Citation:
. Preparation of chloro-functionalized reduced graphene oxide by silver-catalyzed radical reaction[J]. Chinese Chemical Letters,
;2017, 28(2): 407-411.
doi:
10.1016/j.cclet.2016.10.017

-
It is well-known that chemical functionalization of graphene has the great significance. We report the development of a new synthesis method of chloro-functionalized reduced graphene oxide (rGOCl). The rGOCl was prepared by radical reaction, and treatment of carboxyl graphene oxide (GOCOOH) with N-chlorosuccinimide (NCS) at 90℃ for 10 h under an atmosphere of nitrogen, using silver nitrate as catalyst. The morphologies and structures of the prepared materials were investigated by field-emission scanning electron microscopy (FESEM), X-ray diffraction (XRD), Fourier transform infrared spectroscopy (FTIR), Raman spectroscopy and the thermal gravimetric. Results indicated that the rGOCl can be readily obtained from graphene oxide (GO) in three steps.
-
1. Introduction
Graphene, the wonder material with a single atomic layer, has received significant attention over the last 10 years since its discovery [1-3]. Owing to its 2D crystal lattice structure with point band gap properties, graphene has many potential applications in future electronics such as batteries [4], supercapacitors [5], fuel cells [6], photovoltaic devices [7], photocatalysis [8], and sensing platforms [9]. Tapping into these exciting possibilities, however, still requires overcoming a number of significant obstacles such as high-yield production methods, sorting and separation, controlled doping with heteroatoms, development of hierarchically ordered architectures, layer formation, processing, and solubilization. The properties of pristine graphene are not sufficient or suitable for many applications. Thus, considerable research has been conducted in the field of graphene chemical modification/functionalization.
Covalent or noncovalent chemical modifications of graphene, with the possibility of creating functional groups on the edge and/ or basal plane, can open up a new avenue in endowing graphene with novel and tunable properties [10, 11]. Halide graphene is a novel graphene derivative to be explored. For example, fluorinated graphene has been found to exhibit an excellent performance [12], and our group synthesized fluoro-functionalized graphene via the Hunsdiecker reaction [13]. Unlike fluorine, other halogens cannot react directly with graphite. Recently, Wu [60_TD$DIF] et al . [14] and Li et al . [15] reported the chlorination of monolayer graphene by plasma and photochemical methods, respectively. However, as a new but significant research subject, the chemical modification of graphene by other methods, remains to be exploited. In this paper, we report a facile covalent method to prepare chloro-functionalized reduced graphene oxide (rGOCl) by silver-catalyzed radical reaction of this field, and give a clear description of the purpose.
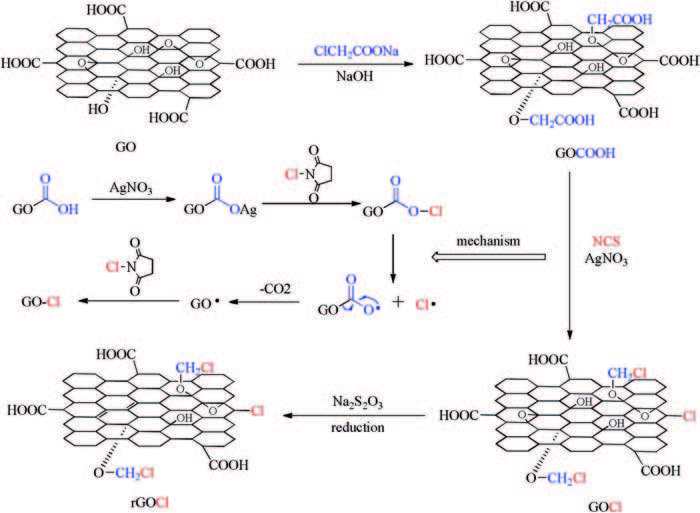
The process of chlorination reaction is in Fig. 1. First, the GOCOOH was synthesized by a substitution reaction, which was caused by the introduction of sodium chloroacetate interacting with hydroxyl groups. The GOCOOH sheets contained a large number of carboxyl groups on both the basal planes and edges. Second, the chloro-functionalized graphene oxide (GOCl) was prepared via radical reaction. We proposed that with the AgNO3 catalyst, the carboxyl groups on both the basal planes and edges of GOCOOH underwent efficient decarboxylative chlorination with the N-chlorosuccinimide (NCS) reagent in aqueous solution. Third, the GOCl was reduced to rGOCl by sodium thiosulfate at room temperature, which also contained another function is removed from the byproduct silver chloride via coordination reaction. A possible mechanism of the second step was proposed [16], as shown in Fig. 1. GOCOOH was initially reacted to the intermediates of GOCOOCl by silver nitrate and NCS. The intermediates of GOCOOCl could rapidly present chlorine and GOCOO radical, and the latter underwent fast decarboxylation to generate GO radical.The GO radical then abstracted the chlorine atom of NCS to yield the GOCl product. Thus, the silver-catalyzed decarboxylative chlorination was a process of radical reaction.
