Citation:
. The exploration of chiral N-cyano sulfiliminyl dicarboxamides on insecticidal activities[J]. Chinese Chemical Letters,
;2017, 28(7): 1499-1504.
doi:
10.1016/j.cclet.2017.02.021

-
Due to new mechanism of action and ecofriendly characteristics, dicarboxamide insecticides have attracted more and more attentions in modern pest management. A series of 20 dual chiral N-cyano sulfilimines containing two centers (carbon and sulfur) were designed and synthesized. All title compounds were determined by 1H NMR, high-resolution mass spectrometry (HRMS) and optical polarimeter. The preliminary results indicated that some of them exhibited favourable insecticidal activities against oriental armyworm (Pseudaletia separata Walker). These isomers exhibited different impact on activity following the sequence as (Sc, Ss)≥(Sc, Rs), and the rule of title compounds' activity against oriental armyworm was 3-CF3≥2-CH3-4-Cl >2, 3, 4-trifluro in the anilide moiety. The results indicated that these groups such as 3-CF3, 2-CH3-4-Cl or 2, 3, 4-trifluro were inefficient to replace heptafluoroisopropyl group for high larvicidal activity, which provided some guidance for the further modifications of sulfiliminyl dicarboxamides.
-
1. Introduction
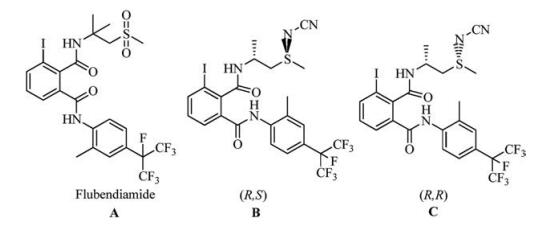
With the modern agro-chemical means for pest control, crop production has made considerable progress. During the last century, a large number of insecticides including the organochlorines, organophosphates, carbamates, pyrethroids and neonicotinoids have been introduced to the market. However, some of these chemicals have brought undesired environmental and health problems [1]. Persistent application of the insecticides with the same mode of action induced insecticide resistance has become a problem. Therefore, it is necessary to discover new insecticides with novel mechanisms of action [2]. As an ideal biological target for insecticide research, ryanodine receptor (RyR) has aroused considerable interests in integrated strategies for the development of agro-chemistry. Recently, the first commercial RyR insecticide, flubendiamide [3, 4] (Fig. 1), was developed by Nihon Nohyaku jointly with Bayer and was brought to the market in 2007 [5, 6]. In particular, flubendiamide [7, 8] has been developed as a highly potent insecticide against lepidopteran insects [9, 10].
图 1
 图 1 The structures of flubendiamide (A) and N-cyano sulfilimine (B).Figure 1. The structures of flubendiamide (A) and N-cyano sulfilimine (B).
图 1 The structures of flubendiamide (A) and N-cyano sulfilimine (B).Figure 1. The structures of flubendiamide (A) and N-cyano sulfilimine (B).In our previous work [11, 12], dual chiral scaffolds containing sulfiliminyl substituents and their larvacidal activities were reported. For oriental armyworm, some compounds reached the same high level as flubendiamide, with LC50 values 0.0504 (B) and 0.0699 (C) mg/L, lower than that of flubendiamide (0.1230 mg/L) [13]. Other sulfilimine derivatives exhibited higher activity against diamondback moths than flubendiamide [14]. The previous results implied that the introduction of the dual chiral N-cyano sulfiliminyl substituent was favorable for the larvacidal activity. The results prompted us to explore the further structural modifications. It is well-known that heptafluoroisopropyl iodide is an expensive material for the synthesis of flubendiamide. To reduce production costs, it is necessary to identify cheaper replacement of heptafluoroisopropyl iodide. What is more, the new structure also maintains excellent insecticidal activity after the replacement of heptafluoroisopropyl group.
Enlightened by the above research, 20 novel dual chiral N-cyano sulfiliminyl phthalamide derivatives with these groups including 3—CF3, 2—CH3—4—Cl or 2, 3, 4-trifluro in the anilide moiety were designed and synthesized. All title compounds were determined by 1H NMR, high-resolution mass spectrometry and optical polarimeter. The preliminary structure-activity relationship (SAR) was discussed as well.
2. Results and discussion
2.1 Synthesis
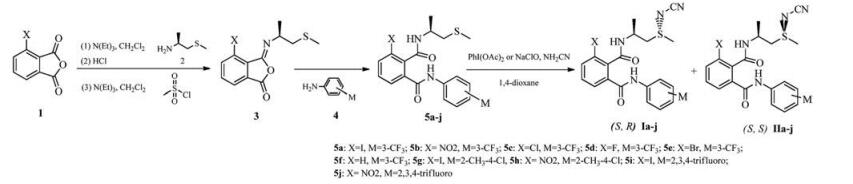
N-cyano sulfilimines can be easily obtained by sulfides in 95%–99% yield with the ratio of diastereoselectivity 6:4. The synthetic method of these title compounds were shown in Scheme 1.
Scheme1
The title compounds have been determined by melting point, 1H NMR, HRMS and optical polarimeter. The 1H NMR data of N-cyano sulfilimines were characteristic between two diastereoisomers. The active proton signals of —NHCO—in the anilide moiety were observed at 9.83–10.88 ppm in DMSO-d6. However, the chemical shifts of the active proton signals on the amide bridge in the aliphatic amide moiety were at δ 8.76–9.02 and δ 8.58–8.87 ppm in DMSO-d6, respectively.
图 2
2.2 Crystal structure analysis
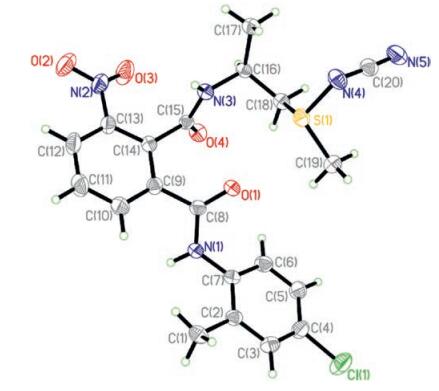
The crystal structure of Ⅰf had been shown in the literature previously reported [13]. The crystal structure (0.20 mm × 0.18 mm × 0.12 mm) of compound Ⅰh was cultivated from the solvent DMSO, which was selected and mounted on Rigaku Saturn 724 CCD diffractometer equipped with a graphite-monochromatic MoKá radiation (ë = 0.71073 Å). The data were collected at 293(2) K to a maximum θ value of 25.02 with the following index ranges: -4 ≤ h ≤ 5, -26 ≤ k ≤ 26 and -27 ≤ l ≤ 27. A total of 21057 integrated reflections were collected and 4473 with Rint = 0.0638 were independent, of which 3581 with I > 2ó (I) were observed. The structure was resolved by direct methods with the SHELXL-97 program [14]. Refinements were done by the full-matrix leastsquares on F2 with SHELXL-97. The crystal is of orthorhombic system with P2(1)2(1)2(1) space group. X-ray single crystal diffraction was shown in Fig. 2 with the following crystallographic parameters: a = 4.8338(10) Å, b = 22.320 (5) Å, c = 23.512(5) Å, α = 90°, β = 90°, γ = 90°, V = 2536.8(9) Å3, Z = 4, Dc = 1.414 mg/m3, μ = 0.358 mm-1, F (000) = 1128, R = 0.0797, wR = 0.1802, final R factor = 6.29%, final wR factor = 16.27%, absolute structure parameter = 0.06 (11). All the bond length and bond angle were in normal range, the N(5)—C(20) bond length of 1.172 Å is intermediate between C=N(1.34–1.38 Å) and C≡N(1.14–1.16 Å) may be due to the conjugation effect of S(1)=N(4). The torsion angles of S(1)—N (4)—C(20)—N(5) is 172.6°, which indicates that the S(1) = N(4) and N(5)≡C(20) are not coplanar in the molecular structure.
