Effects of concave and convex substrate curvature on cell mechanics and the cytoskeleton
- Corresponding author: Lu Qing-Hua, qhlu@sjtu.edu.cn
Citation:
Chen Shuang-Shuang, Lu Xue-Min, Lu Qing-Hua. Effects of concave and convex substrate curvature on cell mechanics and the cytoskeleton[J]. Chinese Chemical Letters,
;2017, 28(4): 818-826.
doi:
10.1016/j.cclet.2016.10.039

Dopamine (3, 4-dihydroxyphenylethylamine, DA) is a catecholamine neurotransmitter, which plays a significant role in both the central and peripheral nervous systems [1]. The disorder concentration of DA has been considered to be the parameter of a series of neural and metabolic diseases [2-4]. Therefore, the accurate detection of DA is of great importance. Additionally, DA can also be oxidized and polymerize to form polydopamine (pDA) under alkaline condition. For example, DA was added to the mixture of Tris-buffer (pH 8.5) and isopropyl alcohol with continuous stirring for 48 h in the dark, producing black pDA [5-7]. As well known, pDA is an effective fluorescent quencher and is utilized to detect many biomolecules [8-10]. However, there are only a few studies that report the detection of DA by the quenching property of pDA [11, 12]. The reason may be that the self-polymerization of DA is time-consuming. Moreover, the detection is insensitive since DA with a low concentration is hard to self-polymerize. Thus, the establishment of a simple, feasible and high-efficiency way to monitor DA by employing pDA as the fluorescent quencher remains a challenge.
Benefiting from the advantages of nanotechnology, the special structures and optical properties of nanoscaled materials have attracted a wide attention [13, 14]. Covalent organic frameworks (COFs) are a kind of popular materials, linked by covalent bonds. Schwab et al. reported a series of COFs by polycondensation between melamine and di- or tri-aldehydes and defined them as Schiff base networks [15]. Thanks to outstanding chemical stability, electron-rich property and unique optical feature, COFs have shown promising attention in the fields of sensing applications. For instance, through the photoinduced electron transfer (PET) mechanism, the fluorescence of COFs was quenched by electron-deficient nitroaromatic explosives including picric acid (PA) [16], 2, 4, 6-trinitrophenol (TNP) [17] and 2, 4, 6-trinitrotoluene (TNT) [18]. However, a majority of biomolecules are electron-rich. Hence, COFs are rarely used to determine biomolecules.
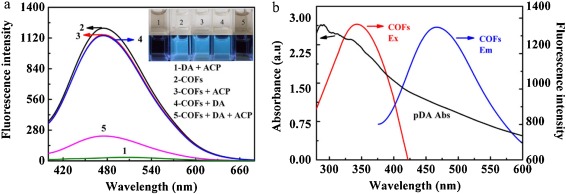
In this work, a fluorescent strategy for DA detection is proposed based on in situ growth of pDA on the surface of COFs with the aid of acid phosphatase (ACP). As shown in Transmission electron microscopy (TEM) image, COFs are nearly spherical nanoparticles and the average diameter is about 45 nm (Fig. S1a in Supporting information). Comparing the Fourier transform infrared (FT-IR) spectra of terephthalaldehyde and melamine with that of COFs (Fig. S1b in Supporting information), the C=O stretching vibration peak of terephthalaldehyde at 1698 cm−1 disappears in the spectrum of COFs [15]. The two peaks at 3468.5 cm-1 and 3418.8 cm−1 of melamine are ascribed to the stretching vibration of NH2, and the peak at 1650 cm−1 attributed to NH2 deformation is also absent in the spectrum of COFs [19]. The result demonstrates that the formation of COFs depends on the dehydration reaction between the amino groups of melamine and aldehyde groups of terephthalaldehyde. Moreover, owing to the C=N vibration of triazine ring of melamine, two disparate bands at 1548 cm−1 and 1480 cm−1 appear in COFs, revealing the frameworks combined by triazine ring units. In the X-ray photoelectron spectroscopy (XPS) spectra of COFs (Figs. S1c-e in Supporting information), two peaks at 399.3 and 284.3 eV corresponding to N 1s and C 1s are observed. The spectrum of C 1s can be further fitted into four peaks. The peak at 284.6 and 285.7 ev are attributed to carbon atoms in the benzene ring (C=C, C-C) of terephthalaldehyde. The peak of linkages (C-N) is located at 286.7 eV, and the peak at 287.6 eV is assigned to the carbon atom in the triazine ring (C=N) of melamine [20]. These results reveal the successful crosslinking between the monomers after pyrolysis [21]. Furthermore, the N 1s can be grouped into two peaks at 398.6 and 399.8 ev corresponding to nitrogen atoms in the triazine ring (C=N) of melamine and amine (NH) moieties of linkages, respectively [22]. The spectra of XPS confirm the formation of COFs, and the functional groups of COFs are triazine rings, benzene rings and aminol groups. Besides, the elemental analysis suggests that COFs are mainly constituted of C, H and N elements, and their content are 63.44%, 4.8% and 31.72%, respectively. It further proves that COFs are composed of light elements. Moreover, COFs display a blue emission centered at 470 nm with the maximum excitation wavelength at 370 nm (Fig. S1f in Supporting information). According to the reported works, the triazine ring units associated with theπ-π* electronic transitions are the main reason for the luminescence of COFs [20, 23]. COFs also show a good stability, and their fluorescence can maintain a high level at least one month and in a wide pH range (Fig. S2 in Supporting information).
