Investigation of L/D-threonine substituted L-serine octamer ions by mass spectrometry and infrared photodissociation spectroscopy
- Corresponding author: Kong Xiang-Lei, kongxianglei@nankai.edu.cn
Citation:
Ren Juan, Wang Yi-Yun, Feng Ru-Xia, Kong Xiang-Lei. Investigation of L/D-threonine substituted L-serine octamer ions by mass spectrometry and infrared photodissociation spectroscopy[J]. Chinese Chemical Letters,
;2017, 28(3): 537-540.
doi:
10.1016/j.cclet.2016.10.032

Nitrogen-containing heterocycles are abundant structural motifs in a large number of alkaloids [1], drug molecules [2] and biologically active substances [3]. Although significant accomplishments have been achieved towards the synthesis of various nitrogen heterocyclic compounds in the past few decades [4], modern organic synthesis still demands more efficient and divergent methodologies to access privileged motifs of biological active compounds [5]. As a prime instance, 1, 3-oxazinan-2-ones are not only a core scaffold within natural products [6] and pharmacologically interesting molecules [7], but also widely utilized as key intermediates [8] in the synthesis of drugs [8c, 8f] and bioactive natural products [7b, 8g]. Numerous synthetic approaches were reported to access various substituted 1, 3-oxazinan-2-ones, including halonium-mediated [6c, 9] or metal-catalyzed cyclization [10], intramolecular Michael addition of functionalized homoallylamines/homoallylic alcohols [11], allylic C-H amination [12], and tethered aminohydroxylation of olefins [13]. However, the effective methods to access 6, 6-disubstituted-1, 3-oxazinan-2-one 1, exemplified with the core structural unit of biologically active compounds such as anti-HIV Efavirenz 2 (Merck) [7e] and 11-β-HSD-1 inhibitor 3 [7c], are rare[14] (Fig. 1).
N-Acyliminium ions, acting as important organic synthetic intermediates, are widely used in the formation of C-C and C-heteroatom bonds [15], mostly through intermolecular addition [16] and intramolecular cyclization [17] with various nucleophilic reagents. For examples, the reactions of N-acyliminium ions with olefins could undergo Lewis acid-catalyzed intramolecular addition-cyclization to construct a series of heterocyclic skeletons (Fig. 2, Eq. 1) [18]. Intermolecular reactions of N-acyliminium ions with olefins were also reported [19]. Kobayashi achieved the ring-opening allylation of semicyclic N, O-acetals with allylic silanes (Fig. 2, Eq. 2a) [19a]. Later, Zhang developed the intermolecular coupling reaction of N-acyliminium ions with styrene (Fig. 2, Eq. 2b) [19b]. Notably, N-acyliminium ions could serve as part of electron-deficient dienes, undergoing [4 + 2] cycloaddition with various dienophiles (alkenes or alkynes) [20, 21]. For example, Yoshida established a cycloaddition process of N-acyliminium ions connecting with an alkoxycarbonyl group with alkenes to afford substituted 1, 3-oxazinan-2-one framework, but the formation of corresponding N-acyliminium dienes required anodic oxidation of α-silyl carbamate substrates (Fig. 2, Eq. 3) [20d]. On the basis of our continuous efforts in exploring chemical transformations of semicyclic N, O-acetals [22], we envisioned that such [4 + 2] cycloaddition could lead to various important units. Herein we present an efficient synthetic approach to 4, 6, 6-trisubstituted-1, 3-oxazinan-2-ones6/7/9/10 through TMSOTf-mediated [4 + 2] cycloaddition of semicyclicN, O-acetals 4 with 1, 1-disubstituted ethylenes 5/8 (Fig. 2, Eq. 4).
Our investigation started with the reaction of semicyclic N, O-acetal 4b with 1, 1-diphenylethene 5a. The reaction could not take place in the absence of Lewis acid (Table 1, entry 1). Several types of iron Lewis acids could lead to only faint products (Table 1, entries 2-6). When Ni(OTf)2, Cu(OTf)2 and Sc(OTf)3 were examined, no product was observed (Table 1, entries 7-9). SnCl4 could afford the desired product 7ba in 34% yield (Table 1, entry 10). Slight improvements in yields were achieved when TiCl4 and BF3˙Et2O were applied, and the desired product 7ba could be obtained in moderate yield (48% and 66% respectively, Table 1, entries 11 and 12). Delightfully, TMSOTf could significantly increase the yield of 7ba to 81% (Table 1, entry 13). It was worth noting that the reaction was conducted at -78 ℃. Either increasing or decreasing the loading of TMSOTf resulted in slight drop of the reaction yield (Table 1, entries 14 and 15). The reaction could also afford the desired product using THF and PhMe as solvents, but the corresponding yields were lower compared with that in dichloromethane (Table 1, entries 16 and 17).