图 1
 图 1 A process and possible mechanism covalent functionalization of graphene with NCS by the radical reaction.Figure 1. A process and possible mechanism covalent functionalization of graphene with NCS by the radical reaction.
图 1 A process and possible mechanism covalent functionalization of graphene with NCS by the radical reaction.Figure 1. A process and possible mechanism covalent functionalization of graphene with NCS by the radical reaction.2. Results and discussion
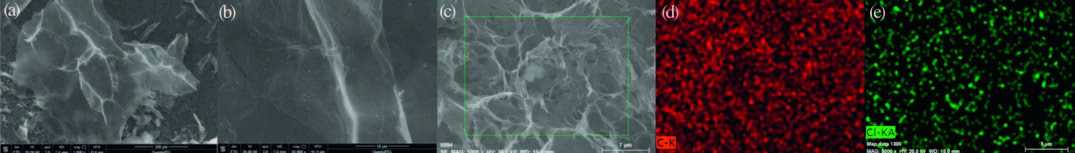
Fig. 2 demonstrates the field emission scanning electron microscopy images of rGOCl (a and b). Fig. 2a and b presents that the rGOCl maintains the 2D-layered flexible structure. These nanosheets, as shown in Fig. 2b, look transparent with a clear wrinkled morphology, indicating the single- or few-layer structure of rGOCl.To verify the composition of the formed compound, energy dispersive X-ray spectroscopic mapping of the obtained material was carried out (Fig. 2d and e) from Fig. 2c. The results confirm that the chlorine atoms are uniformly distributed on the surface of rGOCl sheets. From these results, we may conclude that GOCOOH undergoes an efficient decarboxylative chlorination process.
图 2
Fig. 3 displays the typical FTIR (a) and Raman spectra (b) of GO, GOCOOH and rGOCl. For GO, GOCOOH and rGOCl, the absorption bands at 1646 and 1070 cm-1 are ascribed to the —C=O stretching of the —COOH groups and the C—O stretching of the C—OH/ C—O—C groups, respectively. IR characterization of GO, GOCOOH and rGOCl samples indicated —CH2 vibrations at nearby 2900 cm-1, compared with GOCOOH and rGOCl, GO have weaker vibrations due to less CH2 group of GO. In compared to GOCOOH, the absorption peaks of rGOCl red shift to 2853 cm-1 from 2912 cm-1 due to carboxyl group have stronger performance of withdrawing electron than chlorine functional groups. The remaining peaks of GO, GOCOOH and rGOCl at 3402 and 1400 cm-1 are assigned to the vibration and deformation peaks of the O—H groups. When compared with GO and GOCOOH, the absorption peaks blue shift to 1127cm-1 from 1070cm-1 in rGOCl. The shifts may be attributed to the conjugation effect from C—C double bond of rGOCl, which is generated from the process of sodium thiosulfate reducing GOCl. Fig. 3b shows the Raman spectra of GO, GOCOOH and rGOCl. In the spectrum of GO, a prominent G peak at 1580cm-1 corresponding to anE2g mode of graphite represents the in-planebond-stretching vibration of the sp2-bonded carbon atoms. The D band at 1350 cm-1 is related to the breathing mode of k-point phonons with A1g symmetry and the vibrations of the carbon atoms with dangling bonds in the plane terminations in the disordered and defected graphite. The IG/ID intensity ratio change suggests a change in the average size of the sp2 domains, which is due to a decrease in the O-containing functional groups. The reason for this change that a C—C double bond forms via the dehydration reaction of hydroxyl groups during the formation of GOCOOH and rGOCl. The IG/ID ratios of GO, GOCOOH and rGOCl are 0.89, 0.94 and 1.25, respectively. The increase in the IG/ID ratio of rGOCl compared with those of GO and GOCOOH indicates that the sp2 domains are formed and that a high level of charge is introduced, because of the reduction reaction of rGOCl with sodium thiosulfate.
图 3
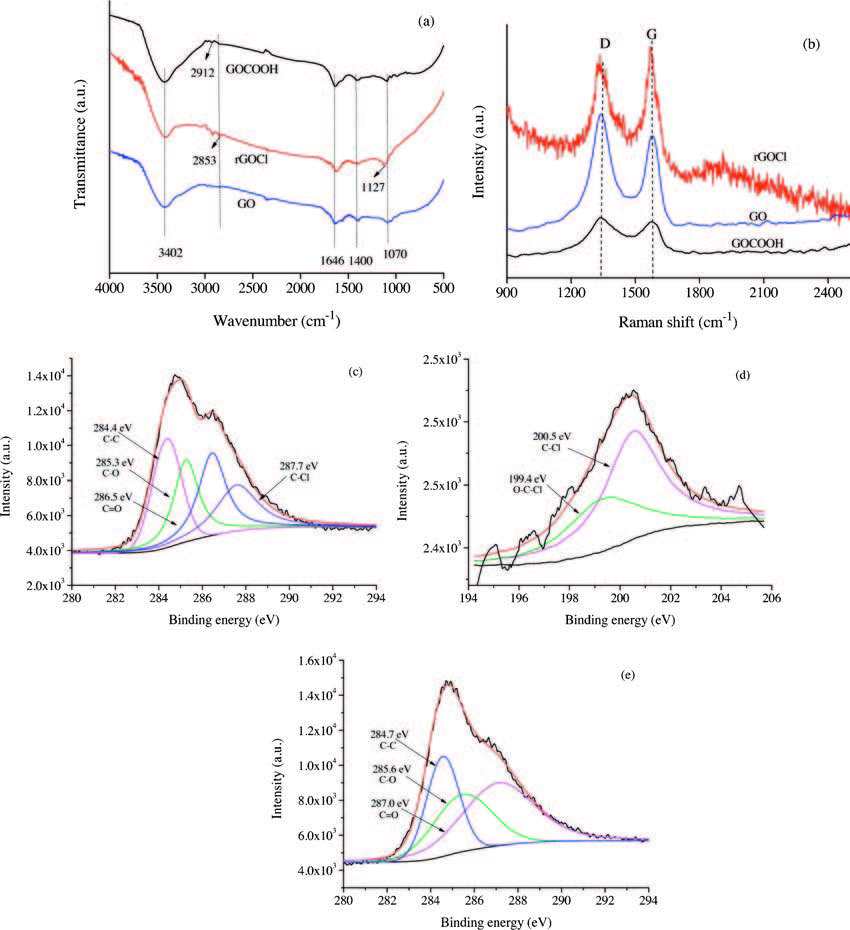 图 3 FTIR (a) and Raman (b) spectra of GO, GOCOOH and rGOCl. The core-level XPS spectra of C1s (c) and Cl 2p (d) spectra of rGOCl, and C1s (e) spectra of GOCOOH.Figure 3. FTIR (a) and Raman (b) spectra of GO, GOCOOH and rGOCl. The core-level XPS spectra of C1s (c) and Cl 2p (d) spectra of rGOCl, and C1s (e) spectra of GOCOOH.
图 3 FTIR (a) and Raman (b) spectra of GO, GOCOOH and rGOCl. The core-level XPS spectra of C1s (c) and Cl 2p (d) spectra of rGOCl, and C1s (e) spectra of GOCOOH.Figure 3. FTIR (a) and Raman (b) spectra of GO, GOCOOH and rGOCl. The core-level XPS spectra of C1s (c) and Cl 2p (d) spectra of rGOCl, and C1s (e) spectra of GOCOOH.To present clearer elemental information for the sample, XPS measurements were carried out on the rGOCl and GOCOOH. Fig. 3c and d shows the high-resolution XPS scans for the C1s and Cl2p peaks of the rGOCl, respectively. The XPS C1s peak can be fitted with four different peaks with binding energies at 284.4, 285.3, 286.5 and 287.7 eV, which are assigned to C—C, C—O, C=O, and C—Cl groups bonds, respectively. The high-resolution Cl2p spectrum of the rGOCl (Fig. 3d) shows two characteristic peaks positioned at 199.4 and 200.5 eV, which can be attributed to the Cl—C—O (basal planes) and C—Cl (edges) bonds, unequivocally indicating the covalent bond formation between Cl and graphene [17]. As shown in Fig. 3e, a C1s peaks of the GOCOOH at around 284.7, 285.6, and 287.0 eV, corresponding to the C—C, C—O and C=O groups, respectively. The O and Cl content of GOCOOH and rGOCl was estimated by XPS spectra, the result suggests that the rGOCl (27.9 atom% O and 2.7 atom% Cl) have the larger Cl and smaller O content than GOCOOH (30.0 atom% O and 0.3 atom% Cl), which can be attributed to the decrease of carboxyl group and increase of chlorine atom in rGOCl.
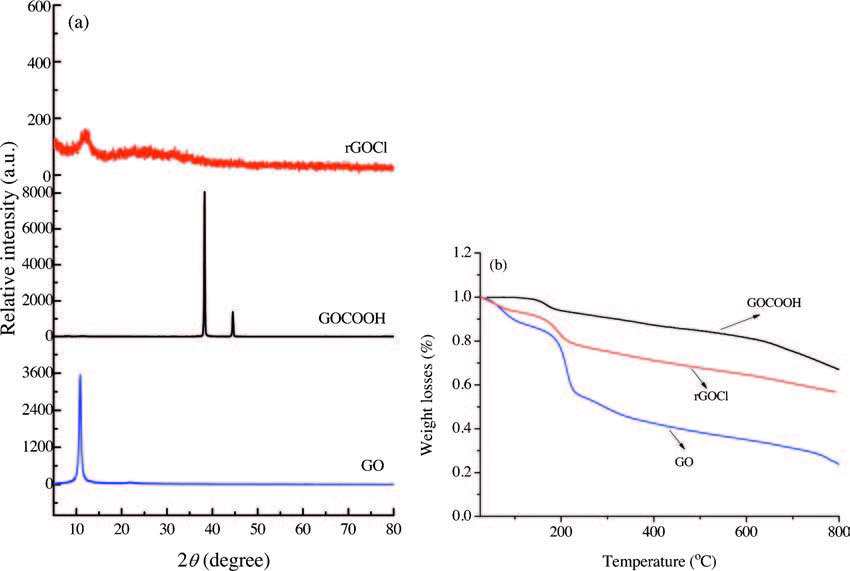
XRD patterns of the GO, GOCOOH and rGOCl samples are shown in Fig. 4a. The as-prepared GOCOOH exhibits a sharp reflection peak at 2θ = 38.3° and 2θ = 44.6°, without the characteristic peak of GO. Compared with GO and GOCOOH, rGOCl is absolutely different from crystalline graphite, which has distinctly weak characteristic peaks at 2θ = 12.0°, suggesting that rGOCl presents an increase in the average interlayer spacing. As shown in Fig. 4b, all of the materials have an initial mass loss of approximately 100 ℃, which is attributed to the evaporation of surface absorbed water molecules. For GO, the rapid weight loss of 27.3% before 200 ℃ corresponds to the decomposition of the O-containing functional groups, producing CO2, CO and H2O. For GOCOOH, the gradual weight loss at approximately between 200 ℃ is ascribed to the decomposition of the O-containing functional groups and the carboxyl groups. For rGOCl, the thermogravimetric analysis profiles of both GO and rGOCl show similar weight loss plots at approximately 200 ℃. However, the rGOCl shows a slow weight loss, which is due to the fact that the rGOCl containings are relatively fewer unstable oxygen functional groups than GO.
图 4
3. Conclusion
In summary, a mild and operationally simple method is developed to synthesize chloro-functionalized reduced graphene oxide via the radical reaction from GO. Experimental investigations concerning the morphology and chemical components indicate that the rGOCl can be readily obtained from GO in three steps. Considering the easily available raw materials and mild reaction conditions, of the rGOCl samples, this work provides enlightening insights into the chemical modification and preparation of functionalized graphene, as well as promotes more research enthusiasm and interest into chloro-functionalized graphene and other graphene materials. Extension of this method to other potential substrates and a study of the detailed reaction mechanism are now in progress in our laboratory.
4. Experimental
4.1 Characterization
X-ray diffractometry (XRD: PANalytical X'pert Powder) was performed using Cu Kα radiation of 40 kV. Fourier transform infrared spectrum (PerkinElmer, BRUKER) analysis was carried out in a FTIR-N3896. Raman measurements were performed with a LabRAM XploRA Raman spectrometer (HORIBA JobinYvon S.A.S) The thermal gravimetric (TG, NETZSCH) analysis was carried out by a STA-449C in a constant flow of Ar and the heating rate of 10 ℃/ min. X-ray photoelectron spectra (XPS) were obtained on a Amicus spectrometer using monochromatic Al Kα radiation. The field emission scanning electron micrographs (FE-SEM) of the samples were obtained on a ZEISS Supra 55 SEM.
4.2 Materials
Flake graphite of 400 mesh were purchased from Meilikun Co., Ltd (Qingdao, China). Aqueous ammonia (28 wt%), potassium permanganate and 98 wt% H2SO4 were bought from Fengchuan Chem. Co., Ltd (Tianjin, China). Silver nitrate, sodium thiosulfate and N-chlorosuccinimide were from Aladdin Co., Ltd. (Shanghai, China). All the reagents were used as received without further purification.
4.3 Preparation of GOCOOH [13]
GO was prepared by a modified Hummers’ method. GO aqueous suspension (1 mg/mL, 250 mL) was dispersed into ultrasonic treatment which was carried out for 1 h. After the GO was entirely dispersed, sodium hydroxide (NaOH, 10 g) and sodium chloroacetate (ClCH2COONa, 14 g) were added to the GO suspension and a magnetic stirring at room temperature for 12 h, and the pH of the solution was adjusted to 5-6 by dilute hydrochloric acid. Then the mixture was filtered and washed with anhydrous ethanol and a large amount of distilled water in sequence. Then the samples were collected and dialyzed against distilled water for 2 days to remove any impurities. To prepare freeze-dried GOCOOH, the obtained GO colloid was directly subjected to freeze-drying for 24 h on a vacuum freeze dryer.
4.4 Preparation of rGOCl
To a stirred solution of GOCOOH (20 mg) in distilled water (50 mL) was added N-chlorosuccinimide (268 mg), silver nitrate (170 mg) and acetonitrile (25 mL), and the reaction mixture was stirred at 90 ℃ for 10 h under an atmosphere of nitrogen. After the removal of acetonitrile under reduced pressure, the solution was extracted with ethyl acetate (3 × 15 mL) to remove organic impurities. Then the water layer was added Na2S2O3 (1.5 g), the mixture was stirred for 1.5 h at room temperature. After the complete disappearance of most of the solid, the solution was repeatedly centrifuged with a large amount of distilled water until the silver ion and chloride ion were not detected in the process. The obtained rGOCl colloid was directly subjected to freeze-drying for 24 h on a vacuum freeze dryer.
Acknowledgments
This work was financially supported by the Natural Science Foundation of Inner Mongolia (No. 2014BS0202), Inner Mongolia University of Science and Technology Innovation Fund (No. 2014QDL018) and Talent Incubation Funding of School of Materials and Metallurgy (No. 2014CY012)
-

-
-
[1]
Tian TIAN , Meng ZHOU , Jiale WEI , Yize LIU , Yifan MO , Yuhan YE , Wenzhi JIA , Bin HE . Ru-doped Co3O4/reduced graphene oxide: Preparation and electrocatalytic oxygen evolution property. Chinese Journal of Inorganic Chemistry, 2025, 41(2): 385-394. doi: 10.11862/CJIC.20240298
-
[2]
Yihong Li , Zhong Qiu , Lei Huang , Shenghui Shen , Ping Liu , Haomiao Zhang , Feng Cao , Xinping He , Jun Zhang , Yang Xia , Xinqi Liang , Chen Wang , Wangjun Wan , Yongqi Zhang , Minghua Chen , Wenkui Zhang , Hui Huang , Yongping Gan , Xinhui Xia . Plasma enhanced reduction method for synthesis of reduced graphene oxide fiber/Si anode with improved performance. Chinese Chemical Letters, 2024, 35(11): 109510-. doi: 10.1016/j.cclet.2024.109510
-
[3]
Fengjun Deng , Tingyu Zhao , Xiaochen Zhang , Kaiyong Feng , Ze Liu , Youlin Xiang , Yingjian Yu . Reduced graphene oxide assembled on the Si nanowire anode enabling low passivation and hydrogen evolution for long-life aqueous Si-air batteries. Chinese Chemical Letters, 2025, 36(6): 109897-. doi: 10.1016/j.cclet.2024.109897
-
[4]
Zhuwen Wei , Jiayan Chen , Congzhen Xie , Yang Chen , Shifa Zhu . Divergent de novo construction of α-functionalized pyrrole derivatives via coarctate reaction. Chinese Chemical Letters, 2024, 35(12): 109677-. doi: 10.1016/j.cclet.2024.109677
-
[5]
Xiao-Bo Liu , Ren-Ming Liu , Xiao-Di Bao , Hua-Jian Xu , Qi Zhang , Yu-Feng Liang . Nickel-catalyzed reductive formylation of aryl halides via formyl radical. Chinese Chemical Letters, 2024, 35(12): 109783-. doi: 10.1016/j.cclet.2024.109783
-
[6]
Manman Ou , Yunjian Zhu , Jiahao Liu , Zhaoxuan Liu , Jianjun Wang , Jun Sun , Chuanxiang Qin , Lixing Dai . Polyvinyl alcohol fiber with enhanced strength and modulus and intense cyan fluorescence based on covalently functionalized graphene quantum dots. Chinese Chemical Letters, 2025, 36(2): 110510-. doi: 10.1016/j.cclet.2024.110510
-
[7]
Ying Chen , Li Li , Junyao Zhang , Tongrui Sun , Xuan Zhang , Shiqi Zhang , Jia Huang , Yidong Zou . Tailored ionically conductive graphene oxide-encased metal ions for ultrasensitive cadaverine sensor. Chinese Chemical Letters, 2024, 35(8): 109102-. doi: 10.1016/j.cclet.2023.109102
-
[8]
Jia-Li Xie , Tian-Jin Xie , Yu-Jie Luo , Kai Mao , Cheng-Zhi Huang , Yuan-Fang Li , Shu-Jun Zhen . Octopus-like DNA nanostructure coupled with graphene oxide enhanced fluorescence anisotropy for hepatitis B virus DNA detection. Chinese Chemical Letters, 2024, 35(6): 109137-. doi: 10.1016/j.cclet.2023.109137
-
[9]
Huining Zhang , Baixiang Wang , Jianping Han , Shaofeng Wang , Xingmao Liu , Wenhui Niu , Zhongyu Shi , Zhiqiang Wei , Zhiguo Wu , Ying Zhu , Qi Guo . Nature’s revelation: Preparation of Graphene-based Biomimetic materials and its application prospects for water purification. Chinese Chemical Letters, 2025, 36(6): 110319-. doi: 10.1016/j.cclet.2024.110319
-
[10]
Jing-Qi Tao , Shuai Liu , Tian-Yu Zhang , Hong Xin , Xu Yang , Xin-Hua Duan , Li-Na Guo . Photoinduced copper-catalyzed alkoxyl radical-triggered ring-expansion/aminocarbonylation cascade. Chinese Chemical Letters, 2024, 35(6): 109263-. doi: 10.1016/j.cclet.2023.109263
-
[11]
Yuhan Liu , Jingyang Zhang , Gongming Yang , Jian Wang . Highly enantioselective carbene-catalyzed δ-lactonization via radical relay cross-coupling. Chinese Chemical Letters, 2025, 36(1): 109790-. doi: 10.1016/j.cclet.2024.109790
-
[12]
Chaozheng He , Jia Wang , Ling Fu , Wei Wei . Nitric oxide assists nitrogen reduction reaction on 2D MBene: A theoretical study. Chinese Chemical Letters, 2024, 35(5): 109037-. doi: 10.1016/j.cclet.2023.109037
-
[13]
Qi Li , Zi-Lu Wang , Yun-He Xu . Copper-catalyzed 1,4-silylcyanation of 1,3-enynes: A silyl radical-initiated approach for synthesis of difunctionalized allenes. Chinese Chemical Letters, 2025, 36(3): 109991-. doi: 10.1016/j.cclet.2024.109991
-
[14]
Guan-Nan Xing , Di-Ye Wei , Hua Zhang , Zhong-Qun Tian , Jian-Feng Li . Pd-based nanocatalysts for oxygen reduction reaction: Preparation, performance, and in-situ characterization. Chinese Journal of Structural Chemistry, 2023, 42(11): 100021-100021. doi: 10.1016/j.cjsc.2023.100021
-
[15]
Zhen Liu , Zhi-Yuan Ren , Chen Yang , Xiangyi Shao , Li Chen , Xin Li . Asymmetric alkenylation reaction of benzoxazinones with diarylethylenes catalyzed by B(C6F5)3/chiral phosphoric acid. Chinese Chemical Letters, 2024, 35(5): 108939-. doi: 10.1016/j.cclet.2023.108939
-
[16]
Yiqian Jiang , Zihan Yang , Xiuru Bi , Nan Yao , Peiqing Zhao , Xu Meng . Mediated electron transfer process in α-MnO2 catalyzed Fenton-like reaction for oxytetracycline degradation. Chinese Chemical Letters, 2024, 35(8): 109331-. doi: 10.1016/j.cclet.2023.109331
-
[17]
Yi-Fan Wang , Hao-Yun Yu , Hao Xu , Ya-Jie Wang , Xiaodi Yang , Yu-Hui Wang , Ping Tian , Guo-Qiang Lin . Rhodium(Ⅲ)-catalyzed diastereo- and enantioselective hydrosilylation/cyclization reaction of cyclohexadienone-tethered α, β-unsaturated aldehydes. Chinese Chemical Letters, 2024, 35(9): 109520-. doi: 10.1016/j.cclet.2024.109520
-
[18]
Shuai Zhu , Mingjie Chen , Haichao Shen , Hanming Ding , Wenbo Li , Junliang Zhang . Palladium/Xu-Phos-catalyzed enantioselective arylalkoxylation reaction of γ-hydroxyalkenes at room temperature. Chinese Chemical Letters, 2024, 35(11): 109879-. doi: 10.1016/j.cclet.2024.109879
-
[19]
Hongliang Zeng , Yuan Ji , Jinfeng Wen , Xu Li , Tingting Zheng , Qiu Jiang , Chuan Xia . Pt nanocluster-catalyzed hydrogen evolution reaction: Recent advances and future outlook. Chinese Chemical Letters, 2025, 36(3): 109686-. doi: 10.1016/j.cclet.2024.109686
-
[20]
Peng Wang , Jianjun Wang , Ni Song , Xin Zhou , Ming Li . Radical dehydroxymethylative fluorination of aliphatic primary alcohols and diverse functionalization of α-fluoroimides via BF3·OEt2-catalyzed C‒F bond activation. Chinese Chemical Letters, 2025, 36(1): 109748-. doi: 10.1016/j.cclet.2024.109748
-
[1]
Metrics
- PDF Downloads(0)
- Abstract views(895)
- HTML views(25)

 Login In
Login In




 下载:
下载:
 DownLoad:
DownLoad: