016.12.006
W.S. Kuo, Y.T. Shao, K.S. Huang, et al., ACS Appl. Mater. Interfaces 10(2018) 14438-14446.
doi: 10.1021/acsami.8b01429
W.A. Saidi, J. Phys. Chem. Lett. 4(2013) 4160-4165.
doi: 10.1021/jz402090d
Q. Chen, Y. Hu, C. Hu, et al., Phys. Chem. Chem. Phys. 16(2014) 19307-19313.
doi: 10.1039/C4CP02761B
K. Lee, H. Lee, Y. Shin, et al., Nano Energy 26(2016) 746-754.
doi: 10.1016/j.nanoen.2016.06.030
R. Vinoth, I.M. Patil, A. Pandikumar, et al., ACS Omega 1(2016) 971-980.
doi: 10.1021/acsomega.6b00275
D. Qu, M. Zheng, J. Li, et al., Light-Sci. Appl. 4(2015) e364.
doi: 10.1038/lsa.2015.137
X. Zheng, J. Xu, K. Yan, et al., Chem. Mater. 26(2014) 2344-2353.
doi: 10.1021/cm500347r
L. Ma, J. Ye, W. Chen, et al., Nano Energy 10(2014) 144-152.
doi: 10.1016/j.nanoen.2014.09.006
S.Y. Tai, C.J. Liu, S.W. Chou, et al., J. Mater. Chem. 22(2012) 24753-24759.
doi: 10.1039/c2jm35447k
D. Kong, H. Wang, J.J. Cha, et al., Nano Lett. 13(2013) 1341-1347.
doi: 10.1021/nl400258t
K.K. Liu, W. Zhang, Y.H. Lee, et al., Nano Lett. 12(2012) 1538-1544.
doi: 10.1021/nl2043612
Y. Zhai, Y. Dou, D. Zhao, et al., Adv. Mater. 23(2011) 4828-4850.
doi: 10.1002/adma.201100984
A. Thomas, A. Fischer, F. Goettmann, et al., J. Mater. Chem. 18(2008) 4893-4908.
doi: 10.1039/b800274f
H. Zhu, M.L. Du, M. Zhang, et al., Chem. Commun. 50(2014) 15435-15438.
doi: 10.1039/C4CC06480A
J. Kibsgaard, Z. Chen, B.N. Reinecke, et al., Nat. Mater. 11(2012) 963-969.
doi: 10.1038/nmat3439
J.D. Benck, Z. Chen, L.Y. Kuritzky, et al., ACS Catal. 2(2012) 1916-1923.
doi: 10.1021/cs300451q
Z. Xing, Q. Liu, A.M. Asiri, et al., ACS Catal. 5(2014) 145-149.
Z. Huang, W. Luo, L. Ma, et al., Angew. Chem. 127(2015) 15396-15400.
doi: 10.1002/ange.201507529
J. Bonde, P.G. Moses, T.F. Jaramillo, et al., Faraday Discuss. 140(2009) 219-231.
doi: 10.1039/B803857K
M.A. Lukowski, A.S. Daniel, F. Meng, et al., J. Am. Chem. Soc. 135(2013) 10274-10277.
doi: 10.1021/ja404523s
J.A. Turner, Science 305(2004) 972-974.
doi: 10.1126/science.1103197
J.K. Norskov, C.H. Christensen, Science 312(2006) 1322-1323.
doi: 10.1126/science.1127180
F.E. Osterloh, Chem. Soc. Rev. 42(2013) 2294-2320.
doi: 10.1039/C2CS35266D
J.X. Feng, H. Xu, Y.T. Dong, et al., Angew. Chem. Int. Ed. 56(2017) 2960-2964.
doi: 10.1002/anie.201611767
J.X. Feng, J.Q. Wu, Y.X. Tong, et al., J. Am. Chem. Soc. 140(2018) 610-617.
doi: 10.1021/jacs.7b08521
J.X. Feng, S.Y. Tong, Y.X. Tong, et al., J. Am. Chem. Soc. 140(2018) 5118-5126.