2.3 Structure-activity relationship (SAR)
2.3.1 Larvicidal activity against oriental armyworm
The larvicidal activities of compounds 5f-5j, Ⅰa-Ⅰj, Ⅱa-Ⅱj and flubendiamide against oriental armyworm were shown in Tables 1 and 2. Most title compounds showed moderate larvicidal activity against oriental armyworm. In general, the sequence of these isomers' activity is (Sc, Ss) ≥ (Sc, Rs), which is consistent with SARs described in our previous report ((Sc, Ss) ≥ (Sc, Rs) >> (Rc, Ss) > (Rc, Rs)) [13]. As shown in Table 1, Ⅱk (Sc, Ss) exhibited 50% insecticidal activity at 10 mg/L, while Ⅰk (Sc, Rs) gave a mortality rate of 30% at 50 mg/L. At concentration of 50 mg/L, Ⅱq (Sc, Ss) (100%) possessed better insecticidal activity than Ⅰq (Rc, Rs) (0%). These observations indicated that the influence of sulfur chirality on biological activities is self-evident.
表 1
 表 1 Insecticidal activities of compounds 5f-5j, Ⅰa-Ⅰj, Ⅱa-Ⅱj and flubendiamide against oriental armyworm.Table 1. Insecticidal activities of compounds 5f-5j, Ⅰa-Ⅰj, Ⅱa-Ⅱj and flubendiamide against oriental armyworm.
表 1 Insecticidal activities of compounds 5f-5j, Ⅰa-Ⅰj, Ⅱa-Ⅱj and flubendiamide against oriental armyworm.Table 1. Insecticidal activities of compounds 5f-5j, Ⅰa-Ⅰj, Ⅱa-Ⅱj and flubendiamide against oriental armyworm.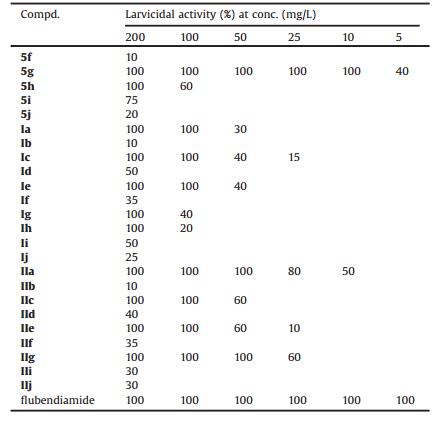
表 2
 表 2 LC50 values of compounds ⅰa, ⅰg, ⅰj, ⅱa, ⅱg, ⅱj and flubendiamide against oriental armyworm.Table 2. LC50 values of compounds ⅰa, ⅰg, ⅰj, ⅱa, ⅱg, ⅱj and flubendiamide against oriental armyworm.
表 2 LC50 values of compounds ⅰa, ⅰg, ⅰj, ⅱa, ⅱg, ⅱj and flubendiamide against oriental armyworm.Table 2. LC50 values of compounds ⅰa, ⅰg, ⅰj, ⅱa, ⅱg, ⅱj and flubendiamide against oriental armyworm.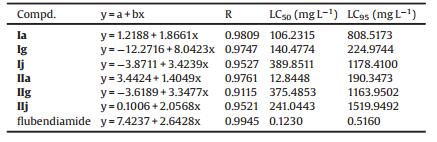
From Table 1, we could find that compounds 5g, Ⅱa and Ⅱg exhibited 100%, 80% and 60% activities at 25 mg/L, respectively. It was worth noting that 5 g gave the mortality of 40% at 5 mg/L. Furthermore, the iodine substituents (5g, Ⅱa and Ⅱg) showed the best larvicidal activity. For compounds with CF3 group in the anilide moiety, I showed the best activity, Br as well as Cl, and F substituent possessed similar biological activity against oriental armyworm. As shown in Table 1, these title compounds exerted the sequence of biological activity as 3—CF3 ≥ 2—CH3—4—Cl > 2, 3, 4-trifluro in the anilide moiety. From Table 2, it was found that the LC50 value of Ⅱa was 12.8448 mg/L, higher than that of flubendiamide (0.1230 mg/L).
2.3.2 CoMFA analysis by SYBYL
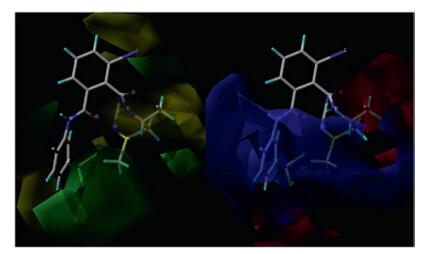
With the purpose of increasing the calculated sample, the published compounds (Ⅰa, Ⅰb, Ⅱa, Ⅱb, Ⅱd) [13] and the novel compounds Ⅰa, Ⅰg, Ⅰj, Ⅱa, Ⅱg, Ⅱj were selected for 3D-QSAR model. The above compounds were prepared with SYBYL program, the conformations with minimum energy were evaluated by Tripos force field and Gasteiger-Hükel charges in advance. The most active molecule published Ⅰa was used as a template to align the training samples by align database, the common skeleton was overlapped very well. The pIC50 was the activity data, and the negative logarithms of LC50 data were shown in Table 2. Then the 3D-QSAR model CoMFA was developed successfully. The Fig. 3 showed the CoMFA steric and electrostatic contour map. Partial least-squares analysis gives a satisfactory R2 value of 0.952 and Q2 value of 0.867 with 2 components, which indicates a valid and stable CoMFA model.