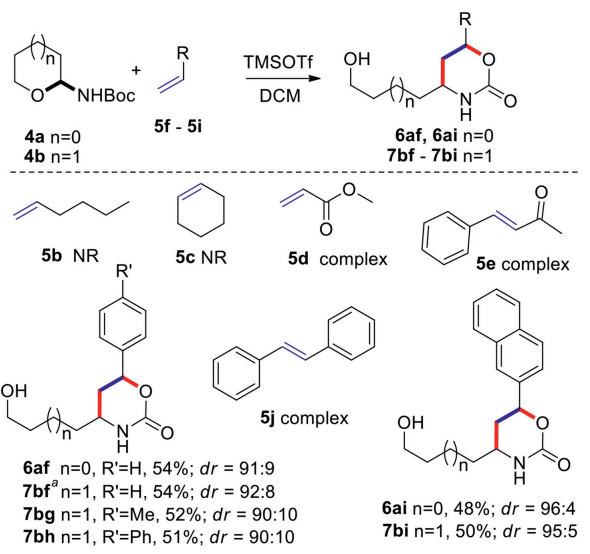
With the above identified optimized reaction conditions, the olefin substrates 5b-5j with different electronic properties were examined and the results were summarized in Scheme 1. First, neither hex-1-ene 5b nor cyclohexene 5c could afford any desired product in the presence of TMSOTf. The dienophiles with electron-withdrawing groups, methyl acrylate 5d and (E)-4-phenylbut-3-en-2-one 5e, led to complicated reaction mixtures. Delightfully, styrene 5f and its derivatives (5g and 5h) could generate the desired products 6af, 7bf-7bh in moderate yields and diastereoselectivities. 2-Vinylnaphthalene could also react with 4a and 4b to give the corresponding products 6ai and 7bi in moderate yields and diastereoselectivities. It was worth mentioning that a complex result was obtained when (E)-1, 2-diphenylethene 5j was investigated, probably due to the steric effect during the intermolecular [4 + 2] cycloaddition process.
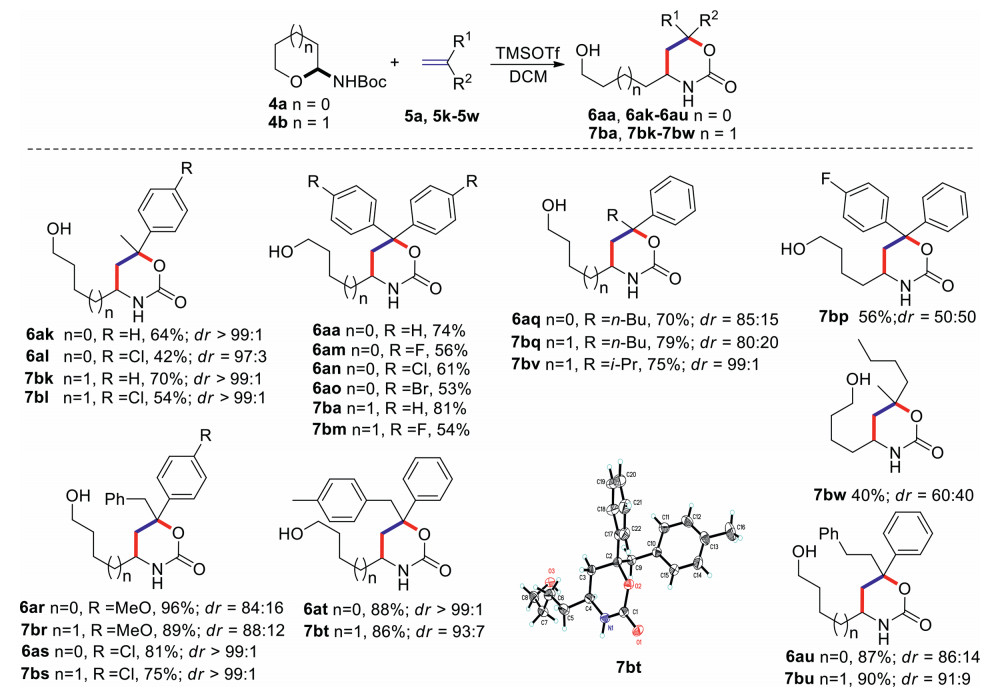
Next, we turned to investigate the scope and limitation of such addition-cyclization of semicyclic N, O-acetal (4a or 4b) with 1, 1-disubstituted ethylenes 5a, 5k-5v (Scheme 2). When prop-1-en-2-ylbenzene 5k was explored, desired products 6ak, 7bk were obtained in moderate yields and excellent diastereoselectivities. 4-Chloro substitution at phenyl ring (5l) led to slight decrease in yields of 6al, 7bl, but with excellent diastereoselectivities (dr up to 99:1). Replacement of the methyl group of 5k with other alkyl substitutions (5q: n-butyl and 5v: isopropyl) was tolerated, and the desired products 6aq, 7bq, 7bv were afforded in moderate yields under the optimized conditions. Although the n-butyl substituted products 6aq, 7bq showed moderate diastereoselectivities, the isopropyl substituted product 7bv was obtained with excellent diastereoselectivities. In addition, a series of diaryl substituted alkenes were surveyed under the optimized conditions. In general, all these substituted alkenes (5a, 5l-5o) could react with semicyclic N, O-acetals 4a and 4b, affording the desired products 6aa, 6am-6ao, 7ba, 7bm, 7bp in moderate yields. Several benzyl and phenyl olefins 5r-5t were also screened, most of them could give the desired products 6ar-6at, 7br-7bt in