doi: 10.1021/jacs.7b12968
M.A. Abbas, J.H. Bang, Chem. Mater. 27(2015) 7218-7235.
doi: 10.1021/acs.chemmater.5b03331
M.S. Faber, S. Jin, Energy Environ. Sci. 7(2014) 3519-3542.
doi: 10.1039/C4EE01760A
N.P. Sweeny, C.S. Rohrer, O.W. Brown, J. Am. Chem. Soc. 80(1958) 799-800.
doi: 10.1021/ja01537a012
J. Wang, F. Xu, H. Jin, et al., Adv. Mater. 29(2017) 1605838.
doi: 10.1002/adma.v29.14
H. Vrubel, X. Hu, Angew. Chem. 124(2012) 12875-12878.
doi: 10.1002/ange.v124.51
W.F. Chen, J.T. Muckerman, E. Fujita, Chem. Commun. 49(2013) 8896-8909.
doi: 10.1039/c3cc44076a
Z. Chen, D. Higgins, A. Yu, et al., Energy Environ. Sci. 4(2011) 3167-3192.
doi: 10.1039/c0ee00558d
X. Zou, Y. Zhang, Chem. Soc. Rev. 44(2015) 5148-5180.
doi: 10.1039/C4CS00448E
J. Xie, H. Zhang, S. Li, et al., Adv. Mater. 25(2013) 5807-5813.
doi: 10.1002/adma.v25.40
A.B. Laursen, S. Kegnæs, S. Dahl, et al., Energy Environ. Sci. 5(2012) 5577-5591.
doi: 10.1039/c2ee02618j
L. Ma, Y. Hu, G. Zhu, et al., Chem. Mater. 28(2016) 5733-5742.
doi: 10.1021/acs.chemmater.6b01980
X. Xu, Y. Sun, W. Qiao, et al., Appl. Surf. Sci. 396(2017) 1520-1527.
doi: 10.1016/j.apsusc.2016.11.201
S. Reddy, R. Du, L. Kang, et al., Appl. Catal. B: Environ. 194(2016) 16-21.
doi: 10.1016/j.apcatb.2016.04.007
H. Zhu, M.L. Du, M. Zhang, et al., Chem. Commun. 50(2014) 015435-015438.
doi: 10.1039/C4CC06480A
B. Guo, K. Yu, H. Li, et al., ACS Appl. Mater. Interfaces 9(2017) 3653-3660.
doi: 10.1021/acsami.6b14035
Z.C. Xiang, Z. Zhang, X.J. Xu, et al., Carbon 98(2016) 84-89.
doi: 10.1016/j.carbon.2015.10.071
I.S. Amiinu, Z. Pu, X. Liu, et al., Adv. Func. Mater. 27(2017) 1702300.
doi: 10.1002/adfm.v27.44
D. He, Y. Xiong, J. Yang, et al., J. Mater. Chem. A 5(2017) 1930-1934.
doi: 10.1039/C5TA09232A
V.N. Patel, N. Tandon, R.K. Pandey, Procedia Technol. 14(2014) 312-319.
doi: 10.1016/j.protcy.2014.08.041
D.J. Li, U.N. Maiti, J. Lim, et al., Nano Lett. 14(2014) 1228-1233.
doi: 10.1021/nl404108a
K.J.Huang, L.Wang, Y.J.Liu, et al., Inter. J. Hydrogen Energy 38(2013) 14027-14034.