图 3
The left of Fig. 3 is the steric field, the green regions mean that bulky substituents can increase the insecticidal activity, while yellow regions mean that the bulky substituents reduce the activity. From Fig. 3, a large green region is found surrounding the CF(CF3)2 group, indicating that bulky substituents are preferred here. For instance, insecticidal activity of published compounds Ⅰa, Ⅰb, Ⅱa, Ⅱb and Ⅱd [13] with CF(CF3)2 group are higher than compounds Ⅰa, Ⅰg, Ⅰj, Ⅱa, Ⅱg, Ⅱj with other less bulky substituents. The right of Fig. 3 is the electrostatic field, the red regions indicate that electronegative substituents will increase the insecticidal activity, while blue regions mean that electropositive substituents will increase the insecticidal activity. Here, red area near the CF3 group, indicating a electronegative substituent will increase the activity, such as compound Ⅱa has higher activities than compound Ⅰg, Ⅰj, Ⅱg and Ⅱj.
3. Conclusion
In conclusion, 20 novel optically active N-cyano sulfilimines were designed, synthesized and evaluated against oriental armyworm (Pseudaletia separata Walker) for their insecticidal activities. The resultsof bioassay indicated that most target compounds possessed favorable activities against oriental armyworm. For oriental armyworm, these isomers exhibited different impact on biological activity following the sequence as (Sc, Ss) ≥ (Sc, Rs), which was in accordance with SARs described in our previous report [13]. And the rule of activity against oriental armyworm was 3-CF3 ≥ 2—CH3—4—Cl > 2, 3, 4-trifluro in the anilide moiety. The results indicated that these groups including 3-CF3, 2—CH3—4—Cl or 2, 3, 4-trifluro in the anilide moiety might not be essential for favorable larvicidal activity, which provided some guidance for the further modifications of sulfiliminyl dicarboxamides.
4. Experimental
4.1 Instruments and materials
The melting points were determined on an X-4 binocular microscope melting point apparatus (Beijing Tech Instrument Co., Beijing, China) and uncorrected. 1H NMR and 13C NMR spectra were recorded at 300 MHz (Bruker AC-P 300 spectrometer) or 400 MHz (Bruker AV 400 spectrometer) in CDCl3 or DMSO-d6 solution with tetramethylsilane as internal standard, and chemical shift (δ) in (ppm) (s = singlet, d = doublet, t = triplet, m = multiplet). Elemental analyses were performed on a Vario EL elemental analyzer. HRMS data were obtained on a Varian QFT-ESI. Optical rotations were measured with Perkin-Elmer 341 polarimeter at 20 ℃. GC-MS were recorded on HP 5973 MSD with 6890 GC Flash chromatography with silica gel (200–300 mesh). Reagents were all analytically pure. All solvents and liquid reagents were dried by standard methods and distilled before use. Insecticide Flubendiamide was used only as a control, synthesized according to the literature [2]. Compounds 3-substituented anhydride 1 [15, 16], (S)-1-(methylthio) propan -2-amine 2 [17] were synthesized according to the methods reported in the literatures.
4.2 General synthetic procedure for compounds 5a-j
5a-j were synthesized in moderate yields by the method previously published in the literature [18]. The melting point and 1H NMR data were consistent with the literature.
(S)-N1-(1-(Methylthio)propan-2-yl)-N2-(3-(trifluoromethyl) phenyl)phthalamide 5f: White solid; yield 72.91%; mp 178–180 ℃; 1H NMR (400 MHz, DMSO-d6): δ 10.65 (s, 1H, Ar-NH), 8.36 (d, 1H, J = 7.7 Hz, —CNH), 8.22 (s, 1H, Ar-H), 7.90 (d, 1H, J = 7.1 Hz, Ar-H), 7.58 (m, 5H, Ar-H), 7.44 (d, 1H, J = 7.0 Hz, Ar-H), 4.08–3.98 (m, 1H, —NCH), 2.66 (dd, 1H, J = 13.0, 6.3 Hz, —SCH2), 2.54-2.53 (m, 1H, -SCH2), 2.05 (s, 3H, —SCH3), 1.19 (d, 3H, J = 6.3 Hz, —CCH3).
((S)-N1-(4-Chloro-2-methylphenyl)-3-iodo-N2-(1-(methylthio) propan-2-yl)phthalamide 5g: White solid; yield 61.74%; mp 209–210 ℃; 1H NMR (400 MHz, CDCl3): δ 8.33 (s, 1H, Ar-NH), 8.01 (d, 1H, J = 9.3 Hz, Ar-H), 7.94 (d, 1H, J = 7.9 Hz, Ar-H), 7.73 (d, 1H, J = 7.7 Hz, Ar-H), 7.18 (t, 3H, J = 8.0 Hz, Ar-H), 6.38 (d, 1H, J = 8.1 Hz, —CNH), 4.35–4.27 (m, 1H, —NCH), 2.63 (dd, 1H, J = 13.5, 6.0 Hz, —SCH2), 2.56 (dd, 1H, J = 13.5, 6.3 Hz, —SCH2), 2.29 (s, 3H, —SCH3), 1.97 (s, 3H, ArCH3), 1.25 (d, 3H, J = 6.6 Hz, —CCH3).
((S)-N1-(4-Chloro-2-methylphenyl)-N2-(1-(methylthio)propan-2-yl)-3-nitrophthalamide 5h: White solid; yield 55.23%%; mp 224–226 ℃; 1H NMR (400 MHz, DMSO-d6): δ 9.87 (s, 1H, Ar-NH), 8.63 (d, 1H, J = 7.6 Hz, —CNH), 8.20 (d, 1H, J = 7.9 Hz, Ar-H), 8.04 (d, 1H, J = 7.3 Hz, Ar-H), 7.78 (t, 1H, J = 7.8 Hz, Ar-H), 7.54 (d, 1H, J = 8.4 Hz, Ar-H), 7.36 (s, 1H, Ar-H), 7.30 (d, 1H, J = 7.9 Hz, Ar-H), 3.97 (m, 1H, —NCH), 2.63 (dd, 1H, J = 12.9, 4.3 Hz, —SCH2), 2.37–2.32 (m, 1H, —SCH2), 2.28 (s, 3H, —SCH3), 2.02 (s, 3H, ArCH3), 1.11 (d, 3H, J = 6.3 Hz, —CCH3).
(S)-3-Iodo-N2-(1-(methylthio)propan-2-yl)-N1-(2, 3, 4-trifluorophenyl)phthalamide 5i: White solid; yield 61.48%; mp 160–162 ℃; 1H NMR (400 MHz, DMSO-d6): δ 10.21 (s, 1H, Ar-NH), 8.44 (d, 1H, J = 7.8 Hz, —CNH), 8.02 (d, 1H, J = 7.8 Hz, ArH), 7.69 (d, 1H, J = 7.4 Hz, Ar-H), 7.51 (s, 1H, Ar-H), 7.39–7.32 (m, 1H, Ar-H), 7.26 (t, 1H, J = 7.7 Hz, Ar-H), 3.99 (dd, 1H, J = 12.7, 6.5 Hz, —NCH), 2.69 (dd, 1H, J = 13.2, 4.8 Hz, —SCH2), 2.37 (dd, 1H, J = 13.1, 8.7 Hz, —SCH2), 2.02 (s, 3H, —SCH3), 1.15 (d, 3H, J = 6.5 Hz, —CCH3).