excellent yields and diastereoselectivities, except for the p-methoxyphenyl substituted olefin 5r. Substituted olefin 5u containing phenyl and phenethyl could also afford the desired products 6au and 7bu in excellent yields with moderate diastereoselectivities. The methyl and butyl substituted ethylene 5w could also react with N, O-acetal 4b to afford the desired product 7bw in 40% yield, but the diastereoselectivities was lower than those of aryl olefins. The chemical structures of 6aa, 6ak-6au, 7ba, 7bk-7bw were unambiguously confirmed based on the X-ray crystallographic analysis of compound 7bt (see Supporting information for detail).
Next, we turned our attention to investigate the reaction of semicyclic N, O-acetal 4a or 4b with exocyclic olefins 8a-8g, aiming for the formation of 1, 3-oxazinan-2-ones containing a spiro quaternary carbon (Scheme 3). The reaction of 2-methylene-1, 2, 3, 4-tetrahydronaphthalene 8a with semicyclic N, O-acetal 4b afforded the desired product 10ba in 70% yield. The 6-bromo substituted olefin 8b led to 10bb in slightly lower yield of 65%, while the 5-methoxy substituted olefin 8c could generate 10bc in slightly higher yield of 78%. However, the diastereoselectivities of 10ba-10bc were low. The symmetric olefin, 2-methylene-2, 3-dihydro-1H-indene 8d, also worked well with semicyclic N, O-acetals 4a and 4b, affording the desired products 9ad and 10bd in moderate yields. Notably, a simple exocyclic olefin methylenecyclopentane 8g also worked well, and the corresponding product 10bg was obtained in 60% yield. Regarding olefin substrates with the exo-double bond adjacent to the phenyl ring, 5-methylene-6, 7, 8, 9-tetrahydro-5H-benzo[7]annulene 8e bearing a fused seven-membered ring could lead to the corresponding products in higher yields than that of 1-methylene-1, 2, 3, 4-tetrahydronaphthalene 8f bearing a fused six-membered ring. In detail, the desired products 9ae and 10be were obtained in 70% and 63% yields, while the yield of 10bf was only 36%. The diastereoselectivities of 9ae, 10be and 10bf were increased slightly, maybe due to the steric hindrance. The structures of 9ad, 9ae, 10ba-10bg were unambiguously confirmed based on the X-ray crystallographic analysis of compound10bb (see Supporting information for detail).