doi: 10.1016/j.ijhydene.2013.08.112
J. Zhang, L. Zhao, A. Liu, et al., Electrochim. Acta 182(2015) 652-658.
doi: 10.1016/j.electacta.2015.09.147
W. Zhou, K. Zhou, D. Hou, et al., ACS Appl. Mater. Interfaces 6(2014) 21534-21540.
doi: 10.1021/am506545g
L. Zhao, C. Hong, L. Lin, et al., Carbon 116(2017) 223-231.
doi: 10.1016/j.carbon.2017.02.010
J. Sun, L. Li, X. Zhang, et al., RSC Adv. 5(2015) 11925-11932.
doi: 10.1039/C4RA13857K
Q. Lian, Z. He, Q. He, et al., Anal. Chim. Acta 823(2014) 32-39.
doi: 10.1016/j.aca.2014.03.032
X. Zhou, Z. He, Q. Lian, et al., Sensor. Actuat. B-Chem. 193(2014) 198-204.
doi: 10.1016/j.snb.2013.11.085
Y. Duan, T. Zeng, T. Sun, et al., Nano 13(2018) 1830003.
doi: 10.1142/S1793292018300037
L.A.Ponomarenko, F.Schedin, M.I.Katsnelson, et al., Science 320(2008) 356-358.
doi: 10.1126/science.1154663
S. Zhu, Y. Song, J. Wang, et al., Nano Today 13(2017) 10-14.
doi: 10.1016/j.nantod.2016.12.006
W.S. Kuo, Y.T. Shao, K.S. Huang, et al., ACS Appl. Mater. Interfaces 10(2018) 14438-14446.
doi: 10.1021/acsami.8b01429
W.A. Saidi, J. Phys. Chem. Lett. 4(2013) 4160-4165.
doi: 10.1021/jz402090d
Q. Chen, Y. Hu, C. Hu, et al., Phys. Chem. Chem. Phys. 16(2014) 19307-19313.
doi: 10.1039/C4CP02761B
K. Lee, H. Lee, Y. Shin, et al., Nano Energy 26(2016) 746-754.
doi: 10.1016/j.nanoen.2016.06.030
R. Vinoth, I.M. Patil, A. Pandikumar, et al., ACS Omega 1(2016) 971-980.
doi: 10.1021/acsomega.6b00275
D. Qu, M. Zheng, J. Li, et al., Light-Sci. Appl. 4(2015) e364.
doi: 10.1038/lsa.2015.137
X. Zheng, J. Xu, K. Yan, et al., Chem. Mater. 26(2014) 2344-2353.
doi: 10.1021/cm500347r
L. Ma, J. Ye, W. Chen, et al., Nano Energy 10(2014) 144-152.
doi: 10.1016/j.nanoen.2014.09.006
S.Y. Tai, C.J. Liu, S.W. Chou, et al., J. Mater. Chem. 22(2012) 24753-24759.
doi: 10.1039/c2jm35447k
D. Kong, H. Wang, J.J. Cha, et al., Nano Lett. 13(2013) 1341-1347.
doi: 10.1021/nl400258t
K.K. Liu, W. Zhang, Y.H. Lee, et al., Nano Lett. 12(2012) 1538-1544.
doi: 10.1021/nl2043612
Y. Zhai, Y. Dou, D. Zhao, et al., Adv. Mater. 23(2011) 4828-4850.
doi: 10.1002/adma.201100984
A. Thomas, A. Fischer, F. Goettmann, et al., J. Mater. Chem. 18(2008) 4893-4908.
doi: 10.1039/b800274f
H. Zhu, M.L. Du, M. Zhang, et al., Chem. Commun. 50(2014) 15435-15438.
doi: 10.1039/C4CC06480A
J. Kibsgaard, Z. Chen, B.N. Reinecke, et al., Nat. Mater. 11(2012) 963-969.
doi: 10.1038/nmat3439
J.D. Benck, Z. Chen, L.Y. Kuritzky, et al., ACS Catal. 2(2012) 1916-1923.
doi: 10.1021/cs300451q
Z. Xing, Q. Liu, A.M. Asiri, et al., ACS Catal. 5(2014) 145-149.
Z. Huang, W. Luo, L. Ma, et al., Angew. Chem. 127(2015) 15396-15400.
doi: 10.1002/ange.201507529
J. Bonde, P.G. Moses, T.F. Jaramillo, et al., Faraday Discuss. 140(2009) 219-231.
doi: 10.1039/B803857K
M.A. Lukowski, A.S. Daniel, F. Meng, et al., J. Am. Chem. Soc. 135(2013) 10274-10277.
doi: 10.1021/ja404523s

Ke Wang , Jia Wu , Shuyi Zheng , Shibin Yin . NiCo Alloy Nanoparticles Anchored on Mesoporous Mo2N Nanosheets as Efficient Catalysts for 5-Hydroxymethylfurfural Electrooxidation and Hydrogen Generation. Chinese Journal of Structural Chemistry, 2023, 42(10): 100104-100104. doi: 10.1016/j.cjsc.2023.100104
Haibin Yang , Duowen Ma , Yang Li , Qinghe Zhao , Feng Pan , Shisheng Zheng , Zirui Lou . Mo doped Ru-based cluster to promote alkaline hydrogen evolution with ultra-low Ru loading. Chinese Journal of Structural Chemistry, 2023, 42(11): 100031-100031. doi: 10.1016/j.cjsc.2023.100031
Ziyang Yin , Lingbin Xie , Weinan Yin , Ting Zhi , Kang Chen , Junan Pan , Yingbo Zhang , Jingwen Li , Longlu Wang . Advanced development of grain boundaries in TMDs from fundamentals to hydrogen evolution application. Chinese Chemical Letters, 2024, 35(5): 108628-. doi: 10.1016/j.cclet.2023.108628
Ping Wang , Ting Wang , Ming Xu , Ze Gao , Hongyu Li , Bowen Li , Yuqi Wang , Chaoqun Qu , Ming Feng . Keplerate polyoxomolybdate nanoball mediated controllable preparation of metal-doped molybdenum disulfide for electrocatalytic hydrogen evolution in acidic and alkaline media. Chinese Chemical Letters, 2024, 35(7): 108930-. doi: 10.1016/j.cclet.2023.108930
Huyi Yu , Renshu Huang , Qian Liu , Xingfa Chen , Tianqi Yu , Haiquan Wang , Xincheng Liang , Shibin Yin . Te-doped Fe3O4 flower enabling low overpotential cycling of Li-CO2 batteries at high current density. Chinese Journal of Structural Chemistry, 2024, 43(3): 100253-100253. doi: 10.1016/j.cjsc.2024.100253
Caili Yang , Tao Long , Ruotong Li , Chunyang Wu , Yuan-Li Ding . Pseudocapacitance dominated Li3VO4 encapsulated in N-doped graphene via 2D nanospace confined synthesis for superior lithium ion capacitors. Chinese Chemical Letters, 2025, 36(2): 109675-. doi: 10.1016/j.cclet.2024.109675
Sanmei Wang , Yong Zhou , Hengxin Fang , Chunyang Nie , Chang Q Sun , Biao Wang . Constant-potential simulation of electrocatalytic N2 reduction over atomic metal-N-graphene catalysts. Chinese Chemical Letters, 2025, 36(3): 110476-. doi: 10.1016/j.cclet.2024.110476
Cheng Guo , Xiaoxiao Zhang , Xiujuan Hong , Yiqiu Hu , Lingna Mao , Kezhi Jiang . Graphene as adsorbent for highly efficient extraction of modified nucleosides in urine prior to liquid chromatography-tandem mass spectrometry analysis. Chinese Chemical Letters, 2024, 35(4): 108867-. doi: 10.1016/j.cclet.2023.108867
Sanmei Wang , Dengxin Yan , Wenhua Zhang , Liangbing Wang . Graphene-supported isolated platinum atoms and platinum dimers for CO2 hydrogenation: Catalytic activity and selectivity variations. Chinese Chemical Letters, 2025, 36(4): 110611-. doi: 10.1016/j.cclet.2024.110611
Wenjing Xiong , Yulin Xu , Fangzhou Zhao , Baokai Xia , Hongqiang Wang , Wei Liu , Sheng Chen , Yongzhi Zhang . Graphene architecture interpenetrated with mesoporous carbon nanosheets promotes fast and stable potassium storage. Chinese Chemical Letters, 2025, 36(4): 109738-. doi: 10.1016/j.cclet.2024.109738
Huining Zhang , Baixiang Wang , Jianping Han , Shaofeng Wang , Xingmao Liu , Wenhui Niu , Zhongyu Shi , Zhiqiang Wei , Zhiguo Wu , Ying Zhu , Qi Guo . Nature’s revelation: Preparation of Graphene-based Biomimetic materials and its application prospects for water purification. Chinese Chemical Letters, 2025, 36(6): 110319-. doi: 10.1016/j.cclet.2024.110319
Xiao Li , Wanqiang Yu , Yujie Wang , Ruiying Liu , Qingquan Yu , Riming Hu , Xuchuan Jiang , Qingsheng Gao , Hong Liu , Jiayuan Yu , Weijia Zhou . Metal-encapsulated nitrogen-doped carbon nanotube arrays electrode for enhancing sulfion oxidation reaction and hydrogen evolution reaction by regulating of intermediate adsorption. Chinese Chemical Letters, 2024, 35(8): 109166-. doi: 10.1016/j.cclet.2023.109166
Xiangyuan Zhao , Jinjin Wang , Jinzhao Kang , Xiaomei Wang , Hong Yu , Cheng-Feng Du . Ni nanoparticles anchoring on vacuum treated Mo2TiC2Tx MXene for enhanced hydrogen evolution activity. Chinese Journal of Structural Chemistry, 2023, 42(10): 100159-100159. doi: 10.1016/j.cjsc.2023.100159
Xinyu Hou , Xuelian Yu , Meng Liu , Hengxing Peng , Lijuan Wu , Libing Liao , Guocheng Lv . Ultrafast synthesis of Mo2N with highly dispersed Ru for efficient alkaline hydrogen evolution. Chinese Chemical Letters, 2025, 36(4): 109845-. doi: 10.1016/j.cclet.2024.109845
Zhuo Li , Peng Yu , Di Shen , Xinxin Zhang , Zhijian Liang , Baoluo Wang , Lei Wang . Low-loading Pt anchored on molybdenum carbide-based polyhedral carbon skeleton for enhancing pH-universal hydrogen production. Chinese Chemical Letters, 2025, 36(4): 109713-. doi: 10.1016/j.cclet.2024.109713
Jinli Chen , Shouquan Feng , Tianqi Yu , Yongjin Zou , Huan Wen , Shibin Yin . Modulating Metal-Support Interaction Between Pt3Ni and Unsaturated WOx to Selectively Regulate the ORR Performance. Chinese Journal of Structural Chemistry, 2023, 42(10): 100168-100168. doi: 10.1016/j.cjsc.2023.100168
Renshu Huang , Jinli Chen , Xingfa Chen , Tianqi Yu , Huyi Yu , Kaien Li , Bin Li , Shibin Yin . Synergized oxygen vacancies with Mn2O3@CeO2 heterojunction as high current density catalysts for Li–O2 batteries. Chinese Journal of Structural Chemistry, 2023, 42(11): 100171-100171. doi: 10.1016/j.cjsc.2023.100171
Xiaoli Deng , Xiangchao Lu , Yang Cao , Qianjin Chen . Electrochemical imaging uncovers the heterogeneity of HER activity by sulfur vacancies in molybdenum disulfide monolayer. Chinese Chemical Letters, 2025, 36(3): 110379-. doi: 10.1016/j.cclet.2024.110379
Rui Deng , Wenjie Jiang , Tianqi Yu , Jiali Lu , Boyao Feng , Panagiotis Tsiakaras , Shibin Yin . Cycad-leaf-like crystalline-amorphous heterostructures for efficient urea oxidation-assisted water splitting. Chinese Journal of Structural Chemistry, 2024, 43(7): 100290-100290. doi: 10.1016/j.cjsc.2024.100290
Wenjie Jiang , Zhixiang Zhai , Xiaoyan Zhuo , Jia Wu , Boyao Feng , Tianqi Yu , Huan Wen , Shibin Yin . Revealing the reactant adsorption role of high-valence WO3 for boosting urea-assisted water splitting. Chinese Journal of Structural Chemistry, 2025, 44(3): 100519-100519. doi: 10.1016/j.cjsc.2025.100519