As well known, ACP can cleave a phosphate group from related substrates. However, the catalytic effect of ACP on DA polymerization is different from it, because there is no phosphate group in DA. The UV–vis characterization of DA polymerization is used to compare the ACP-mediated reaction (PBS buffer, pH 7.0) with pH-induced one (Tris-Buffer, pH 8.5). As illustrated in Fig. 1a and b, the peak at 280 nm is attributed to the π-π* electronic transitions of DA [24], and ACP displays an absorbance peak at 225 nm due to the nature of protein absorption. Especially, the shoulder peak at around 475 nm is developed at first 5 min in both ACP-mediated and pH-induced polymerization, and it is the n-π* electronic transitions of dopaquinone [25]. Dopaquinone is the initial oxidation product of DA. Then, another should peak at 320 nm can be distinguished after 45 min in ACP-mediated reaction and 4 h in pH-induced one. It is ascribed to the formation of 5, 6-dihydroxyindole via intramolecular cyclization [26]. 5, 6-dihydroxyindole is further oxidized into 5, 6-indolequinone. Subsequently, both integrate into dimers or trimers, then polymerization of DA [27]. A useful distinction can be drawn between these two ways. The absorbance at 475 nm changes significantly in pH-induced polymerization, revealing the formation of large amount of dopaquinone. However, the absorbance variation at 320 nm is clear in ACP-mediated reaction, so 5, 6-dihydroxyindole or 5, 6-indolequinone is the main intermediate. It is speculated that the catalysis of ACP accelerates the conversion from DA to 5, 6-dihydroxyindole or 5, 6-indolequinone. Therefore, ACP-mediated oxidation shortens the time of pDA formation, implying higher efficiency. Similarly, the study of Pan's group also suggested the reducing agent suppressed the polymerization of DA catalyzed by ACP, but the inhibitors of ACP had no such effect [28]. It means that the catalysis of ACP in DA polymerization is related to the oxidation. Herein, our assay also supports this view.
For another, the mixture color changes from colorless to brown and then to black after 4 h in ACP-mediated oxidation or 48 h in pH-induced one (inset of Fig. 1), also indicating the complete formation of pDA. As shown in the Scanning electron microscope (SEM) images (Figs. 1c and d), there are some regular-shaped pDA and amounts of irregular agglomerates in ACP-mediated oxidation. However, the obtained pDA nanoparticles are all spherical in pH-induced polymerization. The reason may be that some fragments have not agglomerate to form regular sphere in the short time of ACP-mediated oxidation. Besides, particle size (about 500 nm), functional groups, and charges of the particles formed in these two ways are similar (Figs. S3a and b in Supporting information). On the above discussions, ACP-mediated oxidation provides an efficient choice for the preparation of pDA. Other enzymes and protein cannot catalyze the oxidation and polymerization of DA in neutral medium (Fig. S4 in Supporting information), suggesting the high specificity of ACP for DA in the polymerization.
Based on in situ growth of pDA on the surface of COFs, the emission of COFs is quenched significantly (Fig. 2a), and the color of the solution changes to black, which can be distinguished by bare eyes. However, when DA or ACP is added alone, no obvious change in the fluorescence intensity is observed, and the solution remains transparent under the daylight. Subsequently, the quenching mechanism has been investigated. In the process of ACP-mediated oxidation, the initial oxidation product of DA is dopaquinone [29]. However, dopaquinone makes a negligible effect on the fluorescence of COFs (Fig. S5b in Supporting information). Hence, the fluorescence quenching of COFs is ascribed to pDA rather than dopaquinone. Additionally, it is observed that the absorption spectrum of pDA overlaps well with both of the excitation and emission spectra of COFs (Fig. 2b). The result signifies that the quenching mechanism may be Forster resonance energy transfer (FRET) or inner filter effect (IFE). After incubation with DA and ACP, the fluorescence decay of COFs changes from 12.3416 ns to 9.3742 ns (Fig. S6 in Supporting information). The FRET efficiency is evaluated by the Eq. 1 [30]:
|
|
(1) |
where E is the FRET efficiency, τ (τ = 9.3742 ns) andτ0 (τ0 = 12.3416 ns) are the fluorescence lifetimes of the COFs in the presence and absence of DA and ACP. The calculated FRET efficiency is 24%. Subsequently, the quenching efficiency for IFE of pDA on the fluorescence of COFs is further studied according to Eq. 2 [31]:
|
|
(2) |
where CF represents the correction factor; Fobsd stands for the maximum fluorescence intensity of COFs with addition of DA at 470 nm and Fcor is corrected fluorescence intensity by removing the IFE from Fobsd; Aex and Aem refer to the absorbance of COFs with the addition of DA at 370 and 470 nm, respectively; d represents the width of the cuvette (1.00 cm); g denotes the distance between the edge of the excitation beam and the edge of the cuvette (0.40 cm) and s is the thickness of excitation beam (0.10 cm). In order to obtain more precise measurements, the value of CF should not exceed 3. As shown in Table S1 (Supporting information), the CF of IFE and relevant parameters with addition of different concentrations of DA are calculated at different temperatures. The corrected suppressed efficiency (E%) of pDA is obtained on the basis of the Eqs. 3 and 4:
|
|
(3) |
|
|
(4) |
where Fobsd, 0 or Fcor, 0 represents the observed or the corrected fluorescence intensity of COFs in the absence of DA and ACP. As depicted in Fig. S7 (Supporting information), the quenching effect attributed to IFE at 298 K is 22% after calculation. Almost 46% of quenching effect results from FRET and IFE, revealing other quenching mechanism also coexisting.
Then, Stern-Volmer equation [32] is employed to evaluate the role of static quenching effect (SQE) or dynamic quenching effect (DQE) in fluorescence suppression (Eq. 5).
|
|
(5) |
F0 and F denote the fluorescence intensities of COFs before and after the addition of quencher. Considering IFE, Fcor, 0 and Fcor should take place of the F0 and F. KSV represents the Stern-Volmer quenching constant and [Q] is the concentration of DA; Kq refers the quenching rate constant and τ0 is the fluorescence lifetime of free COFs (τ0 = 12.3416 ns). As presented in Fig. S8 (Supporting information), the corrected fluorescence intensity ratios increase linearly with the concentration of DA at different temperatures, indicating that the existing quenching mechanism is SQE or DQE [33].
