Screening the anti-gout traditional herbs from TCM using an in vitro method
- Corresponding author: Liu Shu, mslab20@ciac.ac.cn Liu Zhong-Ying, Liuzy@jlu.edu.cn
Citation:
Chen Wei-Jia, Wu Yi, Zhao Xin, Liu Shu, Song Feng-Rui, Liu Zhong-Ying, Liu Zhi-Qiang. Screening the anti-gout traditional herbs from TCM using an in vitro method[J]. Chinese Chemical Letters,
;2016, 27(11): 1701-1707.
doi:
10.1016/j.cclet.2016.05.022

Gout is an inflammatory arthritis that is induced by the precipitation of monosodium uratemonohydrate (MSU) crystals in articular joints and periarticular tissues. The incidence of the disease has increased annually, which may be associated with dietary and lifestyle changes [1]. The global epidemiology of gout data showed that the worldwide prevalence of gout ranges from 0.1% to approximately 10%, and developed countries tend to have a higher burden of gout than developing countries, while gout incidence of men is higher than women by 2-6 fold [2]. Most frequently, gout causes recurrent attacks of acute arthritis and tophi depositions, and other chronic diseases, such as common complications of renal [3], metabolic syndrome [4], hypertension and cardiovascular diseases [5, 6]. Therefore, the treatment of gout has become a serious problem to solve.
New advances in gout research have revealed that there are many complexes cellular mechanisms on the powerful inflammatory response induced by MSU-stimulation, mainly involving an early increase of pro-inflammatory cytokines, and followed in few days by their reduction along with an increase of anti-inflammatory cytokines [7]. The high levels of cytokines such as IL-1β, ICAM-1, and tumor necrosis factor-α (TNF-α) have been detected in the synovial fluid of gout patients [8, 9]. Uric acid is also a main risk factor for gout and hyperuricemia and formed by the oxidation of hypoxanthine to xanthine. Xanthine can be further oxidized to uric acid by XOD, releasing peroxide anion in the process. XOD is a key enzyme in the occurrence and development of gout, thus, it is important to inhibit the activity of XOD to control uric acid accumulation and deposit.
At present, a number of anti-gout drugs including antiinflammatory drugs (indomethacin and colchicine) and the urate-lowering drugs (allopurinol and benzbromarone) are used as the primary clinical therapies to treat gout and hyperuricemia. Allopurinol is the most widely used first-line drug to control hyperuricemia via inhibiting XOD activity, and thus inhibiting the generation of uric acid from hypoxanthine [10]. Indomethacin as the most potent non-steroidal anti-inflammatory drugs (NSAIDs) has been widely used in the treatment of acute gout to date [11, 12]. However, few drugs produce satisfactory therapeutic efficacy without causing adverse effects, including gastric damage, renal toxicity and hypersensitivity [13-16], thus limit their clinical uses. Consequently, new drugs lower adverse effects from natural resources have raised hope.
Traditional Chinese medicine (TCM) is still an important component of human health care involving a variety of herbs and herbal combinations, in which, a large number of plant species are used for cure of diseases, such as inflammatory, analgesic and immune diseases [17-19]. TCM has fewer adverse effects, is safe and has ideal effects in treating different diseases compared with modern medicines. Previous pharmacological studies had attributed an anti-inflammatory effect of these herbal extracts to reducing neutrophil migration and the expression of proinflammatory cytokines [20]. It showed that the herbal extracts and Chinese herbal formula have significant curative effect on inflammation. It is beneficial to search XOD inhibitors and antiinflammatory herbs from TCM for the prevention of hyperuricemia and gout.
In this study, the anti-inflammation effects of 33 herbal extracts and 16 standards were detected by an in vitro cell model. Moreover, the XOD inhibitory activity was measured using an UPLC-TQ-MS method. The results would provide a reference for screening more safe and effective herbs to prevent and treat gout.
Sodium urate, XOD, xanthine and Tris were purchased from Sigma (St. Louis, MO, USA); Allopurinol was acquired from Alfa Aesar (Shanghai, China); Dulbecco's modified Eagle's medium (DMEM) was purchased from Hyclone (USA); Fetal bovine serum (FBS) was obtained from Hangzhou Sijiqing Co., Ltd. (Hangzhou, China); 3-(4, 5-dimethyl-2-thiazolyl)-2, 5-diphenyl-2-H-tetrazolium bromide (MTT), dimethyl sulfoxide (DMSO), penicillin and streptomyc were obtained from Dingguo Changsheng Biotechnology Co., Ltd. (Beijing, China); ELISA Kits for human ICAM-1 and IL-1β were taken from the Cloud-Clone Corp (Houston, USA); the herbs were provided by Jilin Pharmacy (Changchun, China); the standards were purchased from the National Institute for the Control of Pharmaceutical and Biological Products (Beijing, China); HPLC grade acetonitrile was bought from Fisher Scientific (Loughborough, UK); water was obtained from Milli-Q water purification system (Milford, MA); all the other chemicals of analytical grade were provided by Beijing Shiji (Beijing, China).
Sodium urate solution was prepared as previously described [21]. After sterilization by autoclaving for 1 h, 10 mg of sodium urate was dissolved in 100 μL of DMSO and then 10 mL of serum free sterile culture medium was added. The solution was homogenized using an ultrasonic homogenizer (kunshangshumei, China) and finally, 1 mg/mL of sodium urate solution (MSU) was obtained. The crude extract (2 g) was extracted by immersing in 70% ethanol for 20 min and ultrasonic extraction for 40 min. The supernatant was lyophilized. The herbal extracts were re-dissolved with DMEM: ethanol=1:1 (200 mg/mL); the solution of standards was 2 mg/mL.
The HUVECs were grown in DMEM culture medium containing 10% FBS, 100 units/mL penicillin and 75 units/mL streptomyc. The environment was controlled at 37 ℃ in a humidified atmosphere of 5% CO2 in CO2 incubator (Therom, USA). The HUVECs were seeded onto a 96-well culture plate at a density of 8000 cells/well and incubated at 37 ℃ for 24 h. The medium was replaced with 100 μL of DMEM culture containing 5% FBS and different concentrations of MSU as the model group, while the control group did not contain MSU. The medium was individually incubated for 24 h and 48 h. The medium and MSU were respectively removed for each well after 24 h and 48 h. MTT was dissolved in phosphate buffered saline (PBS) at a concentration of 1 mg/mL; 100 μL of MTT was added to cell cultures after removing medium. After 4 h, the medium was removed and the formazan crystals were dissolved in 150 μL DMSO. The absorbance at 570 nm was read on a microplatereader (Tecan, Austria). The absorbance of living cells was considered as 100% to access cell viability of model group. The percentage of cell viability was calculated as follows: cell viability%=(model/control) × 100%.
The HUVECs were seeded onto a 6-well culture plate at a density of 2 × 105 cells/well and incubated at 37 ℃ for 24 h. The medium was replaced with 2 mL of DMEM culture containing 5% FBS and different concentrations of MSU (50, 100, 150, and 200 μg/mL) for 24 h. Then, the cell culture supernate was collected for 10 min at 1000 r/min. Particulates was removed and assay was carried out immediately. The cells were washed three times with cold PBS, and then detached with trypsin (0.25%). The cells were collected and centrifuged. The cells were re-suspended in PBS and immediately flash-frozen in liquid nitrogen, after that, the cells were thawed at 37 ℃. After repeating the cycle for three times, the cells were centrifuged at 1500 r/min for 10 min at 4 ℃ to remove cellular debris. The productions of ICAM-1 and IL-1β were determined by ELISA.