A possible mechanism for this TMSOTf-mediated [4 + 2] cycloaddition process is presented in Fig. 3 [19a, 20d, 20f]. When semicyclic N, O-acetals 4 reacted with alkenes 5, diene type of N-acyliminium ions Int-1 was first generated under Lewis acid conditions. The subsequent reaction with alkenes 5 gave a six-membered intermediate Int-2, which would define the stereochemical outcome and give Int-3. Upon the cleavage of t-butyl group, the corresponding cycloadducts 6/7/9/10 were produced, along with the release of 2-methylprop-1-ene.
Finally, we focused on the utility of this intermolecular [4 + 2] process of N-acyliminium ions with alkenes in the synthesis of biologically active molecules. Scheme 4 showed a facile synthesis of norallosedamine 12. As a natural product, norallosedamine 12 was isolated from both the Sedum and Lobelia inflata plant family, and have attracted great interest in synthetic chemistry [23]. Starting from the cycloadduct 7bf, Dess-Martin oxidation (DMP) and subsequent reductive amination (Et3SiH/TMSOTf) could produce bicyclic pyrido [1,2-c][1,3]oxazin-1-one 11 in 75% overall yield. Then the ring opening (KOH) of 11 resulted in (±) norallosedamine 12 in 86% yield (dr = 94:6). The spectroscopic and physical data of the synthetic (±)-norallosedamine 12 were identical to the reported data [23a]. Norallosedamine 12 could be potentially converted to other alkaloids of its family by known process [23b, 23d].
In summary, we established a novel and efficient approach for the synthesis of 4, 6-disubstituted- and 4, 6, 6-trisubstituted-1, 3-oxazinan-2-ones 6aa, 6af-6au, 7ba, 7bf-7bw and 6, 6-spiro containing 1, 3-oxazinan-2-ones 9ad, 9ae, 10ba-10bg. The Lewis acid TMSOTf could activate semicyclic N, O-acetals (4a and 4b), and the resulting N-alkoxycarbonyliminium ions readily underwent a [4 + 2] cycloaddition process with 1, 1-disubstituted ethylenes5a, 5k-5w and 8a-8g. The corresponding products were obtained in moderate to excellent yields and diastereoselectivities. In addition, the utility of this methodology was demonstrated by the facile synthesis of natural product (±)-norallosedamine12 from the cycloadduct 7bf.
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
We thank the National Natural Science Foundation of China (No. 21772027 to B.-G. Wei and 21702032 to C.-M. Si) for financial support. The authors also thank Dr. Han-Qing Dong (Arvinas, Inc.) for helpful suggestions.
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.cclet.2021.05.003.
Cooks R.G., Zhang D.X., Koch K.J., Gozzo F.C., Eberlin M.N.. Chiroselective selfdirected octamerization of serine:implications for homochirogenesis[J]. Anal. Chem., 2001,73:3646-3655. doi: 10.1021/ac010284l
Koch K.J., Gozzo F.C., Nanita S.C.. Chiral transmission between amino acids:chirally selective amino acid substitution in the serine octamer as a possible step in homochirogenesis[J]. Angew. Chem. Int. Ed., 2002,41:1721-1724. doi: 10.1002/(ISSN)1521-3773
Takats Z., Nanita S.C., Schlosser G., Vekey K., Cooks R.G.. Atmospheric pressure gasphase H/D exchange of serine octamers[J]. Anal. Chem., 2003,75:6147-6154. doi: 10.1021/ac034284s
Takats Z., Nanita S.C., Cooks R.G.. Serine octamer reactions:indicators of prebiotic relevance[J]. Angew. Chem. Int. Ed., 2003,42:3521-3523. doi: 10.1002/anie.200351210
Takats Z., Nanita S.C., Cooks R.G., Schlosser G., Vekey K.. Amino acid clusters formed by sonic spray ionization[J]. Anal. Chem., 2003,75:1514-1523. doi: 10.1021/ac0260793
Nanita S.C., Cooks R.G.. Serine octamers:cluster formation, reactions, and implications for biomolecule homochirality[J]. Angew. Chem. Int. Ed., 2006,45:554-559. doi: 10.1002/(ISSN)1521-3773
Counterman A.E., Clemmer D.E.. Magic number clusters of serine in the gas phase[J]. J. Phys. Chem. B, 2001,105:8092-8096. doi: 10.1021/jp011421l
Schalley C.A., Weis P.. Unusually stable magic number clusters of serine with a surprising preference for homochirality[J]. Int. J. Mass Spectrom., 2002,221:9-19. doi: 10.1016/S1387-3806(02)00955-7
Gronert S., O'Hair R.A.J., Fagin A.E.. Ion/molecule reactions of the protonated serine octamer[J]. Chem. Commun., 2004:1944-1945.