(S)-N2-(1-(Methylthio)propan-2-yl)-3-nitro-N1-(2, 3, 4-trifluorophenyl)phthalamide 5j: White solid; yield 67.52%; mp >300 ℃; 1H NMR (400 MHz, DMSO-d6): δ 10.46 (s, 1H, Ar-NH), 8.63 (d, 1H, J = 7.7 Hz, —CNH), 8.22 (d, 1H, J = 8.1 Hz, Ar-H), 8.02 (d, 1H, J = 7.4 Hz, Ar-H), 7.79 (s, 1H, Ar-H), 7.58 (s, 1H, Ar-H), 7.39 (d, 1H, J = 9.0 Hz, Ar-H), 3.95 (d, 1H, J = 6.2 Hz, —NCH), 2.65 (dd, 1H, J = 13.1, 4.6 Hz, —SCH2), 2.35 (dd, 1H, J = 13.0, 8.5 Hz, —SCH2), 2.04 (s, 3H, —SCH3), 1.12 (d, 3H, J = 6.5 Hz, —CCH3).
4.3 General synthetic procedure for title compounds
These phthalamide derivatives were synthesized in moderate yield by the method reported in the literature [13].
(S, R)-3-Iodo-N2-(1-(N-cyano-S-methylsulfinimidoyl)-propan-2-yl)-N1-(3-(trifluoromethyl)phenyl)p-thalamide Ⅰa: White solid; yield 73.41%; mp 194–195 ℃; 1H NMR (400 MHz, DMSO-d6): δ 10.64 (s, 1H, Ar-NH), 8.81 (d, 1H, J = 6.9 Hz, —CNH), 8.15 (s, 1H, Ar-H), 8.05 (d, 1H, J = 6.8 Hz, Ar-H), 7.93 (d, 1H, J = 7.4 Hz, Ar-H), 7.75 (d, 1H, J = 6.3 Hz, Ar-H), 7.59 (d, 1H, J = 6.7, 13.2 Hz, Ar-H), 7.46 (d, 1H, J = 6.6 Hz, Ar-H), 7.32 (d, 1H, J = 5.9, 12.4 Hz, Ar-H), 4.25 (m, 1H, —NCH), 3.26–3.17 (m, 2H, —SCH2), 2.81 (s, 3H, —SCH3), 1.27 (d, 3H, J = 4.4 Hz, —CCH3). HRMS calcd. for C20H18F3IN4O2S ([M+H]+), 563.0220; found, 563.0216. [α]D20 = -86.4(c = 0.5, DMF).
(S, R)-3-Nitro-N2-(1-(N-cyano-S-methylsulfinimidoyl)-propan-2-yl)-N1-(3-(trifluoromethyl)phenyl)phthalamide Ⅰb: White solid; yield 69.94%; mp 108–110 ℃; 1H NMR (400 MHz, DMSO-d6): δ 10.88 (s, 1H, Ar-NH), 9.02 (d, 1H, J = 7.9 Hz, —CNH), 8.25 (d, 1H, J = 8.2 Hz, Ar-H), 8.15 (s, 1H, Ar-H), 8.09 (d, 1H, J = 7.6 Hz, Ar-H), 7.93 (d, 1H, J = 8.3 Hz, Ar-H), 7.83 (t, 1H, J = 8.0 Hz, Ar-H), 7.62 (t, 1H, J = 7.9 Hz, Ar-H), 7.49 (d, 1H, J = 7.6 Hz, Ar-H), 4.22 (dt, 1H, J = 13.5, 6.7 Hz, —NCH), 3.30 (dd, 1H, J = 12.7, 6.1 Hz, —SCH2), 3.17 (dd, 1H, J = 12.7, 7.3 Hz, -SCH2), 2.81 (s, 3H, -SCH3), 1.22 (d, 3H, J = 6.6 Hz, —CCH3). HRMS calcd. for C20H18F3N5O4S ([M+H]+), 482.1105; found, 482.1103. [α]D20 = -12.0(c = 0.07, (CH3)2CO).
(S, R)-3-Chloro-N2-(1-(N-cyano-S-methylsulfinimidoyl)-propan-2-yl)-N1-(3-(trifluoromethyl)phenyl)phthalamide Ⅰc: White solid; yield 68.43%; mp 178–180 ℃; 1H NMR (400 MHz, DMSO-d6): δ 10.71 (s, 1H, Ar-NH), 8.91 (d, 1H, J = 8.1 Hz, —CNH), 8.14 (s, 1H, Ar-H), 7.94 (d, 1H, J = 8.2 Hz, Ar-H), 7.72 (dd, 2H, J = 10.9, 7.9 Hz, Ar-H), 7.59 (dd, 2H, J = 12.9, 5.0 Hz, Ar-H), 7.47 (d, 1H, J = 7.7 Hz, Ar-H), 4.27 (dt, 1H, J = 14.5, 7.4 Hz, —NCH), 3.34–3.31 (m, 1H, —SCH2), 3.21 (dd, 1H, J = 12.6, 7.7 Hz, —SCH2), 2.83 (s, 3H, —SCH3), 1.25 (d, 3H, J = 6.7 Hz, —CCH3). HRMS calcd. for C20H18ClF3N4O2S ([M+H]+), 417.0864; found, 471.0868. [α]D20 = -1.0(c = 0.33, (CH3)2CO)
(S, R)-3-Fluoro-N2-(1-(N-cyano-S-methyl-sulfinimidoyl)-propan-2-yl)-N1-(3-(trifluoromethyl)phenyl)phthalamide Ⅰd: White solid; yield 70.62%; mp 179–181 ℃; 1H NMR (400 MHz, DMSO-d6): δ 10.76 (s, 1H, Ar-NH), 8.97 (d, 1H, J = 8.1 Hz, -CNH), 8.15 (s, 1H, Ar-H), 7.95 (d, 1H, J = 8.5 Hz, Ar-H), 7.64–7.59 (m, 3H, Ar-H), 7.49 (dd, 2H, J = 11.4, 8.6 Hz, Ar-H), 4.34–4.25 (m, 1H, —NCH), 3.35 (m, 1H, —SCH2), 3.22 (dd, 1H, J = 12.7, 7.4 Hz, —SCH2), 2.85 (s, 3H, —SCH3), 1.28 (d, 3H, J = 6.7 Hz, —CCH3). HRMS calcd. for C20H18F4N4O2S ([M+H]+), 455.1160; found, 455.1162. [α]D20 = -61.2 (c = 0.5, (CH3)2CO).