Furthermore, DQE and SQE can be distinguished by their dependence on temperature. In DQE process, the fluorescence quenching is due to collision between the excited-state fluorophore and the quencher, leading to non-radiative transitions to the ground state. Thus, the higher temperature accelerates the collision, resulting in the increase in the KSV [34]. For SQE, the fluorescence quenching is due to the formation of nonfluorescent ground-state complex between the fluorophore and quencher. The higher temperature leads to the lower stability of the complex, so higher temperature is responsible for a smaller value of KSV. The obtained KSV values at 298, 303 and 308 K are 2.019 × 104, 2.267 × 104 and 2.523 × 104 L mol−1, respectively (Fig. S8 in Supporting information). The KSV value is proportional to the increasing temperature, indicating that the fluorescence quenching of COFs by pDA is mainly ascribed to DQE. Additionally, fluorescence lifetime is another important difference in the SQE and DQE. It decreases in the DQE, but it remains constant for SQE with the addition of the quencher [35]. In this assay, the fluorescence lifetime of COFs declines from 12.3416 ns to 9.3742 ns after incubation with DA and ACP, suggesting that the fluorescence quenching is due to DQE. The above proofs indicate that the fluorescence quenching of COFs by pDA is ascribed to the combination of FRET, IFE and DQE.
In order to obtain a sensitive response to DA, several experimental conditions are optimized (Fig. S9 in Supporting information) and detailed description are given in the Supplementary information. Under the optimal conditions, the fluorescence of COFs at 470 nm is quenched linearly with the concentration of DA increasing from 0.5 μmol/L to 50 μmol/L (Fig. 3). The LOD is 0.16 μmol/L which is calculated based on 3σ/K, where σ is the standard deviation of the blank sample and K is the slope of the calibration curve.
A series of interfering substances are tested to assess the selectivity of this method for DA, including 16 kinds of amino acids, bisphenol A, phenol, resorcinol, phloroglucinol, 2, 4-chlorophenol, bovine serum albumin, uric acid (UA), ascorbic acid (AA). As depicted in Fig. S10 (Supporting information), it is obvious that all species do not influence on the fluorescence intensity of COFs without or with addition of DA in the presence of ACP. Especially, UA and AA, as the common coexistence substances in human urine, cannot polymerize with addition of ACP, so they do not interfere with the detection of DA. Furthermore, phenolic compounds are not oxidized under the catalysis of ACP due to absence of O-dihydroxy group. Therefore, the proposed probe shows a high selectivity toward DA.
Moreover, this method is compared with other strategies for DA detection in Table S2 (Supporting information). At present, the predominant methods for determining DA are electrochemical and fluorescent analysis. In electrochemical analysis, the current change generated by 2e/2H+ redox reaction relies on the concentration of DA [36]. The sensitivity of electrochemistry is good, but the selectivity is not satisfactory. In real sample detection, some coexistence substances, such as UA and AA, interferes with the DA detection because their oxidation potentials are close to DA [37]. Furthermore, fluorescent detection of DA is usually realized by the oxidation of DA to dopaquinone. The strong electron-withdrawing ability of dopaquinone makes it a good quencher for various probes. However, tyrosine and some phenolic substrates are also oxidized into quinones [29], so these substances may also interfere with the detection of DA. In this work, a series of evidences show that ACP accelerates the polymerization of DA, but it cannot catalyze the oxidation of UA, AA and other phenolic compounds. The results reveal that the selectivity of this new method is superior to that of electrochemistry and other fluorescent methods, and the sensitivity is not inferior to other ways.
The precision and reproducibility of this method for the detection of DA are studied. The intra-day and inter-day precisions of the assay are determined by estimating the corresponding response 3 times on the same day and on 3 different days over a period of one week. As shown in Table S3 (Supporting information), the relative standard deviation (RSD) of intra-day precision (CV%) ranges from 1.2% to 2.3%, and the RSD of inter-day (CV%) is in the range from 2.1%–3.9%, suggesting a good stability and precision of this method. Subsequently, the proposed method is applied to the determination of DA in human urine samples. The detected concentration of DA in the 2-fold diluted human urine sample is about 0.805 μmol/L, which is corresponding to 1.61 μmol/L of DA in the urine sample (without dilution). The result is in agreement with the normal range of 0.3–2.18 μmol/L [38]. Then, the standard DA solutions with three different concentrations are spiked into urine samples, and the recoveries in the range of 100.3%–101.2% are satisfactory (Table 1). ELISA is chosen as a reference method for DA detection. There is no significant difference between the results of ELISA and those of this strategy, demonstrating the reliability and accuracy of this proposed strategy for determination of DA. Besides, the detection of DA is also performed in cell lysate (Table S4 in Supporting information), and the satisfactory recoveries are obtained. Hence, this method has the potential for applications in complex biological samples.
In summary, this assay provides the first case that the detection of DA is realized by employing in situ growth of pDA on the surface of COFs under the catalysis of ACP. The advantages of this strategy lie on two aspects. One is that ACP-mediated oxidation shortens the time remarkably for the polymerization of DA, so it provides a feasible strategy for DA detection. The other one is the good selectivity toward DA because the common coexistence substances cannot be oxidized under the catalysis of ACP. Thus, the findings not only have the potential to monitor DA in clinical diagnosis but also expand the applications of COFs.
The human urine sample experiments were performed with the approval of the Guidelines for Ethical Committee, Qufu Normal University. All urine samples were from health volunteers with their informed consent. All studies were approved by Ethical Committee, Qufu Normal University.
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
This work was supported by the Natural Science Foundation of Shandong Province, China (No. ZR2019QB010), National Natural Science Foundation of China (Nos. 21705095, 21775088), and the Scientific Research Foundation of Qufu Normal University (No. BS D20130117).
Supplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.cclet.2021.04.010.
Yeung T., Georges P.C., Flanagan L.A.. Effects of substrate stiffness on cell morphology cytoskeletal structure, and adhesion[J]. Cell Motil.Cytoskeleton, 2005,60:24-34. doi: 10.1002/(ISSN)1097-0169
Mullen C.A., Vaughan T.J., Voisin M.C.. Cell morphology and focal adhesion location alters internal cell stress[J]. J.R.Soc.Interface, 2014,1120140885. doi: 10.1098/rsif.2014.0885
Keselowsky B.G., Collard D.M., García A.J.. Surface chemistry modulates fibronectin conformation and directs integrin binding and specificity to control cell adhesion[J]. J.Biomed.Mater.Res.A, 2003,66A:247-259. doi: 10.1002/jbm.a.v66a:2
Gibson L.J., Ashby M.F. The mechanics of three-dimensional cellular materials[J]. Proc.R.Soc.A, 1982,382:43-59. doi: 10.1098/rspa.1982.0088
Chen J., Irianto J., Inamdar S.. Cell mechanics structure, and function are regulated by the stiffness of the three-dimensional microenvironment[J]. Biophys.J., 2012,103:1188-1197. doi: 10.1016/j.bpj.2012.07.054
Bao G., Suresh S. Cell and molecular mechanics of biological materials[J]. Nat. Mater., 2003,2:715-725. doi: 10.1038/nmat1001
Tan J., Saltzman W.M. Biomaterials with hierarchically defined micro-and nanoscale structure[J]. Biomaterials, 2004,25:3593-3601. doi: 10.1016/j.biomaterials.2003.10.034
Ranella A., Barberoglou M., Bakogianni S., Fotakis C., Stratakis E. Tuning cell adhesion by controlling the roughness and wettability of 3D micro/nano silicon structures[J]. Acta Biomater., 2010,6:2711-2720. doi: 10.1016/j.actbio.2010.01.016
Zhao L.Z., Mei S.L., Chu P.K., Zhang Y.M., Wu Z.F. The influence of hierarchical hybrid micro/nano-textured titanium surface with titania nanotubes on osteoblast functions[J]. Biomaterials, 2010,31:5072-5082. doi: 10.1016/j.biomaterials.2010.03.014
Chen C.S., Mrksich M., Huang S., Whitesides G.M., Ingber D.E. Geometric control of cell life and death[J]. Science, 1997,276:1425-1428. doi: 10.1126/science.276.5317.1425
Newhart A., Janicki S.M. Seeing is believing:visualizing transcriptional dynamics in single cells[J]. J.Cell.Physiol., 2014,229:259-265. doi: 10.1002/jcp.24445
Dubey G.P., Ben-Yehuda S.. Intercellular nanotubes mediate bacterial communication[J]. Cell, 2011,144:590-600. doi: 10.1016/j.cell.2011.01.015
Fletcher D.A., Mullins D. Cell mechanics and the cytoskeleton[J]. Nature, 2010,463:485-492. doi: 10.1038/nature08908
Yamaki K., Harada I., Goto M., Cho C.S., Akaike T. Regulation of cellular morphology using temperature-responsive hydrogel for integrin-mediated mechanical force stimulation[J]. Biomaterials, 2009,30:1421-1427. doi: 10.1016/j.biomaterials.2008.11.036
Chen L., Liu X.L., Su B.. Aptamer-mediated efficient capture and release of T lymphocytes on nanostructured surfaces[J]. Adv.Mater., 2011,23:4376-4380. doi: 10.1002/adma.201102435
Frame M.D., Sarelius I.H. Flow-induced cytoskeletal changes in endothelial cells growing on curved surfaces[J]. Microcirculation, 2000,7:419-427. doi: 10.1111/micc.2000.7.issue-6
J. A. Sanz-Herrera, P. Moreo, J. M. García-Aznar, M. Doblaré, On the effect of substrate curvature on cell mechanics, Biomaterials 30(2009)6674-6686.
James J., Goluch E.D., Hu H., Liu C., Mrksich M. Subcellular curvature at the perimeter of micropatterned cells influences lamellipodial distribution and cell polarity[J]. Cell Motil.Cytoskeleton, 2008,65:841-852. doi: 10.1002/cm.v65:11
Tamura M. Inhibition of cell migration, spreading, and focal adhesions by tumor suppressor PTEN[J]. Science, 1998,280:1614-1617. doi: 10.1126/science.280.5369.1614
Kim S.V., Mehal W.Z., Dong X.M.. Modulation of cell adhesion and motility in the immune system by Myo1f[J]. Science, 2006,314:136-139. doi: 10.1126/science.1131920
Cukierman E., Pankov R., Stevens D.R., Yamada K.M. Taking cell-matrix adhesions to the third dimension[J]. Science, 2001,294:1708-1712. doi: 10.1126/science.1064829
Raic A., Rödling L., Kalbacher H., Lee-Thedieck C.. Biomimetic macroporous PEG hydrogels as 3D scaffolds for the multiplication of human hematopoietic stem and progenitor cells[J]. Biomaterials, 2014,35:929-940. doi: 10.1016/j.biomaterials.2013.10.038
Park J., Babensee J.E. Differential functional effects of biomaterials on dendritic cell maturation[J]. Acta Biomater., 2012,8:3606-3617. doi: 10.1016/j.actbio.2012.06.006
A. J. García, Get a grip: integrins in cell-biomaterial interactions, Biomaterials 26(2005)7525-7529.
Whitesides G.M., Grzybowski B. Self-assembly at all scales[J]. Science, 2002,295:2418-2421. doi: 10.1126/science.1070821
Xu Y.X., Sheng K.X., Li C., Shi G.Q. Self-assembled graphene hydrogel via a one-step hydrothermal process[J]. ACS Nano, 2010,4:4324-4330. doi: 10.1021/nn101187z
Zhang S.Y., Regulacio M.D., Han M.Y. Self-assembly of colloidal one-dimensional nanocrystals[J]. Chem.Soc.Rev., 2014,43:2301-2323. doi: 10.1039/c3cs60397k
Yamazaki H., Gotou S., Ito K.. Micropatterned culture of HepG2 spheroids using microwell chip with honeycomb-patterned polymer film[J]. J.Biosci. Bioeng., 2014,118:455-460. doi: 10.1016/j.jbiosc.2014.03.006
A.S.de León, J.Rodríguez-Hernández , Cortajarena A.L. Honeycomb patterned surfaces functionalized with polypeptide sequences for recognition and selective bacterial adhesion[J]. Biomaterials, 2013,34:1453-1460. doi: 10.1016/j.biomaterials.2012.10.074
Zhu Y.D., Sheng R.L., Luo T.. Honeycomb-structured films by multifunctional amphiphilic biodegradable copolymers:surface morphology control and biomedical application as scaffolds for cell growth[J]. ACS Appl.Mater.Interfaces, 2011,3:2487-2495. doi: 10.1021/am200371c
Wu X.H., Wang S.F. Regulating MC3T3-E1 cells on deformable poly (e-caprolactone)honeycomb films prepared using a surfactant-free breath figure method in a water-miscible solvent[J]. ACS Appl.Mater.Interfaces, 2012,4:4966-4975. doi: 10.1021/am301334s
Yap F.L., Zhang Y. Assembly of polystyrene microspheres and its application in cell micropatterning[J]. Biomaterials, 2007,28:2328-2338. doi: 10.1016/j.biomaterials.2007.01.034
M.Hernández-Guerrero , Stenzel M.H. Honeycomb structured polymer films via breath figures[J]. Polym.Chem., 2012,3:563-577. doi: 10.1039/C1PY00219H
Chen S.S., Lu X.M., Zhu D.D., Lu Q.H. Targeted grafting of thermoresponsive polymers from a penetrative honeycomb structure for cell sheet engineering[J]. Soft Matter, 2015,11:7420-7427. doi: 10.1039/C5SM01769F
Chen S.S., Lu X.M., Hu Y., Lu Q.H. Biomimetic honeycomb-patterned surface as the tunable cell adhesion scaffold[J]. Biomater.Sci., 2015,3:85-93. doi: 10.1039/C4BM00233D
Yin Y.D., Alivisatos A.P. Colloidal nanocrystal synthesis and the organic-inorganic interface[J]. Nature, 2005,437:664-670. doi: 10.1038/nature04165
Kawano T., Sato M., Yabu H., Shimomura M. Honeycomb-shaped surface topography induces differentiation of human mesenchymal stem cells (hMSCs):uniform porous polymer scaffolds prepared by the breath figure technique[J]. Biomater.Sci., 2014,2:52-56. doi: 10.1039/C3BM60195A
Biazar E., Khorasani M.T., Joupari M.D. Cell adhesion and surface properties of polystyrene surfaces grafted with poly(N-isopropylacrylamide)[J]. Chin.J.Polym. Sci., 2013,31:1509-1518. doi: 10.1007/s10118-013-1335-3
The software was provided on the website: http://cn.mathworks.com/index.html?s_tid=gn_logo.
Yao X., Peng R., Ding J.D. Cell-material interactions revealed via material techniques of surface patterning[J]. Adv.Mater., 2013,25:5257-5286. doi: 10.1002/adma.201301762
Jeon H., C.G.Simon Jr., Kim G. A mini-review:cell response to microscale, nanoscale, and hierarchical patterning of surface structure[J]. J.Biomed.Mater. Res.Part B Appl.Biomater., 2014,102:1580-1594.
Mosser D.M., Edwards J.P. Exploring the full spectrum of macrophage activation[J]. Nat.Rev.Immunol., 2008,8:958-969. doi: 10.1038/nri2448
Dalby M.J., Gadegaard N., Oreffo R.O.C. Harnessing nanotopography and integrin-matrix interactions to influence stem cell fate[J]. Nat.Mater., 2014,13:558-569. doi: 10.1038/nmat3980
Juan S.H., Tur J.M.M. Tensegrity frameworks:static analysis review[J]. Mech. Mach.Theory, 2008,43:859-881. doi: 10.1016/j.mechmachtheory.2007.06.010
Zhang G.H., Hou R.X., Zhan D.X.. Fabrication of hollow porous PLGA microspheres for controlled protein release and promotion of cell compatibility[J]. Chin.Chem.Lett., 2013,24:710-714. doi: 10.1016/j.cclet.2013.05.011
Heng L.P., Meng X.F., Wang B., Jiang L. Bioinspired design of honeycomb structure interfaces with controllable water adhesion[J]. Langmuir, 2013,29:9491-9498. doi: 10.1021/la401991n
Dembo M., Wang Y.L. Stresses at the cell-to-substrate interface during locomotion of fibroblasts[J]. Biophys.J., 1999,76:2307-2316. doi: 10.1016/S0006-3495(99)77386-8
Ingber D.E. Cellular tensegrity:defining new rules of biological design that govern the cytoskeleton[J]. J.Cell Sci., 1993,104:613-627.
Ingber D.E., Tensegrity I. Cell structure and hierarchical systems biology[J]. J.Cell Sci., 2003,116:1157-1173. doi: 10.1242/jcs.00359
Ingber D.E., Tensegrity I.I. How structural networks influence cellular information processing networks[J]. J.Cell Sci., 2003,116:1397-1408. doi: 10.1242/jcs.00360
Ingber D.E. Tensegrity:the architectural basis of cellular mechanotransduction[J]. Annu.Rev.Physiol., 1997,59:575-599. doi: 10.1146/annurev.physiol.59.1.575
Crawford-Young S.J.. Effects of microgravity on cell cytoskeleton and embryogenesis[J]. Int. J. Dev.Biol., 2006,50:183-191. doi: 10.1387/ijdb.052077sc
Cogoli A., Tschopp A., Fuchs-Bislin P.. Cell sensitivity to gravity[J]. Science, 1984,225:228-230. doi: 10.1126/science.6729481
Yeung T., Georges P.C., Flanagan L.A.. Effects of substrate stiffness on cell morphology cytoskeletal structure, and adhesion[J]. Cell Motil.Cytoskeleton, 2005,60:24-34. doi: 10.1002/(ISSN)1097-0169
Mullen C.A., Vaughan T.J., Voisin M.C.. Cell morphology and focal adhesion location alters internal cell stress[J]. J.R.Soc.Interface, 2014,1120140885. doi: 10.1098/rsif.2014.0885
Keselowsky B.G., Collard D.M., García A.J.. Surface chemistry modulates fibronectin conformation and directs integrin binding and specificity to control cell adhesion[J]. J.Biomed.Mater.Res.A, 2003,66A:247-259. doi: 10.1002/jbm.a.v66a:2
Gibson L.J., Ashby M.F. The mechanics of three-dimensional cellular materials[J]. Proc.R.Soc.A, 1982,382:43-59. doi: 10.1098/rspa.1982.0088
Chen J., Irianto J., Inamdar S.. Cell mechanics structure, and function are regulated by the stiffness of the three-dimensional microenvironment[J]. Biophys.J., 2012,103:1188-1197. doi: 10.1016/j.bpj.2012.07.054
Bao G., Suresh S. Cell and molecular mechanics of biological materials[J]. Nat. Mater., 2003,2:715-725. doi: 10.1038/nmat1001
Tan J., Saltzman W.M. Biomaterials with hierarchically defined micro-and nanoscale structure[J]. Biomaterials, 2004,25:3593-3601. doi: 10.1016/j.biomaterials.2003.10.034
Ranella A., Barberoglou M., Bakogianni S., Fotakis C., Stratakis E. Tuning cell adhesion by controlling the roughness and wettability of 3D micro/nano silicon structures[J]. Acta Biomater., 2010,6:2711-2720. doi: 10.1016/j.actbio.2010.01.016
Zhao L.Z., Mei S.L., Chu P.K., Zhang Y.M., Wu Z.F. The influence of hierarchical hybrid micro/nano-textured titanium surface with titania nanotubes on osteoblast functions[J]. Biomaterials, 2010,31:5072-5082. doi: 10.1016/j.biomaterials.2010.03.014
Chen C.S., Mrksich M., Huang S., Whitesides G.M., Ingber D.E. Geometric control of cell life and death[J]. Science, 1997,276:1425-1428. doi: 10.1126/science.276.5317.1425
Newhart A., Janicki S.M. Seeing is believing:visualizing transcriptional dynamics in single cells[J]. J.Cell.Physiol., 2014,229:259-265. doi: 10.1002/jcp.24445
Dubey G.P., Ben-Yehuda S.. Intercellular nanotubes mediate bacterial communication[J]. Cell, 2011,144:590-600. doi: 10.1016/j.cell.2011.01.015
Fletcher D.A., Mullins D. Cell mechanics and the cytoskeleton[J]. Nature, 2010,463:485-492. doi: 10.1038/nature08908
Yamaki K., Harada I., Goto M., Cho C.S., Akaike T. Regulation of cellular morphology using temperature-responsive hydrogel for integrin-mediated mechanical force stimulation[J]. Biomaterials, 2009,30:1421-1427. doi: 10.1016/j.biomaterials.2008.11.036
Chen L., Liu X.L., Su B.. Aptamer-mediated efficient capture and release of T lymphocytes on nanostructured surfaces[J]. Adv.Mater., 2011,23:4376-4380. doi: 10.1002/adma.201102435
Frame M.D., Sarelius I.H. Flow-induced cytoskeletal changes in endothelial cells growing on curved surfaces[J]. Microcirculation, 2000,7:419-427. doi: 10.1111/micc.2000.7.issue-6
J. A. Sanz-Herrera, P. Moreo, J. M. García-Aznar, M. Doblaré, On the effect of substrate curvature on cell mechanics, Biomaterials 30(2009)6674-6686.
James J., Goluch E.D., Hu H., Liu C., Mrksich M. Subcellular curvature at the perimeter of micropatterned cells influences lamellipodial distribution and cell polarity[J]. Cell Motil.Cytoskeleton, 2008,65:841-852. doi: 10.1002/cm.v65:11
Tamura M. Inhibition of cell migration, spreading, and focal adhesions by tumor suppressor PTEN[J]. Science, 1998,280:1614-1617. doi: 10.1126/science.280.5369.1614
Kim S.V., Mehal W.Z., Dong X.M.. Modulation of cell adhesion and motility in the immune system by Myo1f[J]. Science, 2006,314:136-139. doi: 10.1126/science.1131920
Cukierman E., Pankov R., Stevens D.R., Yamada K.M. Taking cell-matrix adhesions to the third dimension[J]. Science, 2001,294:1708-1712. doi: 10.1126/science.1064829
Raic A., Rödling L., Kalbacher H., Lee-Thedieck C.. Biomimetic macroporous PEG hydrogels as 3D scaffolds for the multiplication of human hematopoietic stem and progenitor cells[J]. Biomaterials, 2014,35:929-940. doi: 10.1016/j.biomaterials.2013.10.038
Park J., Babensee J.E. Differential functional effects of biomaterials on dendritic cell maturation[J]. Acta Biomater., 2012,8:3606-3617. doi: 10.1016/j.actbio.2012.06.006
A. J. García, Get a grip: integrins in cell-biomaterial interactions, Biomaterials 26(2005)7525-7529.
Whitesides G.M., Grzybowski B. Self-assembly at all scales[J]. Science, 2002,295:2418-2421. doi: 10.1126/science.1070821
Xu Y.X., Sheng K.X., Li C., Shi G.Q. Self-assembled graphene hydrogel via a one-step hydrothermal process[J]. ACS Nano, 2010,4:4324-4330. doi: 10.1021/nn101187z
Zhang S.Y., Regulacio M.D., Han M.Y. Self-assembly of colloidal one-dimensional nanocrystals[J]. Chem.Soc.Rev., 2014,43:2301-2323. doi: 10.1039/c3cs60397k
Yamazaki H., Gotou S., Ito K.. Micropatterned culture of HepG2 spheroids using microwell chip with honeycomb-patterned polymer film[J]. J.Biosci. Bioeng., 2014,118:455-460. doi: 10.1016/j.jbiosc.2014.03.006
A.S.de León, J.Rodríguez-Hernández , Cortajarena A.L. Honeycomb patterned surfaces functionalized with polypeptide sequences for recognition and selective bacterial adhesion[J]. Biomaterials, 2013,34:1453-1460. doi: 10.1016/j.biomaterials.2012.10.074
Zhu Y.D., Sheng R.L., Luo T.. Honeycomb-structured films by multifunctional amphiphilic biodegradable copolymers:surface morphology control and biomedical application as scaffolds for cell growth[J]. ACS Appl.Mater.Interfaces, 2011,3:2487-2495. doi: 10.1021/am200371c
Wu X.H., Wang S.F. Regulating MC3T3-E1 cells on deformable poly (e-caprolactone)honeycomb films prepared using a surfactant-free breath figure method in a water-miscible solvent[J]. ACS Appl.Mater.Interfaces, 2012,4:4966-4975. doi: 10.1021/am301334s
Yap F.L., Zhang Y. Assembly of polystyrene microspheres and its application in cell micropatterning[J]. Biomaterials, 2007,28:2328-2338. doi: 10.1016/j.biomaterials.2007.01.034
M.Hernández-Guerrero , Stenzel M.H. Honeycomb structured polymer films via breath figures[J]. Polym.Chem., 2012,3:563-577. doi: 10.1039/C1PY00219H
Chen S.S., Lu X.M., Zhu D.D., Lu Q.H. Targeted grafting of thermoresponsive polymers from a penetrative honeycomb structure for cell sheet engineering[J]. Soft Matter, 2015,11:7420-7427. doi: 10.1039/C5SM01769F
Chen S.S., Lu X.M., Hu Y., Lu Q.H. Biomimetic honeycomb-patterned surface as the tunable cell adhesion scaffold[J]. Biomater.Sci., 2015,3:85-93. doi: 10.1039/C4BM00233D
Yin Y.D., Alivisatos A.P. Colloidal nanocrystal synthesis and the organic-inorganic interface[J]. Nature, 2005,437:664-670. doi: 10.1038/nature04165
Kawano T., Sato M., Yabu H., Shimomura M. Honeycomb-shaped surface topography induces differentiation of human mesenchymal stem cells (hMSCs):uniform porous polymer scaffolds prepared by the breath figure technique[J]. Biomater.Sci., 2014,2:52-56. doi: 10.1039/C3BM60195A
Biazar E., Khorasani M.T., Joupari M.D. Cell adhesion and surface properties of polystyrene surfaces grafted with poly(N-isopropylacrylamide)[J]. Chin.J.Polym. Sci., 2013,31:1509-1518. doi: 10.1007/s10118-013-1335-3
The software was provided on the website: http://cn.mathworks.com/index.html?s_tid=gn_logo.
Yao X., Peng R., Ding J.D. Cell-material interactions revealed via material techniques of surface patterning[J]. Adv.Mater., 2013,25:5257-5286. doi: 10.1002/adma.201301762
Jeon H., C.G.Simon Jr., Kim G. A mini-review:cell response to microscale, nanoscale, and hierarchical patterning of surface structure[J]. J.Biomed.Mater. Res.Part B Appl.Biomater., 2014,102:1580-1594.
Mosser D.M., Edwards J.P. Exploring the full spectrum of macrophage activation[J]. Nat.Rev.Immunol., 2008,8:958-969. doi: 10.1038/nri2448
Dalby M.J., Gadegaard N., Oreffo R.O.C. Harnessing nanotopography and integrin-matrix interactions to influence stem cell fate[J]. Nat.Mater., 2014,13:558-569. doi: 10.1038/nmat3980
Juan S.H., Tur J.M.M. Tensegrity frameworks:static analysis review[J]. Mech. Mach.Theory, 2008,43:859-881. doi: 10.1016/j.mechmachtheory.2007.06.010
Zhang G.H., Hou R.X., Zhan D.X.. Fabrication of hollow porous PLGA microspheres for controlled protein release and promotion of cell compatibility[J]. Chin.Chem.Lett., 2013,24:710-714. doi: 10.1016/j.cclet.2013.05.011
Heng L.P., Meng X.F., Wang B., Jiang L. Bioinspired design of honeycomb structure interfaces with controllable water adhesion[J]. Langmuir, 2013,29:9491-9498. doi: 10.1021/la401991n
Dembo M., Wang Y.L. Stresses at the cell-to-substrate interface during locomotion of fibroblasts[J]. Biophys.J., 1999,76:2307-2316. doi: 10.1016/S0006-3495(99)77386-8
Ingber D.E. Cellular tensegrity:defining new rules of biological design that govern the cytoskeleton[J]. J.Cell Sci., 1993,104:613-627.
Ingber D.E., Tensegrity I. Cell structure and hierarchical systems biology[J]. J.Cell Sci., 2003,116:1157-1173. doi: 10.1242/jcs.00359
Ingber D.E., Tensegrity I.I. How structural networks influence cellular information processing networks[J]. J.Cell Sci., 2003,116:1397-1408. doi: 10.1242/jcs.00360
Ingber D.E. Tensegrity:the architectural basis of cellular mechanotransduction[J]. Annu.Rev.Physiol., 1997,59:575-599. doi: 10.1146/annurev.physiol.59.1.575
Crawford-Young S.J.. Effects of microgravity on cell cytoskeleton and embryogenesis[J]. Int. J. Dev.Biol., 2006,50:183-191. doi: 10.1387/ijdb.052077sc
Cogoli A., Tschopp A., Fuchs-Bislin P.. Cell sensitivity to gravity[J]. Science, 1984,225:228-230. doi: 10.1126/science.6729481

Xi Chen , Xue Zhang , Shuai Yang , Jie Wang , Tian Tang , Maling Gou . An adhesive hydrogel for the treatment of oral ulcers. Chinese Chemical Letters, 2025, 36(3): 110021-. doi: 10.1016/j.cclet.2024.110021
Xiaofen GUAN , Yating LIU , Jia LI , Yiwen HU , Haiyuan DING , Yuanjing SHI , Zhiqiang WANG , Wenmin WANG . Synthesis, crystal structure, and DNA-binding of binuclear lanthanide complexes based on a multidentate Schiff base ligand. Chinese Journal of Inorganic Chemistry, 2024, 40(12): 2486-2496. doi: 10.11862/CJIC.20240122
Yao HUANG , Yingshu WU , Zhichun BAO , Yue HUANG , Shangfeng TANG , Ruixue LIU , Yancheng LIU , Hong LIANG . Copper complexes of anthrahydrazone bearing pyridyl side chain: Synthesis, crystal structure, anticancer activity, and DNA binding. Chinese Journal of Inorganic Chemistry, 2025, 41(1): 213-224. doi: 10.11862/CJIC.20240359
Lulu DONG , Jie LIU , Hua YANG , Yupei FU , Hongli LIU , Xiaoli CHEN , Huali CUI , Lin LIU , Jijiang WANG . Synthesis, crystal structure, and fluorescence properties of Cd-based complex with pcu topology. Chinese Journal of Inorganic Chemistry, 2025, 41(4): 809-820. doi: 10.11862/CJIC.20240171
Jia JI , Zhaoyang GUO , Wenni LEI , Jiawei ZHENG , Haorong QIN , Jiahong YAN , Yinling HOU , Xiaoyan XIN , Wenmin WANG . Two dinuclear Gd(Ⅲ)-based complexes constructed by a multidentate diacylhydrazone ligand: Crystal structure, magnetocaloric effect, and biological activity. Chinese Journal of Inorganic Chemistry, 2025, 41(4): 761-772. doi: 10.11862/CJIC.20240344
Lu LIU , Huijie WANG , Haitong WANG , Ying LI . Crystal structure of a two-dimensional Cd(Ⅱ) complex and its fluorescence recognition of p-nitrophenol, tetracycline, 2, 6-dichloro-4-nitroaniline. Chinese Journal of Inorganic Chemistry, 2024, 40(6): 1180-1188. doi: 10.11862/CJIC.20230489
Zhaodong WANG . In situ synthesis, crystal structure, and magnetic characterization of a trinuclear copper complex based on a multi-substituted imidazo[1,5-a]pyrazine scaffold. Chinese Journal of Inorganic Chemistry, 2025, 41(3): 597-604. doi: 10.11862/CJIC.20240268
Liping GUO . Synthesis and crystal structure characterization of yttrium imido complex: The reactivity of 2-substituted-1-amino-o-carborane with yttrium dialkyl complex. Chinese Journal of Inorganic Chemistry, 2025, 41(7): 1409-1415. doi: 10.11862/CJIC.20250065
Jun Guo , Zhenbang Zhuang , Wanqiang Liu , Gang Huang . "Co-coordination force" assisted rigid-flexible coupling crystalline polymer for high-performance aqueous zinc-organic batteries. Chinese Chemical Letters, 2024, 35(9): 109803-. doi: 10.1016/j.cclet.2024.109803
Zhichao Zhou , Fuqian Chen , Xiaotong Xia , Dong Ye , Rong Zhou , Lei Li , Tao Deng , Zhenhua Ding , Fang Liu . Developing a fluorescence substrate for HRP-based diagnostic assays with superiorities over the commercial ADHP. Chinese Chemical Letters, 2024, 35(6): 108970-. doi: 10.1016/j.cclet.2023.108970
Xinpin Pan , Yongjian Cui , Zhe Wang , Bowen Li , Hailong Wang , Jian Hao , Feng Li , Jing Li . Robust chemo-mechanical stability of additives-free SiO2 anode realized by honeycomb nanolattice for high performance Li-ion batteries. Chinese Chemical Letters, 2024, 35(10): 109567-. doi: 10.1016/j.cclet.2024.109567
Chunhui Zhang , Jie Wang , Jieyang Zhan , Runmin Yang , Guanggang Gao , Jiayuan Zhang , Linlin Fan , Mengqi Wang , Hong Liu . Highly sensitive hydrazine detection through a novel Raman scattering quenching mechanism enabled by a crystalline and noble metal–free polyoxometalate substrate. Chinese Chemical Letters, 2025, 36(3): 109719-. doi: 10.1016/j.cclet.2024.109719
Fenglin Wang , Chengwei Kuang , Zhicheng Zheng , Dan Wu , Hao Wan , Gen Chen , Ning Zhang , Xiaohe Liu , Renzhi Ma . Noble metal clusters substitution in porous Ni substrate renders high mass-specific activities toward oxygen evolution reaction and methanol oxidation reaction. Chinese Chemical Letters, 2025, 36(6): 109989-. doi: 10.1016/j.cclet.2024.109989
Ting Xie , Xun He , Lang He , Kai Dong , Yongchao Yao , Zhengwei Cai , Xuwei Liu , Xiaoya Fan , Tengyue Li , Dongdong Zheng , Shengjun Sun , Luming Li , Wei Chu , Asmaa Farouk , Mohamed S. Hamdy , Chenggang Xu , Qingquan Kong , Xuping Sun . CoSe2 nanowire array enabled highly efficient electrocatalytic reduction of nitrate for ammonia synthesis. Chinese Chemical Letters, 2024, 35(11): 110005-. doi: 10.1016/j.cclet.2024.110005
Haoting Wang , Mengfan Luo , Yuzhong Wang , Jialong Yin , Heng Zhang , Jia Zhao , Bo Lai . Mn(Ⅱ) enhanced permanganate oxidation of trace organic pollutants in water: Critical role of in situ formation of colloidal MnO2. Chinese Chemical Letters, 2025, 36(6): 110348-. doi: 10.1016/j.cclet.2024.110348
Jie Wu , Xiaoqing Yu , Guoxing Li , Su Chen . Engineering particles towards 3D supraballs-based passive cooling via grafting CDs onto colloidal photonic crystals. Chinese Chemical Letters, 2024, 35(4): 109234-. doi: 10.1016/j.cclet.2023.109234
Jiakun Bai , Junhui Jia , Aisen Li . An elastic organic crystal with piezochromic luminescent behavior. Chinese Journal of Structural Chemistry, 2024, 43(6): 100323-100323. doi: 10.1016/j.cjsc.2024.100323
Chao Ma , Cong Lin , Jian Li . MicroED as a powerful technique for the structure determination of complex porous materials. Chinese Journal of Structural Chemistry, 2024, 43(3): 100209-100209. doi: 10.1016/j.cjsc.2023.100209
Yuhang Li , Yang Ling , Yanhang Ma . Application of three-dimensional electron diffraction in structure determination of zeolites. Chinese Journal of Structural Chemistry, 2024, 43(4): 100237-100237. doi: 10.1016/j.cjsc.2024.100237
Hai-Ling Wang , Zhong-Hong Zhu , Hua-Hong Zou . Structure and assembly mechanism of high-nuclear lanthanide-oxo clusters. Chinese Journal of Structural Chemistry, 2024, 43(9): 100372-100372. doi: 10.1016/j.cjsc.2024.100372