Different herbal extract solutions and the MSU (200 mg/mL) were simultaneously added into the 96-well after 24 h. The empirical method was mentioned in 2.3. The percentage of herbal extract solution inhibition was calculated as follows: inhibition (%)=(herb-model)/(control-model) × 100%.
The herbal extracts possessing better anti-inflammation activity were selected to assess the expressions of ICAM-1 and IL-1β after MSU-stimulation, including Ginkgo Folium (Gf), Salviae Miltiorrhizae Radix et Rhizoma (Sm), Rhei Radix et Rhizoma (Rr), Polygoni Cuspidati Rhizoma et Radix (Pc), Selaginellae Herba (Sh), and Paeoniae Radix Rubra (Pr). MSU solution and corresponding herbal extract solutions were incubated and maintained under the conditions and time durations as mentioned in 2.3. The different herbal extract solutions (0.5 mg/mL) and indomethacin (10 μg/mL) as wellas the controlMSU (200 μg/mL) were simultaneouslyadded into the 6-well after 24 h. And then, the cell lysates and cell culture supernates were collected. The productions of ICAM-1 and IL-1β were detected.
Xanthine was dissolved in 50 mmol/L Tris-HCl (pH 8.7) and adjusted to 1 mmol/L. The XOD reaction was performed in a total volume of 200 μL containing 50 mmol/L Tris-HCl, 100 μmol/L xanthine, 20 mU/mL XOD and different concentrations of herbal extract solutions or standards. The reaction was initiated for 5 min at 37 ℃, after that it was stopped by adding 750 mL cold acetonitrile, and 4 μmol/L 5-fluorouracil, which is the internal standard. The final solution volume was 1 mL. The uric acid production was indirectly determined by using an ACQUITYTM UPLC system (Waters Corp., Milford, MA, USA) with a thermo stated autosampler at 4 ℃. The separation was carried out on an Agilent ZORBAX SB-C18 column (5 μm; 4.6 × 150 mm; USA) maintained at 35 ℃. Mobile phase component A was acetonitrile and B was water with 0.1% formic acid, and the flow rate was set at 0.3 mL/min. The elution gradients used were: 0-4 min, 10%-30% (A); 4-6 min, 30%-80% (A); 6-7 min, 80%-100% (A), and then returning to the initial condition for 2 min, followed by reequilibration for 5 min. The injection volume was 5 μL.
Mass spectrometric detection was carried out on Xevo TQ mass spectrometer with an electrospray ionization (ESI) source. Quantification analysis was performed in multiple reaction monitoring (MRM) negative mode. The following settings were used for MRM: capillary voltage 2.0 kV; source temperature 350 ℃; cone gas flow 50 L/h; desolvation gas flow 600 L/h; cone and collision energy are presented in Table 1. The percentage of herbal extract solution inhibition was calculated as follows:inhibition (%)=(blank-herb)/blank × 100%.
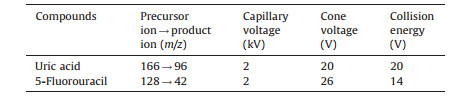
|
The UPLC-TQ-MS data of XOD inhibitor were acquired and processed by Masslynx V4.1 software (Waters, USA). All data were expressed as the mean±SD, and statistical analysis was performed using one-way analysis of SPSS18.0 and significance t test (P < 0.01 or P < 0.05) was carried out.
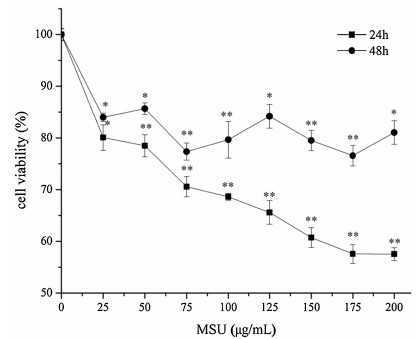
The HUVECs were damaged directly by MSU-stimulation to establish a cell model in vitro [21, 22], and then the antiinflammatory ability of drugs was studied. The cell viability was used as evaluation criterion by an MTT assay. This method is simple, fast and economical on screening anti-gout drugs. In our experimental in vitro study (Fig. 1), the cell viability was monotonously decreased in a dose-dependent manner with the increases of MSU concentration at 24 h. The cell viability remained stable at 175-200 μg/mL (175 μg/mL: 57.32%±1.81%; 200 μg/mL: 57.58%±1.27%). By contrast, the data were irregular fluctuations at 48 h. Moreover, the data revealed that cells were damaged more seriously by MSU-stimulation at 24 h. Thus, we chose the 200 μg/mL MSU and 24 h as the optimal conditions to assess the ability of drugs on inflammation.
The primary pathological feature of gout is generating inflammatory response by MSU-stimulation, including activation of nod-like receptor pyrin domain-containing-3 (NLRP3) to lead to caspase-1 and pro-IL-1β cleavage, and further result in releasing IL-1β [23, 24]. IL-1β is a pivotal mediator of gouty inflammation and pain. Endothelial cell adhesion is the essence of acute gout, which induced ICAM-1 expression [25] and associated with a variety of inflammatory cytokines [26]. Therefore, ICAM-1 and IL-1β could be used as important indicators to assess the model of acute gout.
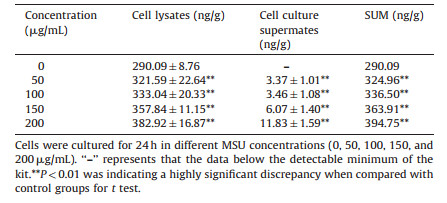
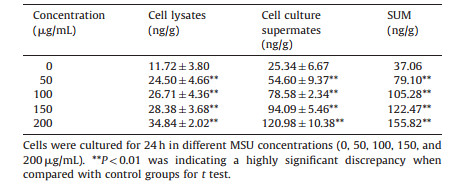
The results for expressions of ICAM-1 and IL-1β are respectively shown in Tables 2 and 3, which demonstrates that both the expressions of ICAM-1 and IL-1β were significantly increased (P < 0.01) in a dose-dependent manner with the different concentrations of MSU in cell lysates and cell culture supernates. We calculated the sum of cell lysates and cell culture supernates at different concentrations. From these tables we can see that both of the expressions of ICAM-1 and IL-1β were also significantly increased (P < 0.01) compared with the control group. ICAM-1 and IL-1β could influence the cell viability under MSU-stimulation. This might be due to the fact that the intracellular substances were activated after stimulating with MSU to produce ICAM-1 and IL-1β and they could damage cells as inflammatory cytokines do. IL-1β could promote the recruitment of neutrophils through the upregulations of E-selectin and ICAM-1 expressions by the endothelial cells [27]. Therefore, this model is useful for studying the pathological mechanism of acute gout on anti-inflammation induced by MSU. To screen the anti-gout traditional herbs from TCM, in this research, the anti-inflammatory mechanism of herbal extracts was observed through the expressions of ICAM-1 and IL-1β.
|
|
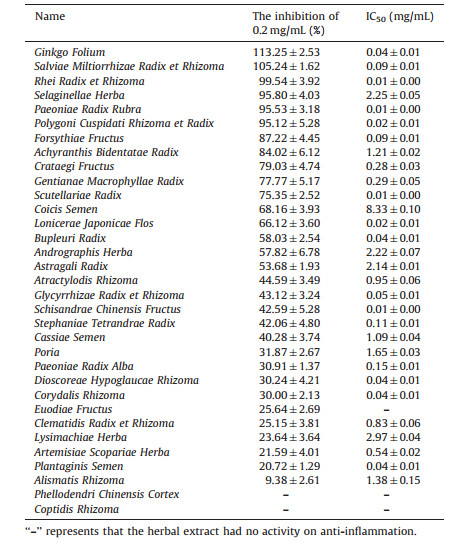
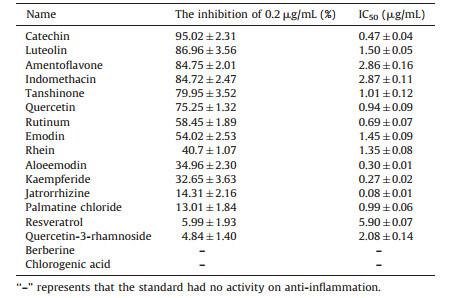
We detected the anti-inflammatory ability of herbal extracts using the convenient method, i.e., in vitro cell model to find out effective herbs in the treatment and prevention of gout. In this study, 33 herbal extracts and 16 standards were detected. From Table 4 we can see that most herbal extracts have the anti-inflammatory activity at 0.2 mg/mL. The inhibition rate of Ginkgo Folium, Salviae Miltiorrhizae Radix et rhizome, Rhei Radix et Rhizoma, Polygoni Cuspidati Rhizoma et Radix, Selaginellae Herba, and Paeoniae Radix Rubra was all more than 90%. Moreover, Scutellarlae Radix, Lonicerae Japonicae Flos, Bupleuri Radix, Glycyrrhizae Radix et Rhizoma and Schisandrae Chinensis Fructus also showed anti-inflammatory activity with lower IC50. The antiinflammation effect was evaluated using indometacin as a comparator. As shown in Table 5, the standards possessed similar ability of anti-inflammation. Catechin, luteolin and amentoflavone were better than the other standards on inflammation at 0.2 μg/mL. Catechin, quercetin, rutinum, aloeemodin, palmatine chloride, jatrorrhizine and kaempferide possessed lower IC50 compared with indometacin. In short, the anti-inflammatory performance of most flavonoid standards was better than that of the other standards.
|
|
These herbs were classified according to the main ingredients. For example, Ginkgo Folium and Selaginellae Herba were classified as flavonoids, Rhei Radix et Rhizoma and Polygoni Cuspidati Rhizoma et Radix were classified as anthraquinones, Phellodendri Chinensis Cortex and Coptidis Rhizoma were classified as alkaloids. Modern pharmacological studies have demonstrated that the herbs and standards of flavonoids [28-32], anthraquinone [33-35], and alkaloids [36, 37] could inhibit the inflammatory cytokine expression and suppress nuclear transcription factor-κB (NF-κB) activation. Besides, glycosides are the main ingredients of Paeoniae Radix Rubra. Paeoniflorin is a potent anti-inflammatory agent that can ameliorate the leukocyte infiltrates and adhesion molecules expression [38, 39]. These data suggest that most herbs from TCM have a better therapeutic effect on inflammation. The capacity of herbal extracts on inflammation may depend upon their bioactive ingredients.
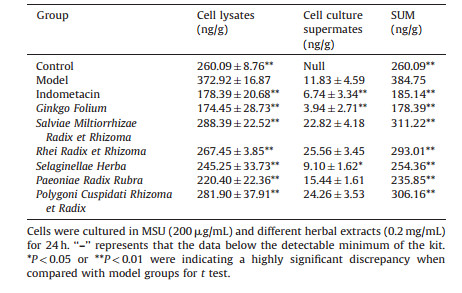
The stronger anti-inflammatory herbs, including Ginkgo Folium (Gf), Salviae Miltiorrhizae Radix et Rhizoma (Sm), Rhei Radix et Rhizoma (Rr), Polygoni Cuspidati Rhizoma et Radix (Pc), Selaginellae Herba (Sh), and Paeoniae Radix Rubra (Pr), were used as examples to assess the effects of them on the expressions of ICAM-1 and IL-1β in cell lysates and cell culture supernates. As shown in Table 6, the expression of ICAM-1 on model group was significantly increased compared with the control group (P < 0.01). And all the herbal extracts were significantly decreased compared with the model group (P < 0.01) in cell lysates. This indicates that the herbal extracts could reduce the intracellular expressions of ICAM-1. In cell culture supernates, In, Gf, and Sh were decreased (P < 0.05 or P < 0.01) compared with the model group, but in contrast, Sm, Rr, Pr and Pc were increased and even more than model group. The sum of ICAM-1 of cell lysates and cell culture supernates showed that each herbal extract was significantly decreased compared with the model group (P < 0.01), and the order of total expression level was In, Gf, Pr, Sh, control, Rr, Pc, Sm, and model.
|
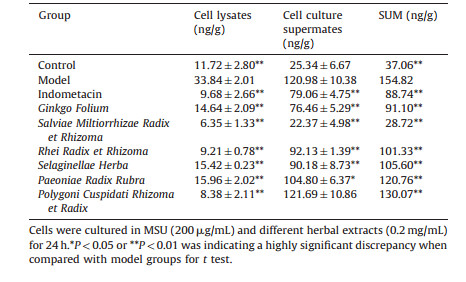
The expression of IL-1β was similar to ICAM-1, thus from Table 7 we can see that the model group was significantly increased compared with the control group (P < 0.01) in cell lysates. In comparing with the model group, the herbal extracts group was significantly decreased (P < 0.01). In cell culture supernates, the herbal extracts group decreased (P < 0.05 or P < 0.01) except Pc compared with the model group. On the other hand, Pc obviously decreased IL-1β in cell lysates that might be released into cell supernates. From Table 7, it can be seen that for the sum of IL-1β expression level of all the herbal extracts was significantly decreased compared with the model group (P < 0.01), of which, the order of total expression level was Sm, Con, In, Gf, Rr, Sh, Pr, Pc, and Mod. These data indicate that the herbal extracts could reduce the release of inflammatory cytokines and protect the cell viability. Especially, Ginkgo Folium seemed to be more effective in inhibiting the expression of ICAM-1 and IL-1β.
|
In conclusion, herbal extracts possess anti-inflammatory bioactivity to protect HUVECs. The possible reason was the active components in the herbal extracts inhibited ICAM-1 and IL-1β expression. Compared with indometacin, Ginkgo Folium and Salviae Miltiorrhizae Radix et Rhizoma could effectively inhibit the releases of ICAM-1 and IL-1β. Ginkgo Folium has been reported that possessed activity on inflammation via inhibiting the production of pro-inflammatory cytokines IL-1β and TNF-α [29, 30].
XOD is a key enzyme for the treatments of gout and hyperuricemia. A large number of the plant species are potential sources of natural XOD inhibitors [40, 41] which could decrease XOD activity in serum or liver of the animal models of hyperuricemia and gout [42, 43]. To date, TCM has been used for inhibiting XOD activity and decreasing the level of uric acid. It can be used as alternatives to allopurinol because of fewer adverse effects [44]. In this research, the UPLC-MS/MS method was used for the detection of XOD activity and screening XOD inhibitors by quantifying products of enzymatic reaction. This method is sensitive, accurate, rapid, and can eliminate false positive and negative results.
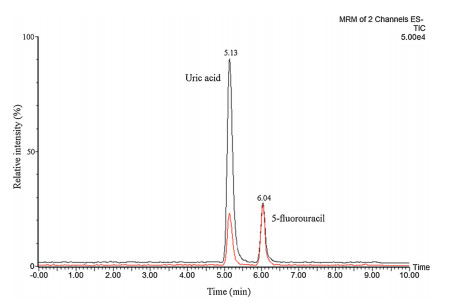
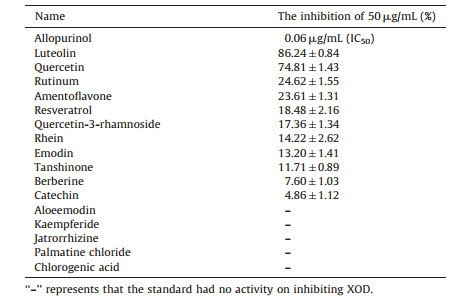
The representative chromatograms of samples are showed in Fig. 2. The black represents the enzyme reaction without inhibitor and red represents the enzyme reaction with allopurinol. From this figure we can see that the peaks of uric acid (TR=5.13 min) and internal standard (TR=6.04 min) were separated. As shown in Table 8, the XOD inhibitory activity of Rhei Radix et Rhizoma, Paeoniae Radix Rubra, Salviae Miltiorrhizae Radix et rhizoma, Selaginellae Herba, Paeoniae Radix Alba, Polygoni Cuspidati Rhizoma et Radix, Ginkgo Folium, and Glycyrrhizae Radix et Rhizoma was over 25% at 5 mg/mL. The activity of the standards ininhibiting XOD is shown in Table 9. Allopurinol is a powerful XOD inhibitor, since it could significantly inhibit the enzyme reaction (IC50 0.06 μg/mL). Luteolin and quercetin, which belong to flavonoids were stronger than the other standards. Flavonoids and anthraquinone can inhibit XOD and reduce serum uric acid levels in vitro and in vivo [45-49], but the exact mechanism is not known and should be further investigated.

|
|
In short, we demonstrated that the herbal extracts and standards could ameliorate the MSU induced cellular damage by inhibiting the release of inflammatory cytokines including ICAM-1 and IL-1β and suppress the oxidation of xanthine to uric acid. Our results support that the compounds of flavonoid and anthraquinone seem to be more effective on inflammation and inhibiting XOD. However, further investigation is needed to probe the possible mechanism. The results obtained in this research provide a theoretical basis for TCM on inflammation and lowering uric acid.At the same time, the results could provide some development directions of new preventive and therapeutic drugs for treating gout.
In the present work, the inhibitory ability of 33 herbal extracts and 16 compounds on anti-inflammatory activity and inhibiting XOD activity were estimated. The results show that the inhibitory activity of Ginkgo Folium and Rhei Radix et Rhizoma on inflammation and inhibiting XOD was stronger than the other herbal extracts, and the anti-inflammatory activity of flavonoids and their XOD inhibitory activity were stronger than that of the other compounds. In short, the compounds of flavonoid and anthraquinone seem to be more effective on inflammation and inhibiting XOD and they could be widely used in the treatment of gout. Since the inhibitory ability of natural products should relate to their bioactive components, so the results could provide a theoretical basis for the further development of natural medicines to treat gout.
The authors would like to thank the National Natural Science Foundation of China (Nos. 21175128, 81303280, 81573574).
Miao Z., Li C., Chen Y.. Dietary and lifestyle changes associated with high prevalence of hyperuricemia and gout in the Shandong coastal cities of Eastern China[J]. J. Rheumatol., 2008,35:1859-1864.
Kuo C.F., Grainge M.J., Zhang C.W., Doherty M.. Global epidemiology of gout: prevalence, incidence and risk factors[J]. Nat. Rev. Rheumatol., 2015,11:649-662. doi: 10.1038/nrrheum.2015.91
Yu K.H., Kuo C.F., Luo S.F.. Risk of end-stage renal disease associated with gout: a nationwide population study[J]. Arthritis Res. Ther., 2012,14R83. doi: 10.1186/ar3806
Puig J.G., Martínez M.A.. Hyperuricemia, gout and the metabolic syndrome[J]. Curr. Opin. Rheumatol., 2008,20:187-191. doi: 10.1097/BOR.0b013e3282f4b1ed
Saag K.G., Choi H.. Epidemiology, risk factors, and lifestyle modifications for gout[J]. Arthritis Res. Ther., 2006,8S2.
Richette P., Bardin T.. Gout[J]. Lancet, 2010,375:318-328. doi: 10.1016/S0140-6736(09)60883-7
Punzi L., Scanu A., Ramonda R., Oliviero F.. Gout as autoinflammatory disease: new mechanisms for more appropriated treatment targets[J]. Autoimmun. Rev., 2012,12:66-71. doi: 10.1016/j.autrev.2012.07.024
Scanu A., Oliviero F., Ramonda R.. Cytokine levels in human synovial fluid during the different stages of acute gout: role of transforming growth factor β1 in the resolution phase[J]. Ann. Rheum. Dis., 2012,71:621-624. doi: 10.1136/annrheumdis-2011-200711
Umar S., Hedaya O., Singh A.K., Ahmed S.. Thymoquinone inhibits TNF-α-induced inflammation and cell adhesion in rheumatoid arthritis synovial fibroblasts by ASK1 regulation[J]. Toxicol. Appl. Pharmacol., 2015,287:299-305. doi: 10.1016/j.taap.2015.06.017
Schumacher Jr. H.R., Becker M.A., Wortmann R.L.. Effects of febuxostat versus allopurinol and placebo in reducing serum urate in subjects with hyperuricemia and gout: a 28-week, phase Ⅲ, randomized, double-blind, parallelgroup trial[J]. Arthritis Rheum., 2008,59:1540-1548. doi: 10.1002/art.v59:11
Shrestha M., Morgan D.L., Moreden J.M.. Randomized double-blind comparison of the analgesic efficacy of intramuscular ketorolac and oral indomethacin in the treatment of acute gouty arthritis[J]. Ann. Emerg. Med., 1995,26:682-686. doi: 10.1016/S0196-0644(95)70037-4
Rubin B.R., Burton R., Navarra S.. Efficacy and safety profile of treatment with etoricoxib 120 mg once daily compared with indomethacin 50 mg three times daily in acute gout: a randomized controlled trial[J]. Arthritis Rheum., 2004,50:598-606. doi: 10.1002/art.v50:2
Arroyo-Lira A.G., Rodríguez-Ramos F., Chávez-Piña A.E.. Synergistic antinociceptive effect and gastric safety of the combination of docosahexaenoic acid and indomethacin in rats[J]. Pharmacol. Biochem. Behav., 2014,122:74-81. doi: 10.1016/j.pbb.2014.03.015
Huh K., Joung J.Y., Jeong H.. Colchicine-induced myoneuropathy in a cyclosporine-treated renal transplant recipient[J]. Kidney Res. Clin. Pract., 2013,32:74-77. doi: 10.1016/j.krcp.2013.04.003
Ryu H.J., Song R., Kim H.W.. Clinical risk factors for adverse events in allopurinol users[J]. J. Clin. Pharmacol., 2013,53:211-216. doi: 10.1177/0091270012439715
Yang C.Y., Chen C.H., Deng S.T.. Allopurinol use and risk of fatal hypersensitivity reactions: a nationwide population-based study in Taiwan[J]. JAMA Intern. Med., 2015,175:1550-1557. doi: 10.1001/jamainternmed.2015.3536
Wang Q.H., Kuang H.X., Su Y.. Naturally derived anti-inflammatory compounds from Chinese medicinal plants[J]. J. Ethnopharmacol., 2013,146:9-39. doi: 10.1016/j.jep.2012.12.013
Shi J., Liu Y.L., Fang Y.X.. Traditional Chinese medicine in treatment of opiate addiction[J]. Acta Pharmacol. Sinica, 2006,27:1303-1308. doi: 10.1111/aphs.2006.27.issue-10
Lü M., Wang T.Y., Tian X.X.. Interaction of anti-thrombotic and antiinflammatory activities of commonly used traditional Chinese medicine for promoting blood circulation and removing blood stasis revealed by network pharmacology analysis[J]. Acta Pharm. Sinica, 2015,50:1135-1141.
Kolasinski S.L.. Food, drink, and herbs: alternative therapies and gout[J]. Curr. Rheumatol. Rep., 2014,16409. doi: 10.1007/s11926-014-0409-8
Kodithuwakku N.D., Pan M., Zhu Y.L.. Anti-inflammatory and antinociceptive effects of Chinese medicine SQ gout capsules and its modulation of proinflammatory cytokines focusing on gout arthritis[J]. J. Ethnopharmacol., 2013,150:1071-1079. doi: 10.1016/j.jep.2013.10.016
Yang Y.H., Yin L., Wang M.Y., Zhao F.M., Duan J.A.. Study on vascular endothelial cell model of acute gout by sodium urate induced[J]. Chin. Arch. Tradit. Chin. Med., 2010,28:592-594.
Martinon F., Burns K., Tschopp J.. The inflammasome: a molecular platform triggering activation of inflammatory caspases and processing of proIL-beta[J]. Mol. Cell, 2002,10:417-426. doi: 10.1016/S1097-2765(02)00599-3
Martinon F., Mayor A., Tschopp J.. The inflammasomes: guardians of the body[J]. Annu. Rev. Immunol., 2009,27:229-265. doi: 10.1146/annurev.immunol.021908.132715
Yagnik D.R., Hillyer P., Marshall D.. Noninflammatory phagocytosis ofmonosodium urate monohydrate crystals by mouse macrophages. Implications for the control of joint inflammation in gout[J]. Arthritis Rheum, 2000,43:1779-1789. doi: 10.1002/(ISSN)1529-0131
Dalbeth N., Haskard D.O.. Mechanisms of inflammation in gout[J]. Rheumatology (Oxford), 2005,44:1090-1096. doi: 10.1093/rheumatology/keh640
Mitroulis I., Kambas K., Ritis K.. Neutrophils, IL-1β, and gout: is there a link?[J]. Semin. Immunopathol., 2013,35:501-512. doi: 10.1007/s00281-013-0361-0
Zhang Z., Sun T., Niu J.G.. Amentoflavone protects hippocampal neurons: anti-inflammatory, antioxidative, and antiapoptotic effects[J]. Neural Regen. Res., 2015,10:1125-1133. doi: 10.4103/1673-5374.160109
Jiao Y.B., Rui Y.C., Li T.J., Yang P.Y., Qiu Y.. Expression of pro-inflammatory and anti-inflammatory cytokines in brain of atherosclerotic rats and effects of Ginkgo biloba extract[J]. Acta Pharmacol. Sinica, 2005,26:835-839. doi: 10.1111/aphs.2005.26.issue-7
Jiao Y.B., Rui Y.C., Yang P.Y., Li T.J., Qiu Y.. Effects of Ginkgo biloba extract on expressions of IL-1beta, TNF-alpha, and IL-10 in U937 foam cells[J]. Acta Pharm. Sinica, 2007,42:930-934.
Yoon J.S., Lee H.J., Choi S.H.. Quercetin inhibits IL-1β-induced inflammation, hyaluronan production and adipogenesis in orbital fibroblasts from Graves' orbitopathy[J]. PLoS ONE, 2011,6e26261. doi: 10.1371/journal.pone.0026261
Chen C.C., Chow M.P., Huang W.C., Lin Y.C., Chang Y.J.. Flavonoids inhibit tumor necrosis factor-a-induced up-regulation of intercellular adhesion molecule-1(ICAM-1) in respiratory epithelial cells through activator protein-1 and nuclear factor-kB: structure-activity relationships[J]. Mol. Pharmacol., 2004,66:683-693.
Kshirsagar A.D., Panchal P.V., Harle U.N.. Anti-inflammatory and antiarthritic activity of anthraquinone derivatives in rodents[J]. Int. J. Inflam., 2014,2014690596.
Han J.W., Shim D.W., Shin W.Y.. Anti-inflammatory effect of emodin via attenuation of NLRP3 inflammasome activation[J]. Int. J. Mol. Sci., 2015,16:8102-8109. doi: 10.3390/ijms16048102
Chen G.L., Zhang J.J., Kao X., Wei L.W., Liu Z.Y.. Emodin ameliorates lipopolysaccharides-induced corneal inflammation in rats[J]. Int. J. Ophthalmol., 2015,8:665-6659.
Lee C.H., Chen J.C., Hsiang C.Y.. Berberine suppresses inflammatory agentsinduced interleukin-1β and tumor necrosis factor-α productions via the inhibition of IkB degradation in human lung cells[J]. Pharmacol. Res., 2007,56:193-201. doi: 10.1016/j.phrs.2007.06.003
Wang Z.G., Chen Z., Yang S.S.. Berberine ameliorates collagen-induced arthritis in rats associated with anti-inflammatory and anti-angiogenic effects[J]. Inflammation, 2014,37:1789-1798. doi: 10.1007/s10753-014-9909-y
Chen T., Guo Z.P., Jiao X.Y.. Protective effects of peoniflorin against hydrogen peroxide-induced oxidative stress in human umbilical vein endothelial cells[J]. Can. J. Physiol. Pharmacol., 2011,89:445-453. doi: 10.1139/y11-034
Chen T., Guo Z.P., Wang L.. Paeoniflorin suppresses vascular damage and the expression of E-selectin and ICAM-1 in a mouse model of cutaneous Arthus reaction[J]. Exp. Dermatol., 2013,22:453-457. doi: 10.1111/exd.12174
Liu S., Xing J.P., Zheng Z.. Ultrahigh performance liquid chromatography-triple quadrupole mass spectrometry inhibitors fishing assay: a novel method for simultaneously screening of xanthine oxidase inhibitor and superoxide anion scavenger in a single analysis[J]. Anal. Chim. Acta, 2012,17:64-70.
Hudaib M.M., Tawaha K.A., Mohammad M.K.. Xanthine oxidase inhibitory activity of the methanolic extracts of selected Jordanian medicinal plants[J]. Pharmacogn. Mag., 2011,7:320-324. doi: 10.4103/0973-1296.90413
Wu X.H., Ruan J.L., Zhang J.. Pallidifloside D, a saponin glycoside constituent from Smilax riparia, resist to hyperuricemia based on URAT1 and GLUT9 in hyperuricemic mice, J[J]. Ethnopharmacology, 2014,157:201-205. doi: 10.1016/j.jep.2014.09.034
Hou C.W., Lee Y.C., Hung H.F., Fu H.W., Jeng K.C.. Longan seed extract reduces hyperuricemia via modulating urate transporters and suppressing xanthine oxidase activity[J]. Am. J. Chin. Med., 2012,40:979-991. doi: 10.1142/S0192415X12500723
Kong L.D., Yang C., Ge F., Wang H.D., Guo Y.S.. A Chinese herbal medicine Ermiao wan reduces serum uric acid level and inhibits liver xanthine dehydrogenase and xanthine oxidase in mice[J]. J. Ethnopharmacol., 2004,93:325-330. doi: 10.1016/j.jep.2004.04.008
Huang J.Q., Wang S.W., Zhu M.Z., Chen J.Z., Zhu X.X.. Effects of Genistein, Apigenin, Quercetin, RutinandAstilbinonserumuricacidlevelsandxanthineoxidaseactivities in normal and hyperuricemic mice[J]. Food Chem. Toxicol., 2011,49:1943-1947. doi: 10.1016/j.fct.2011.04.029
Van Hoorn D.E.C., Nijveldt R.J., Van Leeuwen P.A.M.. Accurate prediction of xanthine oxidase inhibition based on the structure of flavonoids[J]. Eur. J. Pharmacol., 2002,451:111-118. doi: 10.1016/S0014-2999(02)02192-1
Mo S.F., Zhou F., Lv Y.Z.. Hypouricemic action of selected flavonoids in mice: structure-activity relationships[J]. Biol. Pharm. Bull., 2007,30:1551-1556. doi: 10.1248/bpb.30.1551
Sheu S.Y., Chiang H.C.. Inhibition of xanthine oxidase by hydroxylatedanthraquinones and related compounds[J]. Anticancer Res., 1997,17:3293-3297.
Noro T., Noro K., Miyase T.. Inhibition of xanthine oxidase by anthraquinones[J]. Chem. Pharm. Bull., 1987,35:4314-4316. doi: 10.1248/cpb.35.4314
Miao Z., Li C., Chen Y.. Dietary and lifestyle changes associated with high prevalence of hyperuricemia and gout in the Shandong coastal cities of Eastern China[J]. J. Rheumatol., 2008,35:1859-1864.
Kuo C.F., Grainge M.J., Zhang C.W., Doherty M.. Global epidemiology of gout: prevalence, incidence and risk factors[J]. Nat. Rev. Rheumatol., 2015,11:649-662. doi: 10.1038/nrrheum.2015.91
Yu K.H., Kuo C.F., Luo S.F.. Risk of end-stage renal disease associated with gout: a nationwide population study[J]. Arthritis Res. Ther., 2012,14R83. doi: 10.1186/ar3806
Puig J.G., Martínez M.A.. Hyperuricemia, gout and the metabolic syndrome[J]. Curr. Opin. Rheumatol., 2008,20:187-191. doi: 10.1097/BOR.0b013e3282f4b1ed
Saag K.G., Choi H.. Epidemiology, risk factors, and lifestyle modifications for gout[J]. Arthritis Res. Ther., 2006,8S2.
Richette P., Bardin T.. Gout[J]. Lancet, 2010,375:318-328. doi: 10.1016/S0140-6736(09)60883-7
Punzi L., Scanu A., Ramonda R., Oliviero F.. Gout as autoinflammatory disease: new mechanisms for more appropriated treatment targets[J]. Autoimmun. Rev., 2012,12:66-71. doi: 10.1016/j.autrev.2012.07.024
Scanu A., Oliviero F., Ramonda R.. Cytokine levels in human synovial fluid during the different stages of acute gout: role of transforming growth factor β1 in the resolution phase[J]. Ann. Rheum. Dis., 2012,71:621-624. doi: 10.1136/annrheumdis-2011-200711
Umar S., Hedaya O., Singh A.K., Ahmed S.. Thymoquinone inhibits TNF-α-induced inflammation and cell adhesion in rheumatoid arthritis synovial fibroblasts by ASK1 regulation[J]. Toxicol. Appl. Pharmacol., 2015,287:299-305. doi: 10.1016/j.taap.2015.06.017
Schumacher Jr. H.R., Becker M.A., Wortmann R.L.. Effects of febuxostat versus allopurinol and placebo in reducing serum urate in subjects with hyperuricemia and gout: a 28-week, phase Ⅲ, randomized, double-blind, parallelgroup trial[J]. Arthritis Rheum., 2008,59:1540-1548. doi: 10.1002/art.v59:11
Shrestha M., Morgan D.L., Moreden J.M.. Randomized double-blind comparison of the analgesic efficacy of intramuscular ketorolac and oral indomethacin in the treatment of acute gouty arthritis[J]. Ann. Emerg. Med., 1995,26:682-686. doi: 10.1016/S0196-0644(95)70037-4
Rubin B.R., Burton R., Navarra S.. Efficacy and safety profile of treatment with etoricoxib 120 mg once daily compared with indomethacin 50 mg three times daily in acute gout: a randomized controlled trial[J]. Arthritis Rheum., 2004,50:598-606. doi: 10.1002/art.v50:2
Arroyo-Lira A.G., Rodríguez-Ramos F., Chávez-Piña A.E.. Synergistic antinociceptive effect and gastric safety of the combination of docosahexaenoic acid and indomethacin in rats[J]. Pharmacol. Biochem. Behav., 2014,122:74-81. doi: 10.1016/j.pbb.2014.03.015
Huh K., Joung J.Y., Jeong H.. Colchicine-induced myoneuropathy in a cyclosporine-treated renal transplant recipient[J]. Kidney Res. Clin. Pract., 2013,32:74-77. doi: 10.1016/j.krcp.2013.04.003
Ryu H.J., Song R., Kim H.W.. Clinical risk factors for adverse events in allopurinol users[J]. J. Clin. Pharmacol., 2013,53:211-216. doi: 10.1177/0091270012439715
Yang C.Y., Chen C.H., Deng S.T.. Allopurinol use and risk of fatal hypersensitivity reactions: a nationwide population-based study in Taiwan[J]. JAMA Intern. Med., 2015,175:1550-1557. doi: 10.1001/jamainternmed.2015.3536
Wang Q.H., Kuang H.X., Su Y.. Naturally derived anti-inflammatory compounds from Chinese medicinal plants[J]. J. Ethnopharmacol., 2013,146:9-39. doi: 10.1016/j.jep.2012.12.013
Shi J., Liu Y.L., Fang Y.X.. Traditional Chinese medicine in treatment of opiate addiction[J]. Acta Pharmacol. Sinica, 2006,27:1303-1308. doi: 10.1111/aphs.2006.27.issue-10
Lü M., Wang T.Y., Tian X.X.. Interaction of anti-thrombotic and antiinflammatory activities of commonly used traditional Chinese medicine for promoting blood circulation and removing blood stasis revealed by network pharmacology analysis[J]. Acta Pharm. Sinica, 2015,50:1135-1141.
Kolasinski S.L.. Food, drink, and herbs: alternative therapies and gout[J]. Curr. Rheumatol. Rep., 2014,16409. doi: 10.1007/s11926-014-0409-8
Kodithuwakku N.D., Pan M., Zhu Y.L.. Anti-inflammatory and antinociceptive effects of Chinese medicine SQ gout capsules and its modulation of proinflammatory cytokines focusing on gout arthritis[J]. J. Ethnopharmacol., 2013,150:1071-1079. doi: 10.1016/j.jep.2013.10.016
Yang Y.H., Yin L., Wang M.Y., Zhao F.M., Duan J.A.. Study on vascular endothelial cell model of acute gout by sodium urate induced[J]. Chin. Arch. Tradit. Chin. Med., 2010,28:592-594.
Martinon F., Burns K., Tschopp J.. The inflammasome: a molecular platform triggering activation of inflammatory caspases and processing of proIL-beta[J]. Mol. Cell, 2002,10:417-426. doi: 10.1016/S1097-2765(02)00599-3
Martinon F., Mayor A., Tschopp J.. The inflammasomes: guardians of the body[J]. Annu. Rev. Immunol., 2009,27:229-265. doi: 10.1146/annurev.immunol.021908.132715
Yagnik D.R., Hillyer P., Marshall D.. Noninflammatory phagocytosis ofmonosodium urate monohydrate crystals by mouse macrophages. Implications for the control of joint inflammation in gout[J]. Arthritis Rheum, 2000,43:1779-1789. doi: 10.1002/(ISSN)1529-0131
Dalbeth N., Haskard D.O.. Mechanisms of inflammation in gout[J]. Rheumatology (Oxford), 2005,44:1090-1096. doi: 10.1093/rheumatology/keh640
Mitroulis I., Kambas K., Ritis K.. Neutrophils, IL-1β, and gout: is there a link?[J]. Semin. Immunopathol., 2013,35:501-512. doi: 10.1007/s00281-013-0361-0
Zhang Z., Sun T., Niu J.G.. Amentoflavone protects hippocampal neurons: anti-inflammatory, antioxidative, and antiapoptotic effects[J]. Neural Regen. Res., 2015,10:1125-1133. doi: 10.4103/1673-5374.160109
Jiao Y.B., Rui Y.C., Li T.J., Yang P.Y., Qiu Y.. Expression of pro-inflammatory and anti-inflammatory cytokines in brain of atherosclerotic rats and effects of Ginkgo biloba extract[J]. Acta Pharmacol. Sinica, 2005,26:835-839. doi: 10.1111/aphs.2005.26.issue-7
Jiao Y.B., Rui Y.C., Yang P.Y., Li T.J., Qiu Y.. Effects of Ginkgo biloba extract on expressions of IL-1beta, TNF-alpha, and IL-10 in U937 foam cells[J]. Acta Pharm. Sinica, 2007,42:930-934.
Yoon J.S., Lee H.J., Choi S.H.. Quercetin inhibits IL-1β-induced inflammation, hyaluronan production and adipogenesis in orbital fibroblasts from Graves' orbitopathy[J]. PLoS ONE, 2011,6e26261. doi: 10.1371/journal.pone.0026261
Chen C.C., Chow M.P., Huang W.C., Lin Y.C., Chang Y.J.. Flavonoids inhibit tumor necrosis factor-a-induced up-regulation of intercellular adhesion molecule-1(ICAM-1) in respiratory epithelial cells through activator protein-1 and nuclear factor-kB: structure-activity relationships[J]. Mol. Pharmacol., 2004,66:683-693.
Kshirsagar A.D., Panchal P.V., Harle U.N.. Anti-inflammatory and antiarthritic activity of anthraquinone derivatives in rodents[J]. Int. J. Inflam., 2014,2014690596.
Han J.W., Shim D.W., Shin W.Y.. Anti-inflammatory effect of emodin via attenuation of NLRP3 inflammasome activation[J]. Int. J. Mol. Sci., 2015,16:8102-8109. doi: 10.3390/ijms16048102
Chen G.L., Zhang J.J., Kao X., Wei L.W., Liu Z.Y.. Emodin ameliorates lipopolysaccharides-induced corneal inflammation in rats[J]. Int. J. Ophthalmol., 2015,8:665-6659.
Lee C.H., Chen J.C., Hsiang C.Y.. Berberine suppresses inflammatory agentsinduced interleukin-1β and tumor necrosis factor-α productions via the inhibition of IkB degradation in human lung cells[J]. Pharmacol. Res., 2007,56:193-201. doi: 10.1016/j.phrs.2007.06.003
Wang Z.G., Chen Z., Yang S.S.. Berberine ameliorates collagen-induced arthritis in rats associated with anti-inflammatory and anti-angiogenic effects[J]. Inflammation, 2014,37:1789-1798. doi: 10.1007/s10753-014-9909-y
Chen T., Guo Z.P., Jiao X.Y.. Protective effects of peoniflorin against hydrogen peroxide-induced oxidative stress in human umbilical vein endothelial cells[J]. Can. J. Physiol. Pharmacol., 2011,89:445-453. doi: 10.1139/y11-034
Chen T., Guo Z.P., Wang L.. Paeoniflorin suppresses vascular damage and the expression of E-selectin and ICAM-1 in a mouse model of cutaneous Arthus reaction[J]. Exp. Dermatol., 2013,22:453-457. doi: 10.1111/exd.12174
Liu S., Xing J.P., Zheng Z.. Ultrahigh performance liquid chromatography-triple quadrupole mass spectrometry inhibitors fishing assay: a novel method for simultaneously screening of xanthine oxidase inhibitor and superoxide anion scavenger in a single analysis[J]. Anal. Chim. Acta, 2012,17:64-70.
Hudaib M.M., Tawaha K.A., Mohammad M.K.. Xanthine oxidase inhibitory activity of the methanolic extracts of selected Jordanian medicinal plants[J]. Pharmacogn. Mag., 2011,7:320-324. doi: 10.4103/0973-1296.90413
Wu X.H., Ruan J.L., Zhang J.. Pallidifloside D, a saponin glycoside constituent from Smilax riparia, resist to hyperuricemia based on URAT1 and GLUT9 in hyperuricemic mice, J[J]. Ethnopharmacology, 2014,157:201-205. doi: 10.1016/j.jep.2014.09.034
Hou C.W., Lee Y.C., Hung H.F., Fu H.W., Jeng K.C.. Longan seed extract reduces hyperuricemia via modulating urate transporters and suppressing xanthine oxidase activity[J]. Am. J. Chin. Med., 2012,40:979-991. doi: 10.1142/S0192415X12500723
Kong L.D., Yang C., Ge F., Wang H.D., Guo Y.S.. A Chinese herbal medicine Ermiao wan reduces serum uric acid level and inhibits liver xanthine dehydrogenase and xanthine oxidase in mice[J]. J. Ethnopharmacol., 2004,93:325-330. doi: 10.1016/j.jep.2004.04.008
Huang J.Q., Wang S.W., Zhu M.Z., Chen J.Z., Zhu X.X.. Effects of Genistein, Apigenin, Quercetin, RutinandAstilbinonserumuricacidlevelsandxanthineoxidaseactivities in normal and hyperuricemic mice[J]. Food Chem. Toxicol., 2011,49:1943-1947. doi: 10.1016/j.fct.2011.04.029
Van Hoorn D.E.C., Nijveldt R.J., Van Leeuwen P.A.M.. Accurate prediction of xanthine oxidase inhibition based on the structure of flavonoids[J]. Eur. J. Pharmacol., 2002,451:111-118. doi: 10.1016/S0014-2999(02)02192-1
Mo S.F., Zhou F., Lv Y.Z.. Hypouricemic action of selected flavonoids in mice: structure-activity relationships[J]. Biol. Pharm. Bull., 2007,30:1551-1556. doi: 10.1248/bpb.30.1551
Sheu S.Y., Chiang H.C.. Inhibition of xanthine oxidase by hydroxylatedanthraquinones and related compounds[J]. Anticancer Res., 1997,17:3293-3297.
Noro T., Noro K., Miyase T.. Inhibition of xanthine oxidase by anthraquinones[J]. Chem. Pharm. Bull., 1987,35:4314-4316. doi: 10.1248/cpb.35.4314

Yang Li , Ning Sheng , Kun Wang , Yuhuan Li , Jiandong Jiang , Jinlan Zhang . Azvudine alleviates SARS-CoV-2-induced inflammation by targeting myeloperoxidase in NETosis. Chinese Chemical Letters, 2025, 36(5): 110238-. doi: 10.1016/j.cclet.2024.110238
Qi Huang , Jun Liao , Jingjing Li , Zhengyan Gu , Xinkang Zhang , Mingxue Sun , Wenqi Meng , Guanchao Mao , Zhipeng Pei , Shanshan Zhang , Songling Li , Chuan Zhang , Yunqin Wang , Jihao Liu , Tingbin Shu , Min Tao , Ying Lu , Kai Xiao , Qingqiang Xu , Jincai Lu . Curcumin-loaded ceria nanoenzymes for dual-action suppression of inflammation and alleviation of oxidative damage in the treatment of acute lung injury. Chinese Chemical Letters, 2025, 36(4): 109914-. doi: 10.1016/j.cclet.2024.109914
Feifei Wang , Hang Yao , Xinyue Wu , Yijian Tang , Yang Bai , Hui Chong , Huan Pang . Metal–organic framework and its composites modulate macrophage polarization in the treatment of inflammatory diseases. Chinese Chemical Letters, 2024, 35(5): 108821-. doi: 10.1016/j.cclet.2023.108821
Weijian Zhang , Xianyu Deng , Liying Wang , Jian Wang , Xiuting Guo , Lianggui Huang , Xinyi Wang , Jun Wu , Linjia Jiang . Poly(ferulic acid) nanocarrier enhances chemotherapy sensitivity of acute myeloid leukemia by selectively targeting inflammatory macrophages. Chinese Chemical Letters, 2024, 35(9): 109422-. doi: 10.1016/j.cclet.2023.109422
Xing Tian , Di Wu , Wanheng Wei , Guifu Dai , Zhanxian Li , Benhua Wang , Mingming Yu . A lipid droplets-targetable fluorescent probe for polarity detection in cells of iron death, inflammation and fatty liver tissue. Chinese Chemical Letters, 2024, 35(6): 108912-. doi: 10.1016/j.cclet.2023.108912
Yu Yan , Jiawei Song , Dongdong Liu , Zihan Liu , Jialing Cheng , Zhiyang Chen , Yanfang Yang , Weizhe Jiang , Hongliang Wang , Jun Ye , Yuling Liu . Simple and versatile in situ thermo-sensitive hydrogel for rectal administration of SZ-A to alleviate inflammation and repair mucosal barrier in ulcerative colitis. Chinese Chemical Letters, 2024, 35(6): 109736-. doi: 10.1016/j.cclet.2024.109736
Xinyue Han , Yunhan Yang , Jiayin Lu , Yuxiang Lin , Dongxue Zhang , Ling Lin , Liang Qiao . Efficient serum lipids profiling by TiO2-dopamin-assisted MALDI-TOF MS for breast cancer detection. Chinese Chemical Letters, 2025, 36(5): 110183-. doi: 10.1016/j.cclet.2024.110183
Jinqi Yang , Xiaoxiang Hu , Yuanyuan Zhang , Lingyu Zhao , Chunlin Yue , Yuan Cao , Yangyang Zhang , Zhenwen Zhao . Direct observation of natural products bound to protein based on UHPLC-ESI-MS combined with molecular dynamics simulation. Chinese Chemical Letters, 2025, 36(5): 110128-. doi: 10.1016/j.cclet.2024.110128
Guo-Ping Yin , Ya-Juan Li , Li Zhang , Ling-Gao Zeng , Xue-Mei Liu , Chang-Hua Hu . Citrinsorbicillin A, a novel homotrimeric sorbicillinoid isolated by LC-MS-guided with cytotoxic activity from the fungus Trichoderma citrinoviride HT-9. Chinese Chemical Letters, 2024, 35(8): 109035-. doi: 10.1016/j.cclet.2023.109035
Pengyu Chen , Beibei Chen , Man He , Yuxi Zhou , Lei Lei , Jian Han , Bingsheng Zhou , Ligang Hu , Bin Hu . Nanoplastics and nano-ZnO facilitate Cd accumulation in zebrafish larvae via a distinct pathway: Revelation by LA-ICP-MS imaging. Chinese Chemical Letters, 2025, 36(2): 109908-. doi: 10.1016/j.cclet.2024.109908
Dan Zhou , Liangjin Bao , Haoqi Long , Duo Zhou , Yuwei Xu , Bo Wang , Chuanqin Xia , Liang Xian , Chengbin Zheng . Capillary electrophoresis as sample introduction system for highly sensitive and interference-free determination of 99Tc by ICP-MS. Chinese Chemical Letters, 2025, 36(4): 110093-. doi: 10.1016/j.cclet.2024.110093
Wantong Zhang , Zixing Xu , Guofei Dai , Zhijian Li , Chunhui Deng . Removal of Microcystin-LR in lake water sample by hydrophilic mesoporous silica composites under high-throughput MALDI-TOF MS detection platform. Chinese Chemical Letters, 2024, 35(5): 109135-. doi: 10.1016/j.cclet.2023.109135
Jun-Jie Fang , Zheng Liu , Yun-Peng Xie , Xing Lu . Superatomic Ag58 nanoclusters incorporating a [MS4@Ag12]2+ (M = Mo or W) kernel show aggregation-induced emission. Chinese Chemical Letters, 2024, 35(10): 109345-. doi: 10.1016/j.cclet.2023.109345
Simin Wei , Yaqing Yang , Junjie Li , Jialin Wang , Jinlu Tang , Ningning Wang , Zhaohui Li . The Mn/Yb/Er triple-doped CeO2 nanozyme with enhanced oxidase-like activity for highly sensitive ratiometric detection of nitrite. Chinese Chemical Letters, 2024, 35(6): 109114-. doi: 10.1016/j.cclet.2023.109114
Zian Lin , Yingxue Jin . Matrix-Assisted Laser Desorption/Ionization Mass Spectrometry (MALDI-MS) for Disease Marker Screening and Identification: A Comprehensive Experiment Teaching Reform in Instrumental Analysis. University Chemistry, 2024, 39(11): 327-334. doi: 10.12461/PKU.DXHX202403066
Yong-Fang Shi , Sheng-Hua Zhou , Zuju Ma , Xin-Tao Wu , Hua Lin , Qi-Long Zhu . From [Ba3S][GeS4] to [Ba3CO3][MS4] (M = Ge, Sn): Enhancing optical anisotropy in IR birefringent crystals via functional group implantation. Chinese Journal of Structural Chemistry, 2025, 44(1): 100455-100455. doi: 10.1016/j.cjsc.2024.100455
Quan Zhang , Shunjie Xing , Jingqian Han , Li Feng , Jianchun Li , Zhaosheng Qian , Jin Zhou . Organic pollutant sensing for human health based on carbon dots. Chinese Chemical Letters, 2025, 36(1): 110117-. doi: 10.1016/j.cclet.2024.110117
Min Huang , Ru Cheng , Shuai Wen , Liangtong Li , Jie Gao , Xiaohui Zhao , Chunmei Li , Hongyan Zou , Jian Wang . Ultrasensitive detection of microRNA-21 in human serum based on the confinement effect enhanced chemical etching of gold nanorods. Chinese Chemical Letters, 2024, 35(9): 109379-. doi: 10.1016/j.cclet.2023.109379
Chengcheng Xie , Chengyi Xiao , Hongshuo Niu , Guitao Feng , Weiwei Li . Mesoporous organic solar cells. Chinese Chemical Letters, 2024, 35(11): 109849-. doi: 10.1016/j.cclet.2024.109849
Yaohua Li , Qi Cao , Xuanhua Li . Tailoring the configuration of polymer passivators in perovskite solar cells. Chinese Journal of Structural Chemistry, 2025, 44(2): 100413-100413. doi: 10.1016/j.cjsc.2024.100413