(S, R)-3-Bromo-N2-(1-(N-cyano-S-methylsulfinimidoyl)-propan-2-yl)-N1-(3-(trifluoromethyl)phenyl)phthalamide Ⅰe: White solid; yield 71.25%; mp 182–183 ℃; 1H NMR (400 MHz, DMSO-d6): δ 10.71 (s, 1H, Ar-NH), 8.90 (d, 1H, J = 8.2 Hz, —CNH), 8.14 (s, 1H, Ar-H), 7.94 (d, 1H, J = 7.9 Hz, Ar-H), 7.72 (ddd, 2H, J = 9.6, 9.0, 4.2 Hz, Ar-H), 7.59 (dt, 2H, J = 9.8, 8.0 Hz, Ar-H), 7.49 (dd, 1H, J = 15.1, 7.5 Hz, Ar-H), 4.31–4.22 (m, 1H, —NCH), 3.33–3.31 (m, 1H, —SCH2), 3.20 (dd, 1H, J = 12.6, 7.7 Hz, —SCH2), 2.82 (s, 3H, —SCH3), 1.25 (d, 3H, J = 6.7 Hz, —CCH3). HRMS calcd. for C20H18BrF3N4O2S ([M+H]+), 515.0359; found, 515.0356. [α]D20 = -0.6(c = 0.18, DMF).
(S, R)-N2-(1-(N-Cyano-S-methylsulfinimidoyl)-propan-2-yl)-N1-(3-(trifluoromethyl)phenyl)phthalamide Ⅰf: White solid; yield 59.86%; mp 74–78 ℃; 1H NMR (400 MHz, DMSO-d6): δ 10.69 (s, 1H, Ar-NH), 8.76 (d, 1H, J = 7.6 Hz, —CNH), 8.21 (s, 1H, Ar-H), 7.91 (d, 1H, J = 7.8 Hz, Ar-H), 7.66–7.57 (m, 5H, Ar-H), 7.44 (d, 1H, J = 7.4 Hz, ArH), 4.35–4.25 (m, 1H, —NCH), 3.38-3.36 (m, 1H, —SCH2), 3.29–3.21 (m, 1H, —SCH2), 2.83 (s, 3H, —SCH3), 1.29 (d, 3H, J = 5.6 Hz, —CCH3). HRMS calcd. for C20H19F3N4O2S ([M+H]+), 437.1254; found, 437.1252. [α]D20 = -29.8(c = 1, MeOH)
(S, R)-3-Iodo-N2-(1-(N-cyano-S-methylsulfinimidoyl)-propan-2-yl)-N1-(4-chloro-2-methylphenyl)phthalamide Ⅰg: White solid; yield 64.24%; mp 133–134 ℃; 1H NMR (400 MHz, DMSO-d6): δ 9.83 (s, 1H, Ar-NH), 8.80 (d, 1H, J = 7.2 Hz, —CNH), 8.03 (d, 1H, J = 7.4 Hz, Ar-H), 7.77 (d, 1H, J = 6.6 Hz, Ar-H), 7.44 (d, 1H, J = 8.2 Hz, Ar-H), 7.35 (s, 1H, Ar-H), 7.29 (m, 2H, Ar-H), 4.25 (m, 1H, -NCH), 3.29 (m, 1H, —SCH2), 3.20 (d, 1H, J = 6.5 Hz, -SCH2), 2.71 (s, 3H, —SCH3), 2.25 (s, 3H, ArCH3), 1.26 (d, 3H, J = 5.8 Hz, —CCH3). HRMS calcd. for C20H20ClIN4O2S ([M + H]+), 543.0113; found, 543.0107. [α]D20 = -32.2 (c = 1, MeOH).
(S, R)-3-Nitro-N2-(1-(N-cyano-S-methylsulfinimidoyl)-propan-2-yl)-N1-(4-chloro-2-methylphenyl)phthalamide Ⅰh: White solid; yield 63.84%; mp 184–185 ℃; 1H NMR (400 MHz, DMSO-d6): δ 10.09 (s, 1H, Ar-NH), 9.02 (d, 1H, J = 7.6 Hz, —CNH), 8.25 (d, 1H, J = 8.1 Hz, Ar-H), 8.11 (d, 1H, J = 7.5 Hz, Ar-H), 7.83 (t, 1H, J = 7.9 Hz, ArH), 7.48 (d, 1H, J = 8.4 Hz, Ar-H), 7.38 (s, 1H, Ar-H), 7.32 (d, 1H, J = 8.2 Hz, Ar-H), 4.24 (dt, 1H, J = 13.1, 6.5 Hz, —NCH), 3.28 (dd, 1H, J = 12.7, 5.8 Hz, —SCH2), 3.17 (dd, 1H, J = 12.7, 6.7 Hz, —SCH2), 2.74 (s, 3H, -SCH3), 2.30 (s, 3H, ArCH3), 1.23 (d, 3H, J = 6.6 Hz, —CCH3). HRMS calcd. for C20H20ClN5O4S ([M+H]+), 462.0998; found, 462.0994. [α]D20 = -44.8(c = 0.5, MeOH).
(S, R)-3-Iodo-N2-(1-(N-cyano-S-methylsulfinimidoyl)-propan-2-yl)-N1-(2, 3, 4-trifluorophenyl)phthalamide Ⅰi: White solid; yield 71.09%; mp 159–162 ℃; 1H NMR (400 MHz, DMSO-d6): δ 10.35 (s, 1H, Ar-NH), 8.78 (d, 1H, J = 6.7 Hz, —CNH), 8.05 (d, 1H, J = 7.2 Hz, ArH), 7.75 (s, 1H, Ar-H), 7.45 (s, 1H, Ar-H), 7.39–7.26 (m, 3H, Ar-H), 4.24 (m, 1H, —NCH), 3.32–3.28 (m, 1H, -SCH2), 3.23–3.18 (m, 1H, —SCH2), 2.78 (s, 3H, —SCH3), 1.27 (d, 3H, J = 5.7 Hz, —CCH3). HRMS calcd. for C19H16F3IN4O2S ([M+H]+), 549.0064; found, 549.0067. [α]D20 = +1.2(c = 1, DMF).
(S, R)-3-Nitro-N2-(1-(N-cyano-S-methylsulfinimidoyl)-propan-2-yl)-N1-(2, 3, 4-trifluorophenyl)phthalamide, Ⅰj: White solid; yield 63.48%; mp 160–163 ℃; 1H NMR (400 MHz, DMSO-d6): δ 10.63 (s, 1H, Ar-NH), 9.00 (d, 1H, J = 7.7 Hz, —CNH), 8.26 (d, 1H, J = 8.1 Hz, ArH), 8.08 (d, 1H, J = 7.5 Hz, Ar-H), 7.82 (t, 1H, J = 7.9 Hz, Ar-H), 7.54 (s, 1H, Ar-H), 7.41–7.34 (m, 1H, Ar-H), 4.25–4.17 (m, 1H, —NCH), 3.29 (dd, 1H, J = 12.7, 5.9 Hz, —SCH2), 3.17 (dd, 1H, J = 12.6, 7.0 Hz, —SCH2), 2.80 (s, 3H, —SCH3), 1.23 (d, 3H, J = 6.6 Hz, —CCH3). HRMS calcd. for C19H16F3N5O4S ([M+H]+), 468.0948; found, 468.0946. [α]D20 = +10.0(c = 1, DMF).
(S, S)-3-Iodo-N2-(1-(N-cyano-S-methylsulfinimidoyl)-propan-2-yl)-N1-(3-(trifluoromethyl)phenyl)phthalamide, Ⅱa: White solid; yield 26.59%; mp 147–149 ℃; 1H NMR (400 MHz, DMSO) δ 10.63 (s, 1H, Ar-NH), 8.64 (d, J = 7.1 Hz, 1H, —CNH), 8.15 (s, 1H, Ar-H), 8.04 (d, J = 7.5 Hz, 1H, Ar-H), 7.93 (d, J = 7.2 Hz, 1H, Ar-H), 7.73 (d, J = 7.2 Hz, 1H, Ar-H), 7.59 (t, J = 7.3 Hz, 1H, Ar-H), 7.46 (d, J = 7.0 Hz, 1H, Ar-H), 7.30 (t, J = 7.3 Hz, 1H, Ar-H), 4.23 (m, 1H, —NCH), 3.31–3.16 (m, 2H, —SCH2), 2.76 (s, 3H, —SCH3), 1.26 (d, J = 5.8 Hz, 3H, —CCH3). HRMS calcd for C20H18F3IN4O2S ([M+H]+), 563.0220; found, 563.0216. [α]20 D = +28.6(c = 0.2, MeOH).
(S, S)-3-Nitro-N2-(1-(N-cyano-S-methylsulfinimidoyl)-propan-2-yl)-N1-(3-(trifluoromethyl)phenyl)phthalamide, Ⅱb: White solid; yield 30.06%; mp 105–107 ℃; 1H NMR (400 MHz, DMSOd6): δ 10.87 (s, 1H, Ar-NH), 8.87 (d, 1H, J = 7.7 Hz, -CNH), 8.25 (d, 1H, J = 8.2 Hz, Ar-H), 8.17 (s, 1H, Ar-H), 8.08 (d, 1H, J = 7.6 Hz, Ar-H), 7.94 (d, 1H, J = 8.1 Hz, Ar-H), 7.83 (t, 1H, J = 8.0 Hz, Ar-H), 7.62 (t, 1H, J = 8.0 Hz, Ar-H), 7.49 (d, 1H, J = 7.7 Hz, Ar-H), 4.21 (m, 1H, —NCH), 3.25 (dd, 1H, J = 13.2, 9.4 Hz, —SCH2), 3.16 (dd, 1H, J = 13.3, 4.4 Hz, —SCH2), 2.77 (s, 3H, —SCH3), 1.23 (d, 3H, J = 6.7 Hz, —CCH3). HRMS calcd. for C20H18F3N5O4S ([M+H]+), 482.1105; found, 482.1103. [α]D20 = +8.0(c = 0.1, MeOH).
(S, S)-3-Chloro-N2-(1-(N-cyano-S-methylsulfinimidoyl)-propan-2-yl)-N1-(3-(trifluoromethyl)phenyl)phthalamide, Ⅱc: White solid; yield 31.57%; mp 148–150 ℃; 1H NMR (400 MHz, DMSO-d6): δ 10.64 (s, 1H, Ar-NH), 8.72 (d, J = 4.2 Hz, 1H, —CNH), 8.15 (s, 1H, Ar-H), 7.89 (d, J = 7.9 Hz, 1H, Ar-H), 7.71–7.66 (m, 2H, Ar-H), 7.62–7.54 (m, 2H, Ar-H), 7.46 (d, J = 7.7 Hz, 1H, Ar-H), 4.24-4.21 (m, 1H, —NCH), 3.29–3.17 (m, 2H, —SCH2), 2.78 (s, 3H, —SCH3), 1.22 (d, J = 6.7 Hz, 3H, —CCH3). HRMS calcd. for C20H18ClF3N4O2S ([M+H]+), 471.0864; found, 471.0868. [α]D20 = -+ 51.6(c = 0.5, (CH3)2CO).
(S, S)-3-Fluoro-N2-(1-(N-cyano-S-methylsulfinimidoyl)-propan-2-yl)-N1-(3-(trifluoromethyl)phenyl)phthalamide, Ⅱd: White solid; yield 29.38%; mp 110–112 ℃; 1H NMR (400 MHz, DMSO-d6): δ 10.74 (s, 1H, Ar-NH), 8.76 (d, 1H, J = 7.9 Hz, —CNH), 8.15 (s, 1H, Ar-H), 7.94 (d, 1H, J = 8.0 Hz, Ar-H), 7.60 (dd, 3H, J = 10.5, 6.0 Hz, Ar-H), 7.47 (t, 2H, J = 8.9 Hz, Ar-H), 4.29-4.22 (m, 1H, —NCH), 3.28 (dd, 1H, J = 13.2, 9.5 Hz, —SCH2), 3.21 (dd, 1H, J = 13.2, 4.5 Hz, —SCH2), 2.80 (s, 3H, —SCH3), 1.26 (d, 3H, J = 6.7 Hz, —CCH3). HRMS calcd. for C20H18F4N4O2S ([M+H]+), 455.1160; found, 455.1162. [α]D20 = +76.8(c = 0.5, (CH3)2CO).
(S, S)-3-Bromo-N2-(1-(N-cyano-S-methylsulfinimidoyl)-propan-2-yl)-N1-(3-(trifluoromethyl)phenyl)phthalamide, Ⅱe: White solid; yield 28.75%; mp 201–203 ℃; 1H NMR (400 MHz, DMSO-d6): δ 10.72 (s, 1H, Ar-NH), 8.73 (s, 1H, —CNH), 8.15 (s, 1H, Ar-H), 7.93 (d, 1H, J = 8.9 Hz, Ar-H), 7.73–7.66 (m, 2H, Ar-H), 7.63–7.54 (m, 2H, Ar-H), 7.46 (d, 1H, J = 7.7 Hz, Ar-H), 4.29–4.20 (m, 1H, —NCH), 3.32–3.30 (m, 1H, —SCH2), 3.23 (dd, 1H, J = 13.4, 7.1 Hz, -SCH2), 2.78 (s, 3H, —SCH3), 1.24 (d, 3H, J = 6.7 Hz, —CCH3). HRMS calcd. for C20H18BrF3N4O2S ([M+H]+), 515.0359; found, 515.0356. [α]D20 = +59.2(c = 0.29, DMF).
(S, S)-N2-(1-(N-Cyano-S-methylsulfinimidoyl)-propan-2-yl)-N1-(3-(trifluoromethyl)phenyl)phthalamide, Ⅱf: White solid; yield 40.14%; mp 85–87 ℃; 1H NMR (400 MHz, DMSO-d6): δ 10.68 (s, 1H, Ar-NH), 8.58 (d, 1H, J = 7.2 Hz, —CNH), 8.20 (s, 1H, Ar-H), 7.89 (s, 1H, Ar-H), 7.59 (d, 5H, J = 5.9 Hz, Ar-H), 7.44 (d, 1H, J = 7.2 Hz, Ar-H), 4.27 (m, 1H, —NCH), 3.29 (d, 2H, J = 9.6 Hz, —SCH2), 2.81 (s, 3H, —SCH3), 1.27 (d, 3H, J = 6.6 Hz, -CCH3). C20H19F3N4O2S. HRMS calcd. for C20H19F3N4O2S ([M+H]+), 437.1254; found, 437.1252. [α]D20 = +45.1 (c = 1, MeOH).
(S, S)-3-Iodo-N2-(1-(N-cyano-S-methylsulfinimidoyl)-propan-2-yl)-N1-(4-chloro-2-methylphenyl)phthalamide, Ⅱg: White solid; yield 35.76%; mp 109–111 ℃; 1H NMR (400 MHz, DMSO-d6): δ 9.81 (s, 1H, Ar-NH), 8.64 (d, 1H, J = 6.3 Hz, —CNH), 8.02 (d, 1H, J = 6.3 Hz, Ar-H), 7.74 (s, 1H, Ar-H), 7.47 (d, 1H, J = 7.7 Hz, Ar-H), 7.35 (s, 1H, ArH), 7.29 (m, 2H, Ar-H), 4.22 (m, 1H, —NCH), 3.29–3.22 (m, 1H, —SCH2), 3.16 (d, 1H, J = 11.9 Hz, —SCH2), 2.71 (s, 3H, —SCH3), 2.26 (s, 3H, ArCH3), 1.27 (d, 3H, J = 4.1 Hz, —CCH3). HRMS calcd. for C20H20ClIN4O2S ([M+H]+), 543.0113; found, 543.0107. [α]D20 = +115.6 (c = 1, MeOH)
(S, S)-3-Nitro-N2-(1-(N-cyano-S-methylsulfinimidoyl)-propan-2-yl)-N1-(4-chloro-2-methylphenyl)phthalamide, Ⅱh: White solid; yield 36.16%; mp 129–130 ℃; 1H NMR (400 MHz, DMSO-d6): δ 10.06 (s, 1H, Ar-NH), 8.84 (d, 1H, J = 7.5 Hz, —CNH), 8.23 (d, 1H, J = 8.1 Hz, Ar-H), 8.08 (d, 1H, J = 7.5 Hz, Ar-H), 7.82 (d, 1H, J = 7.9 Hz, Ar-H), 7.50 (d, 1H, J = 8.4 Hz, Ar-H), 7.36 (s, 1H, Ar-H), 7.30 (d, 1H, J = 8.3 Hz, Ar-H), 4.18 (m, 1H, —NCH), 3.26–3.18 (m, 1H, —SCH2), 3.13 (dd, 1H, J = 13.2, 4.1 Hz, —SCH2), 2.71 (s, 3H, —SCH3), 2.28 (s, 3H, ArCH3), 1.24 (d, 3H, J = 6.6 Hz, —CCH3). HRMS calcd. for C20H20ClN5O4S ([M+H]+), 462.0998; found, 462.0994. [α]D20 = +24.0 (c = 0.2, MeOH).
(S, S)-3-Nitro-N2-(1-(N-cyano-S-methylsulfinimidoyl)-propan-2-yl)-N1-(2, 3, 4-trifluorophenyl)phthalamide, Ⅱj: White solid; yield 36.52%; mp 168–170 ℃; 1H NMR (400 MHz, DMSO-d6): δ 10.60 (s, 1H, Ar-NH), 8.85 (d, 1H, J = 7.5 Hz, -CNH), 8.25 (d, 1H, J = 8.1 Hz, Ar-H), 8.06 (d, 1H, J = 7.5 Hz, Ar-H), 7.81 (t, 1H, J = 7.9 Hz, Ar-H), 7.58 (s, 1H, Ar-H), 7.37 (dd, 1H, J = 17.7, 8.7 Hz, Ar-H), 4.18 (m, 1H, —NCH), 3.27–3.20 (m, 2H, —SCH2), 3.15 (dd, 1H, J = 13.2, 4.3 Hz, -SCH2), 2.77 (s, 3H, —SCH3), 1.24 (d, 3H, J = 6.6 Hz, —CCH3). HRMS calcd for C19H16F3N5O4S ([M+H]+), 468.0948; found, 468.0946. [α]D20 = -86.4(c = 0.5, DMF).
4.4 X-ray diffraction
The crystals of compound Ⅰf and Ⅰh were established, and X-ray intensity data were measured at 298 K on a Bruker SMART 1000 CCD area detector diffraction meter with graphite monochromated Mo Kα radiation (λ=0.71073 Å). All hydrogen atoms were observed and placed at their calculated positions with a fixed value of their isotropic displacement parameters.
4.5 Biological assay
All biological assays were carried out on representative test organisms prepared in the laboratory. The bioassay experiments were repeated at 25 ±1 ℃ according to statistical needs. Assessments were conducted on the basis of dead/alive, and mortality rates were corrected applying Abbott's formula [19]. Percentage mortalities were evaluated based on a percentage scale of 0-100, in which 0 indicates no activity and 100 indicates total kill. Error of the bioassay experiments was 5%. LC50 values were calculated by probit analysis [20].
4.5.1 Larvicidal activity against oriental armyworm (Mythimna separate Walker)
The insecticidal activity of compounds 5f-5j, Ⅰa-Ⅰj, Ⅱa-Ⅱj and Flubendiamide were evaluated using the reported procedure [21]. The insecticidal activity is outlined in Table 1. LC50 Values of Compounds Ⅰa, Ⅰg, Ⅰj, Ⅱa, Ⅱg, Ⅱj and Flubendiamide are shown in Table 2.
Acknowledgments
This work was supported by National Natural Science Foundation of China (No. 21602118), Innovation Center of Chemical Science and Engineering (Tianjin) and the National Natural Science Foundation of China (No. 21302104).
-

-
-
[1]
Hong-Tao Ji , Yu-Han Lu , Yan-Ting Liu , Yu-Lin Huang , Jiang-Feng Tian , Feng Liu , Yan-Yan Zeng , Hai-Yan Yang , Yong-Hong Zhang , Wei-Min He . Nd@C3N4-photoredox/chlorine dual catalyzed synthesis and evaluation of antitumor activities of 4-alkylated sulfonyl ketimines. Chinese Chemical Letters, 2025, 36(2): 110568-. doi: 10.1016/j.cclet.2024.110568
-
[2]
Chengyao Zhao , Jingyuan Liao , Yuxiang Zhu , Yiying Zhang , Lianjie Zhai , Junrong Huang , Hengzhi You . Polystyrene-supported phosphoric-acid catalyzed atroposelective construction of axially chiral N-aryl benzimidazoles. Chinese Chemical Letters, 2025, 36(6): 110337-. doi: 10.1016/j.cclet.2024.110337
-
[3]
Quan Zhou , Xiao-Min Chen , Xujie Qin , Zhe-Ning Chen , Jun Chen , Wei Zhuang . The counterintuitive aromaticity of bent metallabenzenes: A theoretical exploration. Chinese Chemical Letters, 2025, 36(4): 109770-. doi: 10.1016/j.cclet.2024.109770
-
[4]
Jaeyong Ahn , Zhenping Li , Zhiwei Wang , Ke Gao , Huagui Zhuo , Wanuk Choi , Gang Chang , Xiaobo Shang , Joon Hak Oh . Surface doping effect on the optoelectronic performance of 2D organic crystals based on cyano-substituted perylene diimides. Chinese Chemical Letters, 2024, 35(9): 109777-. doi: 10.1016/j.cclet.2024.109777
-
[5]
Luyao Lu , Chen Zhu , Fei Li , Pu Wang , Xi Kang , Yong Pei , Manzhou Zhu . Ligand effects on geometric structures and catalytic activities of atomically precise copper nanoclusters. Chinese Journal of Structural Chemistry, 2024, 43(10): 100411-100411. doi: 10.1016/j.cjsc.2024.100411
-
[6]
Run-Han Li , Tian-Yi Dang , Wei Guan , Jiang Liu , Ya-Qian Lan , Zhong-Min Su . Evolution exploration and structure prediction of Keggin-type group IVB metal-oxo clusters. Chinese Chemical Letters, 2024, 35(5): 108805-. doi: 10.1016/j.cclet.2023.108805
-
[7]
Yuqing Liu , Yu Yang , Yuhan E , Changlong Pang , Di Cui , Ang Li . Insight into microbial synthesis of metal nanomaterials and their environmental applications: Exploration for enhanced controllable synthesis. Chinese Chemical Letters, 2024, 35(11): 109651-. doi: 10.1016/j.cclet.2024.109651
-
[8]
Zhiwen Li , Jingjing Zhang , Gao Li . Dynamic assembly of chiral golden knots. Chinese Journal of Structural Chemistry, 2024, 43(7): 100300-100300. doi: 10.1016/j.cjsc.2024.100300
-
[9]
Ji Zhang , Tong Zhang , Qiao An , Peng Zhang , Cai-Yan Tian , Chun-Mao Yuan , Ping Yi , Zhan-Xing Hu , Xiao-Jiang Hao . Five quinolizidine alkaloids with anti-tobacco mosaic virus activities from two species of Sophora. Chinese Chemical Letters, 2024, 35(6): 108927-. doi: 10.1016/j.cclet.2023.108927
-
[10]
Ting Li , Xinxin Zheng , Lejing Qu , Yuanyuan Ou , Sai Qiao , Xue Zhao , Yajun Zhang , Xinfeng Zhao , Qian Li . A chromatographic method for pursuing potential GPCR ligands with the capacity to characterize their intrinsic activities of regulating downstream signaling pathway. Chinese Chemical Letters, 2024, 35(10): 109792-. doi: 10.1016/j.cclet.2024.109792
-
[11]
Chao LIU , Jiang WU , Zhaolei JIN . Synthesis, crystal structures, and antibacterial activities of two zinc(Ⅱ) complexes bearing 5-phenyl-1H-pyrazole group. Chinese Journal of Inorganic Chemistry, 2024, 40(10): 1986-1994. doi: 10.11862/CJIC.20240153
-
[12]
Linfang ZHANG , Wenzhu YIN , Gui YIN . A 2-dicyanomethylene-3-cyano-4,5,5-trimethyl-2,5-dihydrofuran-based near-infrared fluorescence probe for the detection of hydrogen sulfide and imaging of living cells. Chinese Journal of Inorganic Chemistry, 2025, 41(3): 540-548. doi: 10.11862/CJIC.20240405
-
[13]
Fenglin Wang , Chengwei Kuang , Zhicheng Zheng , Dan Wu , Hao Wan , Gen Chen , Ning Zhang , Xiaohe Liu , Renzhi Ma . Noble metal clusters substitution in porous Ni substrate renders high mass-specific activities toward oxygen evolution reaction and methanol oxidation reaction. Chinese Chemical Letters, 2025, 36(6): 109989-. doi: 10.1016/j.cclet.2024.109989
-
[14]
Long Jin , Jian Han , Dongmei Fang , Min Wang , Jian Liao . Pd-catalyzed asymmetric carbonyl alkynylation: Synthesis of axial chiral ynones. Chinese Chemical Letters, 2024, 35(6): 109212-. doi: 10.1016/j.cclet.2023.109212
-
[15]
Chuan-Zhi Ni , Ruo-Ming Li , Fang-Qi Zhang , Qu-Ao-Wei Li , Yuan-Yuan Zhu , Jie Zeng , Shuang-Xi Gu . A chiral fluorescent probe for molecular recognition of basic amino acids in solutions and cells. Chinese Chemical Letters, 2024, 35(10): 109862-. doi: 10.1016/j.cclet.2024.109862
-
[16]
Wenying Cui , Zhetong Jin , Wentao Fu , Chengshuo Shen . Flag-hinge-like highly luminescent chiral nanographenes with twist geometry. Chinese Chemical Letters, 2024, 35(11): 109667-. doi: 10.1016/j.cclet.2024.109667
-
[17]
Genlin Sun , Yachun Luo , Zhihong Yan , Hongdeng Qiu , Weiyang Tang . Chiral metal-organic frameworks-based materials for chromatographic enantioseparation. Chinese Chemical Letters, 2024, 35(12): 109787-. doi: 10.1016/j.cclet.2024.109787
-
[18]
Teng-Yu Huang , Junliang Sun , De-Xian Wang , Qi-Qiang Wang . Recent progress in chiral zeolites: Structure, synthesis, characterization and applications. Chinese Chemical Letters, 2024, 35(12): 109758-. doi: 10.1016/j.cclet.2024.109758
-
[19]
Chuang LIU , Lichao SUN , Qingfeng ZHANG . Chiral inorganic nanocatalysts for electrochemical and enzyme-mimicked biosensing. Chinese Journal of Inorganic Chemistry, 2025, 41(1): 59-78. doi: 10.11862/CJIC.20240406
-
[20]
Cong Gao , Zijian Zhu , Siwei Li , Zheng Xi , Qingqing Sun , Jie Han , Rong Guo . Chiral supramolecular catalysts of helical nanoribbon: More twist, higher enantioselectivity. Chinese Chemical Letters, 2025, 36(3): 109968-. doi: 10.1016/j.cclet.2024.109968
-
[1]
Metrics
- PDF Downloads(2)
- Abstract views(787)
- HTML views(14)

 Login In
Login In




 下载:
下载:
 DownLoad:
DownLoad: