Self-assembly of homopolymer of PAA-NH4
-
* Corresponding authors.
E-mail addresses: zhaoning@iccas.ac.cn (N. Zhao), jxu@iccas.ac.cn (J. Xu)
Citation:
Sun Tongbing, Yang Xiaoli, Zhu Caizhen, Zhao Ning, Dong Haixia, Xu Jian. Self-assembly of homopolymer of PAA-NH4[J]. Chinese Chemical Letters,
;2019, 30(2): 477-480.
doi:
10.1016/j.cclet.2018.07.014

Phase-transition materials have recently been paid substantial attention because of their potential applications in tropical regions, such as construction of telecom shelters, production of switchable dielectric devices, and enhanced data storage, sensing, signal processing and so on. Triggered by external stimuli like temperature, pressure, or light, they usually transform as temperature changes, accompanied by the dramatic variations of some of their physical properties, such as dielectric anomaly, ferroelectricity, and ferromagnetism[1-6]. Althoughmuch progress has been achieved on the design of specific molecular packing, synthesizing new molecularbased phase-transition materials is not easy due to the difficulty in achieving balance among the noncovalent (van derWaals, hydrogen bonding, π-π packing, etc.) interactions in the compound [7-11]. Therefore, various approaches have been developed to construct molecular-based phase-transition materials, the most efficient of which is to introduce changeable molecular modules that reorient themselves with changing temperature (examples include metalorganic framework, inorganic acid, and polyoxometalate). Recent studies on phase-transition materials in organic and inorganic hybrid complexes are of great interest in the exploration of novel physical properties, as well as in the understanding of structureproperty relationships. Structural phase transitions usually include reconstructive phase transition, proton-transfer phase transition, order-disorder phase transition, and atom displacement phase transition. Among these, molecular-motion/atomic order-disorder phase transition, which can generate large deformations coupled with functional dielectric or ferroelectric properties, plays an important role in photo-electronic fields. For example, Xiong et al. have obtained one class of attractive phase-transition materials, [(CH3)4P][FeX4] (X=Cl- and Br-) that simultaneously display dielectric, magnetic, and optical properties, accompanied by sequential phase transitions above room temperature [12-16].
Inorganic-organic hybrid compounds with molecular dielectrics have been investigated for the explorations of ideal phasetransition materials. In molecular-based crystal structure, the moieties with smaller volume and higher intramolecular symmetry are prone to reorientation due to their lower energy barrier. During the past decade, crown ethers were considered as ideal candidates for the movement of molecular stators in designing phase change materials and ferroelectric molecular materials. For instance, 18-crown-6 macrocyclic ether can accommodate multiple N-H…O strong H-bonds, and easily anchor the protonated R-NH3+ cation (R=aryl ring) into the cavity of 18-crown-6. On the other hand, inorganic anions like ClO4-, BF4 -, and IO4 - are stable and capable of changing position with varying temperature and weak interactions in crystals because of their moderate size and highly symmetric shape. When the temperature decreases, reorientation of inorganic or organicmoietiesmay cause breakage in symmetry, thus, causing a phase transition. However, some phase-transition materials do not undergo space group change and symmetry change, such as [C6H14N2][(CrO3Cl) Cl], (4-ethoxyanilinium) ([18]-crown-6)[Ni (dmit)2], and (DABCO) Cl (H2O)2 [17-20]. This kind of phase transition is common with the varying changes in physical properties, such as specific heat and dielectric constant [21-23]. Taking all these factors into consideration, our emphasis was to investigate the effects of suitable structural variations on the crystal structures and physical properties like phase transition properties, dielectric behaviors, and melting points, and thus, to obtain multifunctional phasetransition materials that are more suitable for practical applications. We have already reported that a one-dimensional supramolecular hybrid compound of [(4-nitroanilinium)(18-crown-6)][BF4](CH3CN) exhibited a strong dielectric response above 300 K, in which the molecular structure is formed by the N-H…O contacts made through the cavity of 18-crown-6. As a continuation of our systematic studies, we report here the introduction of an inorganic-organic supramolecule with 3-nitroanilinium and 18-crown-6 to IO4 - salts, [(3-nitroanilinium+)(18-crown-6)][IO4 -](CH3OH) (1). As expected, the structural modifications led to the formation of distinct crystal structures, H-bond conformations, reversible structural phase transition temperatures, and manifestation of dielectric properties. Compound 1 underwent reversible phase transition at around 220 K due to the order-disorder rotation of methanol molecules. In addition, the details of differential scanning calorimetry (DSC), single crystal Xray diffraction, rotation-potential-energy calculations and dielectric constant measurements are discussed.