Hodyss R., Julian R.R., Beauchamp J.L.. Spontaneous chiral separation in noncovalent molecular clusters[J]. Chirality, 2001,13:703-706. doi: 10.1002/(ISSN)1520-636X
Julian R.R., Hodyss R., Kinnear B., Jarrold M.F., Beauchamp J.L.. Nanocrystalline aggregation of serine detected by electrospray ionization mass spectrometry:origin of the stable homochiral gas-phase serine octamer[J]. J. Phys. Chem. B, 2002,106:1219-1228. doi: 10.1021/jp012265l
Ustyuzhanin P., Ustyuzhanin J., Lifshitz C.. An electrospray ionization-flow tube study of H/D exchange in protonated serine[J]. Int. J. Mass Spectrom. 223-, 2003,224:491-498.
Mazurek U., Geller O., Lifshitz C.. Protonated serine octamer cluster:structure elucidation by gas-phase H/D exchange reactions[J]. J. Phys. Chem. A, 2005,109:2107-2112. doi: 10.1021/jp0451344
Oh H.B., Lin C., Hwang H.Y.. Infrared photodissociation spectroscopy of electrosprayed ions in a Fourier transform mass spectrometer[J]. J. Am. Chem. Soc., 2005,127:4076-4083. doi: 10.1021/ja040136n
Kong X.L., Tsai I.A., Sabu S.. Progressive stabilization of zwitterionic structures in[H (Ser)2-8]+ studied by infrared photodissociation spectroscopy[J]. Angew. Chem. Int. Ed., 2006,45:4130-4134. doi: 10.1002/(ISSN)1521-3773
Kong X.L., Lin C., Infusini G.. Numerous isomers of serine octamer ions characterized by infrared photodissociation spectroscopy[J]. ChemPhysChem, 2009,10:2603-2606. doi: 10.1002/cphc.v10:15
Sunahori F.X., Yang G.C., Kitova E.N., Klassen J.S., Xu Y.J.. Chirality recognition of the protonated serine dimer and octamer by infrared multiphoton dissociation spectroscopy[J]. Phys. Chem. Chem. Phys., 2013,15:1873-1886. doi: 10.1039/C2CP43296J
Liao G.H., Yang Y.J., Kong X.L.. Chirality effects on proline-substituted serine octamers revealed by infrared photodissociation spectroscopy[J]. Phys. Chem. Chem. Phys., 2014,16:1554-1558. doi: 10.1039/C3CP53469C
Yin H., Kong X.L.. Structure of protonated threonine dimers in the gas phase:saltbridged or charge-solvated[J]. J. Am. Soc. Mass Spectrom., 2015,26:1455-1461. doi: 10.1007/s13361-015-1194-y
Kong X.L.. Reinvestigation of the structure of protonated lysine dimer[J]. J. Am. Soc. Mass Spectrom., 2014,25:422-426. doi: 10.1007/s13361-013-0801-z
Feng R.X., Mu L., Yang S.M., Kong X.L.. Structure of Pro4H+ investigated by infrared photodissociation (IRPD) spectroscopy and theoretical calculations[J]. Chin. Chem. Lett., 2016,27:593-596. doi: 10.1016/j.cclet.2016.02.027
Cody R.B., Hein R.E., Goodman S.D., Marshall A.G.. Stored waveform inverse Fourier transform excitation for obtaining increased parent ion selectivity in collisionally activated dissociation:preliminary results[J]. Rapid Commun. Mass Spectrom., 1987,1:99-102. doi: 10.1002/(ISSN)1097-0231
Cooks R.G., Zhang D.X., Koch K.J., Gozzo F.C., Eberlin M.N.. Chiroselective selfdirected octamerization of serine:implications for homochirogenesis[J]. Anal. Chem., 2001,73:3646-3655. doi: 10.1021/ac010284l
Koch K.J., Gozzo F.C., Nanita S.C.. Chiral transmission between amino acids:chirally selective amino acid substitution in the serine octamer as a possible step in homochirogenesis[J]. Angew. Chem. Int. Ed., 2002,41:1721-1724. doi: 10.1002/(ISSN)1521-3773
Takats Z., Nanita S.C., Schlosser G., Vekey K., Cooks R.G.. Atmospheric pressure gasphase H/D exchange of serine octamers[J]. Anal. Chem., 2003,75:6147-6154. doi: 10.1021/ac034284s
Takats Z., Nanita S.C., Cooks R.G.. Serine octamer reactions:indicators of prebiotic relevance[J]. Angew. Chem. Int. Ed., 2003,42:3521-3523. doi: 10.1002/anie.200351210
Takats Z., Nanita S.C., Cooks R.G., Schlosser G., Vekey K.. Amino acid clusters formed by sonic spray ionization[J]. Anal. Chem., 2003,75:1514-1523. doi: 10.1021/ac0260793
Nanita S.C., Cooks R.G.. Serine octamers:cluster formation, reactions, and implications for biomolecule homochirality[J]. Angew. Chem. Int. Ed., 2006,45:554-559. doi: 10.1002/(ISSN)1521-3773
Counterman A.E., Clemmer D.E.. Magic number clusters of serine in the gas phase[J]. J. Phys. Chem. B, 2001,105:8092-8096. doi: 10.1021/jp011421l
Schalley C.A., Weis P.. Unusually stable magic number clusters of serine with a surprising preference for homochirality[J]. Int. J. Mass Spectrom., 2002,221:9-19. doi: 10.1016/S1387-3806(02)00955-7
Gronert S., O'Hair R.A.J., Fagin A.E.. Ion/molecule reactions of the protonated serine octamer[J]. Chem. Commun., 2004:1944-1945.
Hodyss R., Julian R.R., Beauchamp J.L.. Spontaneous chiral separation in noncovalent molecular clusters[J]. Chirality, 2001,13:703-706. doi: 10.1002/(ISSN)1520-636X
Julian R.R., Hodyss R., Kinnear B., Jarrold M.F., Beauchamp J.L.. Nanocrystalline aggregation of serine detected by electrospray ionization mass spectrometry:origin of the stable homochiral gas-phase serine octamer[J]. J. Phys. Chem. B, 2002,106:1219-1228. doi: 10.1021/jp012265l
Ustyuzhanin P., Ustyuzhanin J., Lifshitz C.. An electrospray ionization-flow tube study of H/D exchange in protonated serine[J]. Int. J. Mass Spectrom. 223-, 2003,224:491-498.
Mazurek U., Geller O., Lifshitz C.. Protonated serine octamer cluster:structure elucidation by gas-phase H/D exchange reactions[J]. J. Phys. Chem. A, 2005,109:2107-2112. doi: 10.1021/jp0451344
Oh H.B., Lin C., Hwang H.Y.. Infrared photodissociation spectroscopy of electrosprayed ions in a Fourier transform mass spectrometer[J]. J. Am. Chem. Soc., 2005,127:4076-4083. doi: 10.1021/ja040136n
Kong X.L., Tsai I.A., Sabu S.. Progressive stabilization of zwitterionic structures in[H (Ser)2-8]+ studied by infrared photodissociation spectroscopy[J]. Angew. Chem. Int. Ed., 2006,45:4130-4134. doi: 10.1002/(ISSN)1521-3773
Kong X.L., Lin C., Infusini G.. Numerous isomers of serine octamer ions characterized by infrared photodissociation spectroscopy[J]. ChemPhysChem, 2009,10:2603-2606. doi: 10.1002/cphc.v10:15
Sunahori F.X., Yang G.C., Kitova E.N., Klassen J.S., Xu Y.J.. Chirality recognition of the protonated serine dimer and octamer by infrared multiphoton dissociation spectroscopy[J]. Phys. Chem. Chem. Phys., 2013,15:1873-1886. doi: 10.1039/C2CP43296J
Liao G.H., Yang Y.J., Kong X.L.. Chirality effects on proline-substituted serine octamers revealed by infrared photodissociation spectroscopy[J]. Phys. Chem. Chem. Phys., 2014,16:1554-1558. doi: 10.1039/C3CP53469C
Yin H., Kong X.L.. Structure of protonated threonine dimers in the gas phase:saltbridged or charge-solvated[J]. J. Am. Soc. Mass Spectrom., 2015,26:1455-1461. doi: 10.1007/s13361-015-1194-y
Kong X.L.. Reinvestigation of the structure of protonated lysine dimer[J]. J. Am. Soc. Mass Spectrom., 2014,25:422-426. doi: 10.1007/s13361-013-0801-z
Feng R.X., Mu L., Yang S.M., Kong X.L.. Structure of Pro4H+ investigated by infrared photodissociation (IRPD) spectroscopy and theoretical calculations[J]. Chin. Chem. Lett., 2016,27:593-596. doi: 10.1016/j.cclet.2016.02.027
Cody R.B., Hein R.E., Goodman S.D., Marshall A.G.. Stored waveform inverse Fourier transform excitation for obtaining increased parent ion selectivity in collisionally activated dissociation:preliminary results[J]. Rapid Commun. Mass Spectrom., 1987,1:99-102. doi: 10.1002/(ISSN)1097-0231

Yang Feng , Yang-Qing Tian , Yong-Qiang Zhao , Sheng-Jun Chen , Bi-Feng Yuan . Dynamic deformylation of 5-formylcytosine and decarboxylation of 5-carboxylcytosine during differentiation of mouse embryonic stem cells into mouse neurons. Chinese Chemical Letters, 2024, 35(11): 109656-. doi: 10.1016/j.cclet.2024.109656
Tian Feng , Yun-Ling Gao , Di Hu , Ke-Yu Yuan , Shu-Yi Gu , Yao-Hua Gu , Si-Yu Yu , Jun Xiong , Yu-Qi Feng , Jie Wang , Bi-Feng Yuan . Chronic sleep deprivation induces alterations in DNA and RNA modifications by liquid chromatography-mass spectrometry analysis. Chinese Chemical Letters, 2024, 35(8): 109259-. doi: 10.1016/j.cclet.2023.109259
Cheng Guo , Xiaoxiao Zhang , Xiujuan Hong , Yiqiu Hu , Lingna Mao , Kezhi Jiang . Graphene as adsorbent for highly efficient extraction of modified nucleosides in urine prior to liquid chromatography-tandem mass spectrometry analysis. Chinese Chemical Letters, 2024, 35(4): 108867-. doi: 10.1016/j.cclet.2023.108867
Junmeng Luo , Qiongqiong Wan , Suming Chen . Chemistry-driven mass spectrometry for structural lipidomics at the C=C bond isomer level. Chinese Chemical Letters, 2025, 36(1): 109836-. doi: 10.1016/j.cclet.2024.109836
Keqiang Shi , Xiujuan Hong , Dongyan Xu , Tao Pan , Huiwen Wang , Hongru Feng , Cheng Guo , Yuanjiang Pan . Analysis of RNA modifications in peripheral white blood cells from breast cancer patients by mass spectrometry. Chinese Chemical Letters, 2025, 36(3): 110079-. doi: 10.1016/j.cclet.2024.110079
Qiongqiong Wan , Yanan Xiao , Guifang Feng , Xin Dong , Wenjing Nie , Ming Gao , Qingtao Meng , Suming Chen . Visible-light-activated aziridination reaction enables simultaneous resolving of C=C bond location and the sn-position isomers in lipids. Chinese Chemical Letters, 2024, 35(4): 108775-. doi: 10.1016/j.cclet.2023.108775
Yao-Hua Gu , Yu Chen , Qing Li , Neng-Bin Xie , Xue Xing , Jun Xiong , Min Hu , Tian-Zhou Li , Ke-Yu Yuan , Yu Liu , Tang Tang , Fan He , Bi-Feng Yuan . Metabolome profiling by widely-targeted metabolomics and biomarker panel selection using machine-learning for patients in different stages of chronic kidney disease. Chinese Chemical Letters, 2024, 35(11): 109627-. doi: 10.1016/j.cclet.2024.109627
Yimin Guo , Yiting Luo , Shuwen Hua , Chuan-Fan Ding , Yinghua Yan . Application of magnetic nanomaterials in peptidomics: A review in the past decade. Chinese Chemical Letters, 2025, 36(6): 110070-. doi: 10.1016/j.cclet.2024.110070
Wei Shao , Wanqun Zhang , Pingping Zhu , Wanqun Hu , Qiang Zhou , Weiwei Li , Kaiping Yang , Xisheng Wang . Design and Practice of Ideological and Political Cases in the Course of Instrument Analysis Experiment: Taking the GC-MS Experiment as an Example. University Chemistry, 2024, 39(2): 147-154. doi: 10.3866/PKU.DXHX202309048
Lu Huang , Jiang Wang , Hong Jiang , Lanfang Chen , Huanwen Chen . On-line determination of selenium compounds in tea infusion by extractive electrospray ionization mass spectrometry combined with a heating reaction device. Chinese Chemical Letters, 2025, 36(1): 109896-. doi: 10.1016/j.cclet.2024.109896
Yanhua Chen , Xian Ding , Jun Zhou , Zhaoying Wang , Yunhai Bo , Ying Hu , Qingce Zang , Jing Xu , Ruiping Zhang , Jiuming He , Fen Yang , Zeper Abliz . Plasma metabolomics combined with mass spectrometry imaging reveals crosstalk between tumor and plasma in gastric cancer genesis and metastasis. Chinese Chemical Letters, 2025, 36(1): 110351-. doi: 10.1016/j.cclet.2024.110351
Haiyan Lu , Jiayue Ye , Yiping Wei , Hua Zhang , Konstantin Chingin , Vladimir Frankevich , Huanwen Chen . Tracing molecular margins of lung cancer by internal extractive electrospray ionization mass spectrometry. Chinese Chemical Letters, 2025, 36(2): 110077-. doi: 10.1016/j.cclet.2024.110077
Wen Su , Siying Liu , Qingfu Zhang , Zhongyan Zhou , Na Wang , Lei Yue . Temperature-controlled electrospray ionization tandem mass spectrometry study on protein/small molecule interaction. Chinese Chemical Letters, 2025, 36(5): 110237-. doi: 10.1016/j.cclet.2024.110237
Feng-Qing Huang , Yu Wang , Ji-Wen Wang , Dai Yang , Shi-Lei Wang , Yuan-Ming Fan , Raphael N. Alolga , Lian-Wen Qi . Chemical isotope labeling-assisted liquid chromatography-mass spectrometry enables sensitive and accurate determination of dipeptides and tripeptides in complex biological samples. Chinese Chemical Letters, 2024, 35(11): 109670-. doi: 10.1016/j.cclet.2024.109670
Haixia Wu , Kailu Guo . Sulfur reduction reaction mechanism elucidated with in situ Raman spectroscopy. Chinese Chemical Letters, 2025, 36(6): 110654-. doi: 10.1016/j.cclet.2024.110654
Yanjing Li , Jiayin Li , Yuqi Chang , Yunfeng Lin , Lei Sui . Tetrahedral framework nucleic acids promote the proliferation and differentiation potential of diabetic bone marrow mesenchymal stem cell. Chinese Chemical Letters, 2024, 35(9): 109414-. doi: 10.1016/j.cclet.2023.109414
Zhiwen Li , Jingjing Zhang , Gao Li . Dynamic assembly of chiral golden knots. Chinese Journal of Structural Chemistry, 2024, 43(7): 100300-100300. doi: 10.1016/j.cjsc.2024.100300
Ling-Hao Zhao , Hai-Wei Yan , Jian-Shuang Jiang , Xu Zhang , Xiang Yuan , Ya-Nan Yang , Pei-Cheng Zhang . Effective assignment of positional isomers in dimeric shikonin and its analogs by 1H NMR spectroscopy. Chinese Chemical Letters, 2024, 35(5): 108863-. doi: 10.1016/j.cclet.2023.108863
Chengde Wang , Liping Huang , Shanshan Wang , Lihao Wu , Yi Wang , Jun Dong . A distinction of gliomas at cellular and tissue level by surface-enhanced Raman scattering spectroscopy. Chinese Chemical Letters, 2024, 35(5): 109383-. doi: 10.1016/j.cclet.2023.109383
Manyu Zhu , Fei Liang , Lie Wu , Zihao Li , Chen Wang , Shule Liu , Xiue Jiang . Revealing the difference of Stark tuning rate between interface and bulk by surface-enhanced infrared absorption spectroscopy. Chinese Chemical Letters, 2025, 36(2): 109962-. doi: 10.1016/j.cclet.2024.109962