General: Elemental analyses were carried out using a vario EL Elementar Analysensysteme GmbH at the TRW Research Collaboration Center, YanZhou, Shandong. IR spectra (400-4000 cm-1) were measured using an Affinity-1 spectrophotometer (Fig. S1 in Supporting information). Thermogravimetric analysis was carried out using a SHIMADZU DTG-60 thermal analysis station with Al2O3 as reference, within the temperature range of 300-900 K, and a heating rate of 8 K min-1 under flowing nitrogen (Fig. S2 in Supporting information). DSC measurements were performed by heating and cooling the samples (16 mg) within the temperature range of 210-280 K on a TA Q2000 DSC instrument under nitrogen conditions at atmospheric pressure in aluminum crucibles, and with a heating rate of 5 K min-1. Crystal dielectric constantsweremeasured with TH2828 Precision LCR meter using the frequency range of 500 Hz to 1 MHz, an applied voltage of 1.0 V, and an approximate temperature sweeping rate of 2 K min-1.
Preparation of crystal [(3-nitroanilinium+)(18-crown-6)][IO4 -] (CH3OH) (1): Single crystal of 1 were prepared through the slow evaporation of a mixture containing HIO4 (50 mg), 3-nitroaniline (20 mg), and 18-crown-6 (200 mg) in methanol (50 mL), as shown in Scheme 1. The methanol solution was allowed to stand for approximately five days under r.t. conditions. The single crystals of salt 1 were yellow block crystals obtained with a 54% yield. The chemical formulas of salt 1 were determined using elemental and X-ray crystallographic analyses. Anal. Calcd. C37H66IN4O25 for salt 1: C, 36.37%; H, 5.26%; N, 4.71%. Found: C, 36.28%; H, 5.13%; N, 4.62%.
Single crystal X-ray diffraction: Crystallographic data for single crystal 1 were collected at 100 and 296 K, respectively, using a Bruker AXS CCD acre-detector diffractometer with a graphite monochromatic Mo Kα radiation (λ=0.71073 Å). Structure refinements were made using the full-matrix least-squares method on F2. The parameters were refined using anisotropic temperature factors, except for the hydrogen atoms, which were refined using the riding model with a fixed C-H bond distance of 0.95 Å [24, 25]. The crystallographic data for salts 1 at 100 and 296 K are summarized in Table 1.
 |
Calculation of rotational potential energy: The relative energies of the structures were calculated using a semi-empirical method with the RHF/6-31(d) basis set [26]. The nearest-neighboring molecules around the 3-nitroanilinium+ and methanol molecule were included in the calculation. The structural units of salt 1 used in the calculation were (3-nitroanilinium+)2(18-crown-6)2(IO4 -)2 and (CH3OH)(18-crown-6)2(IO4 -)2 (Figs. S4 and S5 in Supporting information). The atomic coordinates of salt 1 based on the X-ray crystal structural analysis at low temperature were used in the calculations.
To detect whether a compound displays a phase transition that is triggered by temperature, DSC measurements are usually performed to confirm the existence of heat anomaly during the heating and cooling processes. When a compound undergoes a structural phase transition accompanied by thermal entropy changes, heat anomalies can be observed. When the compound is subjected to order-disorder transitions of molecules or ions, reversible heat anomalies can be detected by DSC upon heating and cooling. DSC results of the compound shows mainly an endothermic peak at approximately 226 K upon heating, and mainly an exothermic peak upon cooling at 216 K, indicating that 1 exhibits a reversible phase transition at around 220 K (Fig. 1). The result is in good agreement with the TGA/DTA measurement, with the title compound decomposing at 410 K (Fig. S3 in Supporting information). The entropy change (ΔS) of the phase transition is estimated to be 2.10 J mol-1 K-1, and the thermal hysteresis is about 10 K based on the DSC results. According to the Boltzmann equation, which provided that ΔS=R lnN, where N represents the ratio of possible configurations and R is the gas constant. We calculated N=1.12, suggesting that the phase transition is an order-disorder type [27, 28].
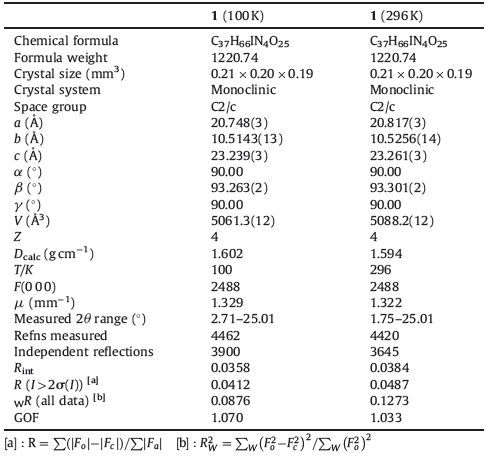
The phase transition of compound 1 was confirmed through the determination of low-and room-temperature crystal structures used to investigate the structural changes during the process of phase transition. Crystallographic data, and details of collection and refinement at 100 K and 296 K are listed in Table 1. The packing diagram for crystal 1 along the b-axis at 100 K is exhibited in Fig. 2. Anionic IO4 - and (3-nitroanilinium+)(18-crown-6) cationic layers are arranged alternately along the a + c-axes (Fig. S3). In the cationic layers, the 3-nitroanilinium and 18-crown-6 formed 1:1 adducts of supramolecular cations through N-H…O hydrogen bonding, and by using a stand-up configuration of the C-N bond of 3-nitroanilinium with respect to the mean plane of the six oxygen atoms in 18-crown-6. The conformation of a CH3OH molecule in crystal 1 shows an orientation disorder caused by the in-plane rotation of CH3OH molecules at low-and room-temperatures (Fig. 3). The distances between the nitrogen atoms of the 3-nitroanilinium, and six oxygen atoms of 18-crown-6 of compound 1 at 100 and 296 K are summarized in Table 2. The average distances between the nitrogen of the cation and the oxygen atoms of the crown ether of crystal 1 were 2.907 and 2.905 Å at 296 and 100 K, respectively, indicating the formation of typical hydrogen bonding. Aside from the aforementioned six N-H…O hydrogen bonds, a seventh hydrogen bond exists between the -NH3 + group of 3-nitroanilinium and adjacent oxygen atoms of CH3OH molecular interaction through the cavity of 18-crown-6. The structural unit of compound 1 contains two 3-nitroanilinium, two 18-crown-6 ethers, and one CH3OH molecule. We divided the structural unit into two parts to facilitate the explanation of the formation of the seventh hydrogen bond. Apparently, two supramolecular cations, 3-nitroanilinium+ and 18-crown-6, are linked by a methanol molecule. This linkage results in a new, one-dimensional N-H…O bonded chain structure along the a + c-axes (Fig. 4). However, the distance between donor (N1) of 3-nitroanilinium and acceptor atoms (O13) of CH3OH molecules is almost identical (N1-H … O13 hydrogen bonding, 3.052 and 3.060 Å at 296 and 100 K, respectively). These results indicate that the H protons are hardly transferred in a one-dimensional array along the a + c-axes.
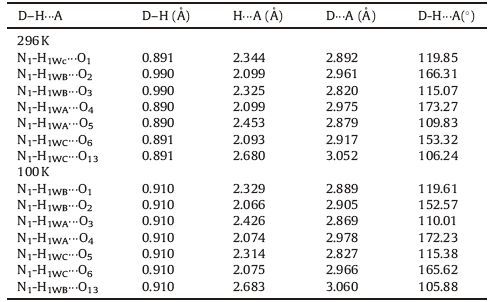 |
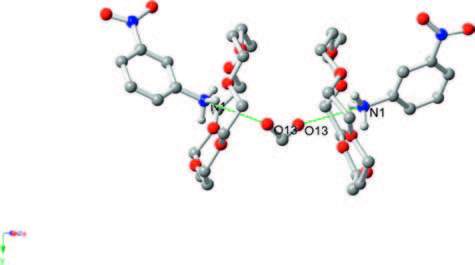
Previously, we gained insight into the structure of compound 1 at low and room temperatures. At different temperatures, the crystal structures of 1 are both monoclinic with the space grouping C2/c and with almost no change in the cell parameters. To determine the relationship between phase transition and the order-disorder rotation of methanol molecules, we evaluated the calculated potential-energy value for the rotation of the phenyl ring and the methanol molecules in the space of two 18-crown-6 molecules. The neighboring IO4 - anions and 18-crown-6 molecules were included in the calculation model due to the strong atom contacts observed in these molecules, as shown in Fig. S4 in Supporting information. Relative energies as a function of the rotational angle (φ) around the C-NH3 + bond of the 3-nitroanilinium were calculated at every 30°-angle rotation. The potential energy for the rotation of 3-nitroanilinium around the C-N was plotted against the angle of rotation, which shows an asymmetrical and double minimum type of potential energy curve (Fig. 5a). The rotation energy barriers of the phenyl ring in compound 1 are 1189 and 1345 kJ mol-1 at 90° and 270°, respectively. Such a high energy barrier makes it difficult to achieve the rotation of the phenyl ring in compound 1 because of the high barrier rendered by neighboring atoms or molecules. For compound 1, the potential energy for the in-plane rotation of the methanol molecules was also calculated. The model structure for the calculation was identical to the one used in the calculation of the rotation of 3-nitroanilinium cation (Fig. S5 in Supporting information). The potential energy curve for in-plane rotation of methanol molecules is exhibited in Fig. 5b. The energy curve of methanol molecules shows an asymmetric double maximum, with 91 and 63 kJ mol-1 at 120° and 300°, respectively. The lower energy barrier suggests thatmethanolmolecules participate in inplane rotation easily, resulting in the formation of phase transition in compound 1.
The adjustable temperature and frequency dependence of the dielectric response is another effective method of investigating the phase transition of single crystals under changes in external conditions. Therefore, the dielectric property of 1 was further investigated, and the plots of dielectric constant (ε) versus temperature at different frequencies along the c-axis are displayed in Fig. 6. As expected, compound 1 displays a clear dielectric anomaly at around 223.5 K of heating, which matches the phase transition phenomena at around 220 K. However, there is no distinct dielectric anomaly with the decrease in temperature. The figure shows that the dielectric constant rapidly increases when the temperature rises to above 185 K, and no maximum peak appears in the plots of dielectric constant versus temperature below 223.5 K. This result is probably caused by the weakening molecular vibrations as the temperature decreased within the measured temperature range. At a relatively high temperature, molecular vibrations were favorable for molecular rotation and were activated for the formation of 1. In addition, the increased range of the dielectric constant with temperature depends strongly on the frequency of the electric field, which is obvious when the frequency is above 500 Hz. In addition, the dielectric constant gradually decreased to 46.14, 29.43, 10.47, 8.00, 6.23, and 6.07 when the frequency was increased to 500 Hz, 1 kHz, 5 kHz, 10 kHz, 100 kHz and 1 MHz, respectively. Dielectric constant of 1 along the a and b-axes was also measured depending on the frequency (500 Hz to 1 MHz). Results show that the same dielectric response on the c-axis existed on the b-axis, but weak dielectric abnormal responses existed along the a-axis (Figs. S6 and S7 in Supporting information). These results suggest the relationship between the dielectric anisotropy and polarity change upon rotation of the molecules. The b and c-axis of compound 1 present the strong dielectric anomaly parallel with the in-plane rotation of the molecules, and the a-axis shows the weak dielectric anomaly due to the perpendicular in-plane rotation of the molecules. Therefore, the significant dielectric anomaly and phase transition for compound 1 may be attributed to the in-plane rotation of methanol molecules in the crystal structure.
A novel inorganic-organic hybrid supramolecular compound [(3-nitroanilinium+)(18-crown-6)][IO4 -](CH3OH) was synthesized and structurally characterized. In this supramolecular assembly, (3-nitroanilinium+)(18-crown-6) cation with IO4 -anion was used to constitute a one-dimensional chain structure via N-H…O hydrogen bonds. In addition, H-bonds interactions were observed between the-NH3 + group of 3-nitroanilinium, and adjacent oxygen atoms of CH3OH molecules through the cavity of 18-crown-6. Although the space grouping of compound 1 at different temperatures was retained, compound 1 underwent a phase transition at about 220 K. The lower energy barrier of methanol molecule in compound 1 suggests thatmolecular in-plane rotation led to the phase transition. The frequency-dependent, dielectric anomaly response of b-and caxes was observed at temperatures ranging from 100 K to 298K, which was probably due to the polarity change caused by the rotation of methanol molecules under the measured temperatures.
The present work was supported by National Natural Science Foundation of China (No. 21561030) and Prophase-sustentation Fund of Xinjiang Agricultural University (Nos. XJAU201410 and XJAU201511).
Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.cclet.2016.07.013.
Y.X. Ni, J. Sun, Y.H. Wei, et al., Biomacromolecules 18(2017) 3367-3374.
doi: 10.1021/acs.biomac.7b01014
J. Jonikaite-Svegzdiene, A. Kudresova, S. Paukstis, M. Skapas, R. Makuska, Polym. Chem. 8(2017) 5621-5632.
doi: 10.1039/C7PY01335C
L.I. Atanase, J. Desbrieresc, G. Riess, Prog. Polym. Sci. 73(2017) 32-60.
doi: 10.1016/j.progpolymsci.2017.06.001
X.S. Fan, X.Y. Wang, M.Y. Cao, et al., Polym. Chem. 8(2017) 5611-5620.
doi: 10.1039/C7PY00999B
J.F. Pan, X. Jin, Q.B. Mou, C. Zhang, Chin. Chem. Lett. 28(2017) 1822-1828.
doi: 10.1016/j.cclet.2017.08.022
L.H. Pang, J.M. Li, X.M. Lu, Q.H. Lu, Chin. Chem. Lett. 28(2017) 1358-1364.
doi: 10.1016/j.cclet.2017.04.009
B. Jeong, Y.H. Bae, D.S. Lee, S.W. Kim, Nature 388(1997) 860-862.
doi: 10.1038/42218
K. Kataoka, A. Harada, Y. Nagasaki, Adv. Drug. Deliver. Rev. 47(2001) 113-131.
doi: 10.1016/S0169-409X(00)00124-1
C. Allen, D. Maysinger, A. Eisenberg, Colloids Surf. B-Biointerfaces 16(1999) 3-27.
doi: 10.1016/S0927-7765(99)00058-2
T.B. Sun, Y. Jin, R. Qi, S.J. Peng, B.Z. Fan, Polym. Chem. 4(2013) 4017-4023.
doi: 10.1039/c3py00406f
J. Saunier, C.A. Chodur, L. Larbes, O. Mrad, N. Yagoubi, J. Appl. Polym. Sci. 104(2007) 585-593.
Y. Lu, Y. Mei, M. Schrinner, et al., J. Phys. Chem. C 111(2007) 7676-7681.
doi: 10.1021/jp070973m
X. Li, J. Gao, L. Xue, Y. Han, Adv. Funct. Mater. 20(2010) 259-265.
doi: 10.1002/adfm.v20:2
T.P. Smart, A.J. Ryan, J.R. Howse, G. Battaglia, Langmuir 26(2009) 7425-7430.
W.D. He, X.L. Sun, W.M. Wan, C.Y. Pan, Macromolecules 44(2011) 3358-3365.
doi: 10.1021/ma2000674
R.J. Hickey, A.S. Haynes, J.M. Kikkawa, S.J. Park, J. Am. Chem. Soc. 133(2011) 1517-1525.
doi: 10.1021/ja1090113
M. Antonietti, S. Förster, Adv. Mater. 15(2003) 1323-1333.
Y. Li, X. Chen, M. Zhang, et al., Macromolecules 41(2008) 4873-4880.
doi: 10.1021/ma702800p
L. Shen, J. Du, S.P. Armes, S. Liu, Langmuir 24(2008) 10019-10025.
doi: 10.1021/la801190z
J.Y. Zhang, J. Xu, S.Y. Liu, J. Phys. Chem. B 112(2008) 11284-11291.
doi: 10.1021/jp803700n
O. Terreau, L. Luo, A. Eisenberg, Langmuir 19(2003) 5601-5607.
doi: 10.1021/la0269715
L. Zhang, A. Eisenberg, Science 268(1995) 1728-1731.
doi: 10.1126/science.268.5218.1728
L. Zhang, A. Eisenberg, Macromolecules 29(1996) 8805-8815.
doi: 10.1021/ma961376t
L. Zhang, A. Eisenberg, J. Am. Chem. Soc. 118(1996) 3168-3181.
doi: 10.1021/ja953709s
O. Terreau, C. Bartels, A. Eisenberg, Langmuir 20(2004) 637-645.
doi: 10.1021/la035557h
H.W. Shen, L.F. Zhang, A. Eisenberg, J. Am. Chem. Soc. 121(1999) 2728-2740.
doi: 10.1021/ja983712m
L.F. Zhang, K. Yu, A. Eisenberg, Science 272(1996) 1777-1779.
doi: 10.1126/science.272.5269.1777
M. Changez, N.G. Kang, H.D. Koh, J.S. Lee, Langmuir 26(2010) 9981-9985.
doi: 10.1021/la100106b
Z. Shi, Y. Zhou, D. Yan, Macromol. Rapid Comm. 29(2008) 412-418.
J.N. Cha, H. Birkedal, L.E. Euliss, et al., J. Am. Chem. Soc. 125(2003) 8285-8289.
doi: 10.1021/ja0279601
M. Sedlak, C. Konak, J. Dybal, Macromolecules 42(2009) 7439-7446.
doi: 10.1021/ma901504h
M. Sedlak, J. Phys. Chem. B 116(2012) 2356-2364.
doi: 10.1021/jp208445p
J. Liu, W. Huang, Y. Pang, et al., Angew. Chem. Int. Ed. 50(2011) 9162-9166.
doi: 10.1002/anie.201102280
Y. Wan, S.H. Yu, J. Phys. Chem. C 112(2008) 3641-3647.
doi: 10.1021/jp710990b
Y.X. Ni, J. Sun, Y.H. Wei, et al., Biomacromolecules 18(2017) 3367-3374.
doi: 10.1021/acs.biomac.7b01014
J. Jonikaite-Svegzdiene, A. Kudresova, S. Paukstis, M. Skapas, R. Makuska, Polym. Chem. 8(2017) 5621-5632.
doi: 10.1039/C7PY01335C
L.I. Atanase, J. Desbrieresc, G. Riess, Prog. Polym. Sci. 73(2017) 32-60.
doi: 10.1016/j.progpolymsci.2017.06.001
X.S. Fan, X.Y. Wang, M.Y. Cao, et al., Polym. Chem. 8(2017) 5611-5620.
doi: 10.1039/C7PY00999B
J.F. Pan, X. Jin, Q.B. Mou, C. Zhang, Chin. Chem. Lett. 28(2017) 1822-1828.
doi: 10.1016/j.cclet.2017.08.022
L.H. Pang, J.M. Li, X.M. Lu, Q.H. Lu, Chin. Chem. Lett. 28(2017) 1358-1364.
doi: 10.1016/j.cclet.2017.04.009
B. Jeong, Y.H. Bae, D.S. Lee, S.W. Kim, Nature 388(1997) 860-862.
doi: 10.1038/42218
K. Kataoka, A. Harada, Y. Nagasaki, Adv. Drug. Deliver. Rev. 47(2001) 113-131.
doi: 10.1016/S0169-409X(00)00124-1
C. Allen, D. Maysinger, A. Eisenberg, Colloids Surf. B-Biointerfaces 16(1999) 3-27.
doi: 10.1016/S0927-7765(99)00058-2
T.B. Sun, Y. Jin, R. Qi, S.J. Peng, B.Z. Fan, Polym. Chem. 4(2013) 4017-4023.
doi: 10.1039/c3py00406f
J. Saunier, C.A. Chodur, L. Larbes, O. Mrad, N. Yagoubi, J. Appl. Polym. Sci. 104(2007) 585-593.
Y. Lu, Y. Mei, M. Schrinner, et al., J. Phys. Chem. C 111(2007) 7676-7681.
doi: 10.1021/jp070973m
X. Li, J. Gao, L. Xue, Y. Han, Adv. Funct. Mater. 20(2010) 259-265.
doi: 10.1002/adfm.v20:2
T.P. Smart, A.J. Ryan, J.R. Howse, G. Battaglia, Langmuir 26(2009) 7425-7430.
W.D. He, X.L. Sun, W.M. Wan, C.Y. Pan, Macromolecules 44(2011) 3358-3365.
doi: 10.1021/ma2000674
R.J. Hickey, A.S. Haynes, J.M. Kikkawa, S.J. Park, J. Am. Chem. Soc. 133(2011) 1517-1525.
doi: 10.1021/ja1090113
M. Antonietti, S. Förster, Adv. Mater. 15(2003) 1323-1333.
Y. Li, X. Chen, M. Zhang, et al., Macromolecules 41(2008) 4873-4880.
doi: 10.1021/ma702800p
L. Shen, J. Du, S.P. Armes, S. Liu, Langmuir 24(2008) 10019-10025.
doi: 10.1021/la801190z
J.Y. Zhang, J. Xu, S.Y. Liu, J. Phys. Chem. B 112(2008) 11284-11291.
doi: 10.1021/jp803700n
O. Terreau, L. Luo, A. Eisenberg, Langmuir 19(2003) 5601-5607.
doi: 10.1021/la0269715
L. Zhang, A. Eisenberg, Science 268(1995) 1728-1731.
doi: 10.1126/science.268.5218.1728
L. Zhang, A. Eisenberg, Macromolecules 29(1996) 8805-8815.
doi: 10.1021/ma961376t
L. Zhang, A. Eisenberg, J. Am. Chem. Soc. 118(1996) 3168-3181.
doi: 10.1021/ja953709s
O. Terreau, C. Bartels, A. Eisenberg, Langmuir 20(2004) 637-645.
doi: 10.1021/la035557h
H.W. Shen, L.F. Zhang, A. Eisenberg, J. Am. Chem. Soc. 121(1999) 2728-2740.
doi: 10.1021/ja983712m
L.F. Zhang, K. Yu, A. Eisenberg, Science 272(1996) 1777-1779.
doi: 10.1126/science.272.5269.1777
M. Changez, N.G. Kang, H.D. Koh, J.S. Lee, Langmuir 26(2010) 9981-9985.
doi: 10.1021/la100106b
Z. Shi, Y. Zhou, D. Yan, Macromol. Rapid Comm. 29(2008) 412-418.
J.N. Cha, H. Birkedal, L.E. Euliss, et al., J. Am. Chem. Soc. 125(2003) 8285-8289.
doi: 10.1021/ja0279601
M. Sedlak, C. Konak, J. Dybal, Macromolecules 42(2009) 7439-7446.
doi: 10.1021/ma901504h
M. Sedlak, J. Phys. Chem. B 116(2012) 2356-2364.
doi: 10.1021/jp208445p
J. Liu, W. Huang, Y. Pang, et al., Angew. Chem. Int. Ed. 50(2011) 9162-9166.
doi: 10.1002/anie.201102280
Y. Wan, S.H. Yu, J. Phys. Chem. C 112(2008) 3641-3647.
doi: 10.1021/jp710990b

Zhibin Ren , Shan Li , Xiaoying Liu , Guanghao Lv , Lei Chen , Jingli Wang , Xingyi Li , Jiaqing Wang . Penetrating efficiency of supramolecular hydrogel eye drops: Electrostatic interaction surpasses ligand-receptor interaction. Chinese Chemical Letters, 2024, 35(11): 109629-. doi: 10.1016/j.cclet.2024.109629
Mingqi Wang , Shixin Fa , Jiate Yu , Guoxian Zhang , Yi Yan , Qing Liu , Qiuyu Zhang . Light-controlled protein imprinted nanospheres with variable recognition specificity. Chinese Chemical Letters, 2025, 36(2): 110124-. doi: 10.1016/j.cclet.2024.110124
Hao Zhang , Haonan Qu , Ehsan Bahojb Noruzi , Haibing Li , Feng Liang . A nanocomposite film with layer-by-layer self-assembled gold nanospheres driven by cucurbit[7]uril for the selective transport of L-tryptophan and lysozyme. Chinese Chemical Letters, 2025, 36(1): 109731-. doi: 10.1016/j.cclet.2024.109731
Siqi Sun , Cheng Zhao , Zhaohuan Zhang , Ding Wang , Xinru Yin , Jingting Han , Jinlei Wei , Yong Zhao , Yongheng Zhu . Highly selective QCM sensor based on functionalized hierarchical hollow TiO2 nanospheres for detecting ppb-level 3-hydroxy-2-butanone biomarker at room temperature. Chinese Chemical Letters, 2025, 36(5): 109939-. doi: 10.1016/j.cclet.2024.109939
Jiahao Xie , Jin Liu , Bin Liu , Xin Meng , Zhuang Cai , Xiaoqin Xu , Cheng Wang , Shijie You , Jinlong Zou . Yolk shell-structured pyrite-type cobalt sulfide grafted by nitrogen-doped carbon-needles with enhanced electrical conductivity for oxygen electrocatalysis. Chinese Chemical Letters, 2024, 35(7): 109236-. doi: 10.1016/j.cclet.2023.109236
Xiaonan LI , Hui HAN , Yihan ZHANG , Jing XIONG , Tingting GUO , Juanzhi YAN . A viologen‐based Cd(Ⅱ) coordination polymer: Self‐assembly, thermochromism, and electrochemical property. Chinese Journal of Inorganic Chemistry, 2025, 41(7): 1439-1444. doi: 10.11862/CJIC.20240376
Saisai Yuan , Yiming Chen , Xijuan Wang , Degui Zhao , Tengyang Gao , Caiyun Wei , Chuanxiang Chen , Yang Yang , Wenjing Hong . Decouple the intermolecular interaction by encapsulating an insulating sheath. Chinese Chemical Letters, 2025, 36(6): 110816-. doi: 10.1016/j.cclet.2025.110816
Yuan Zhang , Shenghao Gong , A.R. Mahammed Shaheer , Rong Cao , Tianfu Liu . Plasmon-enhanced photocatalytic oxidative coupling of amines in the air using a delicate Ag nanowire@NH2-UiO-66 core-shell nanostructures. Chinese Chemical Letters, 2024, 35(4): 108587-. doi: 10.1016/j.cclet.2023.108587
Cheng Wang , Ji Wang , Dong Liu , Zhi-Ling Zhang . Advances in virus-host interaction research based on microfluidic platforms. Chinese Chemical Letters, 2024, 35(12): 110302-. doi: 10.1016/j.cclet.2024.110302
Hengying Xiang , Nanping Deng , Lu Gao , Wen Yu , Bowen Cheng , Weimin Kang . 3D core-shell nanofibers framework and functional ceramic nanoparticles synergistically reinforced composite polymer electrolytes for high-performance all-solid-state lithium metal battery. Chinese Chemical Letters, 2024, 35(8): 109182-. doi: 10.1016/j.cclet.2023.109182
Jinli Chen , Shouquan Feng , Tianqi Yu , Yongjin Zou , Huan Wen , Shibin Yin . Modulating Metal-Support Interaction Between Pt3Ni and Unsaturated WOx to Selectively Regulate the ORR Performance. Chinese Journal of Structural Chemistry, 2023, 42(10): 100168-100168. doi: 10.1016/j.cjsc.2023.100168
Wen Su , Siying Liu , Qingfu Zhang , Zhongyan Zhou , Na Wang , Lei Yue . Temperature-controlled electrospray ionization tandem mass spectrometry study on protein/small molecule interaction. Chinese Chemical Letters, 2025, 36(5): 110237-. doi: 10.1016/j.cclet.2024.110237
Yanyu Jin , Wenzhe Si , Xing Yuan , Hongjun Cheng , Bin Zhou , Li Cai , Yu Wang , Qibao Wang , Junhua Li . Tuning TM–O interaction by acid etching in perovskite catalysts boosting catalytic performance. Chinese Chemical Letters, 2025, 36(5): 110260-. doi: 10.1016/j.cclet.2024.110260
Ruofan Qi , Jing Zhang , Wang Sun , Bai Yu , Zhenhua Wang , Kening Sun . Solid-acid-Lewis-base interaction accelerates lithium ion transport for uniform lithium deposition. Chinese Chemical Letters, 2025, 36(6): 110009-. doi: 10.1016/j.cclet.2024.110009
Xinlong Han , Huiying Zeng , Chao-Jun Li . Trifluoromethylative homo-coupling of carbonyl compounds. Chinese Chemical Letters, 2025, 36(1): 109817-. doi: 10.1016/j.cclet.2024.109817
Bing Niu , Honggao Huang , Liwei Luo , Li Zhang , Jianbo Tan . Coating colloidal particles with a well-defined polymer layer by surface-initiated photoinduced polymerization-induced self-assembly and the subsequent seeded polymerization. Chinese Chemical Letters, 2025, 36(2): 110431-. doi: 10.1016/j.cclet.2024.110431
Shuwen SUN , Gaofeng WANG . Design and synthesis of a Zn(Ⅱ)-based coordination polymer as a fluorescent probe for trace monitoring 2, 4, 6-trinitrophenol. Chinese Journal of Inorganic Chemistry, 2025, 41(4): 753-760. doi: 10.11862/CJIC.20240399
Yu BAI , Jijiang WANG , Long TANG , Erlin YUE , Chao BAI , Xiao WANG , Yuqi ZHANG . A cadmium(Ⅱ) coordination polymer based on a semirigid tetracarboxylate ligand for highly selective detection of Fe3+ and 4-nitrophenol. Chinese Journal of Inorganic Chemistry, 2025, 41(6): 1217-1226. doi: 10.11862/CJIC.20240457
Ruiying Liu , Li Zhao , Baishan Liu , Jiayuan Yu , Yujie Wang , Wanqiang Yu , Di Xin , Chaoqiong Fang , Xuchuan Jiang , Riming Hu , Hong Liu , Weijia Zhou . Modulating pollutant adsorption and peroxymonosulfate activation sites on Co3O4@N,O doped-carbon shell for boosting catalytic degradation activity. Chinese Journal of Structural Chemistry, 2024, 43(8): 100332-100332. doi: 10.1016/j.cjsc.2024.100332
Yan-Bo Li , Yi Li , Liang Yin . Copper(Ⅰ)-catalyzed diastereodivergent construction of vicinal P-chiral and C-chiral centers facilitated by dual "soft-soft" interaction. Chinese Chemical Letters, 2024, 35(7): 109294-. doi: 10.1016/j.cclet.2023.109294