Citation:
Long-Fei Yuan, Yu-Jian He, Hong Zhao, Ying Zhou, Pei Gu. Colorimetric detection of D-amino acids based on anti-aggregation of gold nanoparticles[J]. Chinese Chemical Letters,
;2014, 25(7): 995-1000.
doi:
10.1016/j.cclet.2014.06.002

-
A new method has been proposed to realize the visual detection of D-amino acids (DAAs) via the antiaggregation of 4-mercaptobenzoic acid modified gold nanoparticles (AuNPs) in the presence of D-amino acid oxidase (DAAO). The negatively charged AuNPs were prepared using sodium citrate as a reducer and stabilizer. The presence of 4-mercaptobenzoic acid (4-MBA) and Cu2+ induces the aggregation of AuNPs, resulting in a color change from ruby red to royal purple. However, DAAO could oxidize DAAs to generate H2O2. In the presence of H2O2, the mercapto (-SH) group in 4-mercaptobenzoic acid can be oxidized to form a disulfide (-S-S-) bond. Based on these facts, the pre-incubation of DAAs and 4-mercaptobenzoic acid with DAAO would significantly reduce the concentration of free 4-mercaptobenzoic acidmolecules, thus the aggregation of AuNPs was interrupted since due to the lack of inducer. As the concentration of DAAs increases, the color of the AuNPs solution would progress from royal purple to ruby red. Consequently, DAAs could be monitored by the colorimetric response of AuNPs using a UV-vis spectrophotometer or even naked eyes. This DAAO mediated visual detectionmethod could determine Dalanine (D-Ala) as a representative DAA with concentrations ranging from 1.5×10-7 mol L-1 to 3.0×10-5 mol L-1, and the detection limit was as low as 7.5×10-8 mol L-1. The proposed method is convenient, low-cost and free of complex equipment, making it feasible to analyze the concentration of D-Ala in real samples of β-amyloid peptide (Aβ1-42).
-
Efficient synthesis of novel macrocyclic hosts with unique structures and good host–guest properties is a permanent and challenging topic in the field of supramolecular chemistry [1-7]. During the last decade, considerable effort has been devoted to the development of macrocyclic molecular and a number of new macrocyclic receptors with novel properties have been reported, such as heterocalix[n]aromatics [8-10], pillar[n]arene [11-16], helicarenes [17-19], naphthotubes [20-24], Ex-box and Ex-cage [25-32], and others [33-37]. However, most of the reported macrocyclic hosts showed poor solubility and weak host–guest interactions in water. To develop a new macrocyclic molecule with good water solubility and molecular recognition properties not only helps us to understand and mimic the biological processes, but also enriches the toolbox of supramolecular chemists. Water soluble groups including sulfonate [38-40], carboxylate [41-43] and quaternary amine groups [44, 45] have been modified onto the macrocyclic hosts to increase their water solubility. These approaches often suffer from long synthetic steps and low yields, which restrict their further application in the complicated supramolecular self-assembly. Undoubtedly, it is important to develop novel water-soluble macrocyclic hosts that could be obtained in high yields and show good host–guest properties in water. Recently, Li and coworkers [46, 47] reported the efficient synthesis of water-soluble macrocyclic hosts using dynamic covalent chemistry (DCC) approach. In their method, both macrocycles and [2] catenanes could be obtained in high yields under the thermodynamic control. However, the purification of [2] catenanes need chromatographic. Previously, our group [42, 43] prepared a novel water-soluble cylindrical macrotricyclic host, and found that the host could bind two N-methylquinolinium salts to form 1:2 complexes in water. Inspired by these results, we deduced that whether we could find a new strategy to construct novel water-soluble macrocyclic host with significant host–guest properties in high yields.
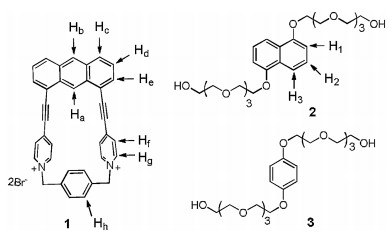
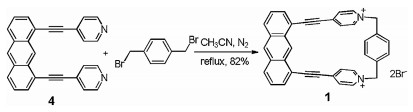
So far as we know, 1, 8-bis(4-pyridylethynyl)anthracene, which looks like a molecule "clip", has been used as donor building block to prepare trigonal prisms [48]. Thus, the anthracene-based "clip" could also serve as half part of a macrocycle and a macrocyclic host could be obtained when a suitable building block was introduced to this clip. Herein, we report the efficient synthesis of a novel water soluble macrocyclic host 12+·2Br− and its complexation with neutral guests in water. By the utilization of the anthracene-based "clip" to react with 1, 4-bis(bromomethyl)benzene, host 12+·2Br− can be obtain via simple filtration in a high yield of 82%. Moreover, it was found that host 12+·2Br− could form 1:1 complexes with guests 2 and 3 in water solution (Fig. 1). Interestingly, we discovered that host 12+·2Br− could only selectively accommodate naphthalene among a variety of polycyclic aromatic hydrocarbons in water. This selective host-guest recognition could be employed for the further removal of naphthalene from sewage.
Figure 1
Synthesis of host 12+·2Br− was outlined in Scheme 1. Compound 4 was first prepared according to the literature procedure [48]. By the reaction of 4 and commercially available 1, 4-bis(bromomethyl)benzene in acetonitrile at 90 ℃, host 12+·2Br− could be easily synthesized in 82% yield. Macrocyclic host 12+·2Br− showed moderate solubility in water, and its structure was confirmed by 1H NMR, 13C NMR, HRMS spectra and crystal structure analysis (Supporting information).
Scheme 1
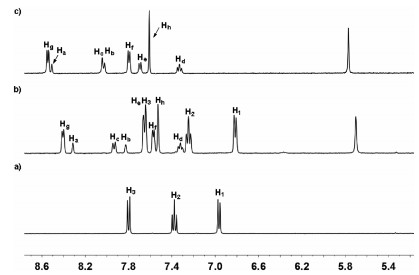
Firstly, the binding properties of host 12+·2Br− toward neutral guests 2 and 3 were investigated by 1H NMR spectra in water solution. Unlike the previous results that reported by our group [43], after electron-poor host 12+·2Br− (4.0 mmol/L) and electron-rich guest 2 with 1:1 molar ratio were mixed in water, no obviously color change was observed. The similar phenomenon was also observed for the aqueous solution between host 12+·2Br− and guests 3. These results led us to doubt that whether host 12+·2Br− could form of complexes with guests 2 and 3. Consequently, the 1H NMR experiments were carried out to further investigate the complexation between host 12+·2Br− and guest 2 in water. As shown in Fig. 2, the 1H NMR spectrum of a 1:1 mixture of 12+·2Br− and 2 in D2O showed a great difference with those for free host 12+·2Br− and free guest 2. Upfield shifts of the resonances of protons H1, H2 and H3 corresponding to guest 2 were observed, which indicated that the naphthalene unit of 2 experienced a shielded magnetic environment in the aromatic cavity of 12+·2Br−. Moreover, the signal of protons Hf and Hg in host 12+·2Br− also shifted upfield, implying that the electron-poor pyridine unit of 12+·2Br− was in shielded magnetic environment and a new complex 1·2 could be formed. Meanwhile, by increasing the amount of guest 2, the spectrum of complex 1·2 showed only one set of resonances, which indicated that the complexation and decomplexation between host 12+·2Br− and guest 2 were a fast exchange process on the NMR time scale at room temperature. The formation of complex 1·2 was also supported by 2D NOESY spectral experiment. As shown in the Fig. S4 (Supporting information), the clear correlation signals between protons H1-Hg, H2-Hg and the protons of crown ether units of host 12+·2Br− were observed, which was also consistent with the formation of complex 1·2. Moreover, 1H NMR titrations and nonlinear fitting were then performed to quantitatively estimate the 1:1 binding manner between host 12+·2Br− and guest 2. Consequently, it was found that 1:1 complex 1·2 were formed by the mole ratio plot. The binding constant (K) of the complex 2⊂12+·2Br− was calculated to be 184±4 L/mol by the Scatchard plot [49]. By the counteranion exchange of 12+·2Br−, we also prepared oil-soluble 12+·2PF6− and investigated its complexation with 2 in MeCN. As shown in Fig. S3 (Supporting information), the 1H NMR spectrum of the 1:1 mixture of 12+·2PF6− and 2 was essentially the sum of the two components, indicating that no obvious complexation between 12+·2PF6− and 2 occurred and the major driving force for the formation of 2⊂12+·2Br− in water could be hydrophobic interactions. In addition, the binding constant of the complex 3⊂12+·2Br− was measured to be around 64±2 L/mol in D2O at 298 K. Compared to guest 2 containing a naphthalene unit, guest 3 has a smaller phenyl hydrophobic moiety, which accounted for its lower binding constant within host 12+·2Br−.
Figure 2
The attempts to obtain the single crystal of 2⊂12+·2Br− and 12+·2Br− were unsuccessful. Fortunately, a yellow single crystal of 12+·2PF6− was obtained by slow vapor diffusion of ether to a solution of 12+·2PF6− in CH3CN, providing unambiguous evidence for the formation of 12+·2Br−. As shown in Fig. 3a, the two pyridinium residues orientate in a face-to-face nanner and the distance between two N and C atoms of pyridinium residues are measured to be 5.812 Å (h) and 5.647 Å (g), indicating the moderate-sized cavity of host 12+·2PF6−. Interestingly, it was found that PF6− ion showed not only multiple CH···F hydrogen bonds but also anion···π interactions with pyridinium rings with the distance of 2.989 (d), 3.062 (e) and 3.159 (f), respectively. Moreover, π···π interaction between two anthracene groups of host 12+·2PF6− with a distance of 3.846 Å (i) was also observed (Fig. 3b). Because of these multiple noncovalent interactions, the macrocyclic molecule 12+·2PF6− could self-assemble to form a 1D tubular channel with PF6− ions inside the channels.
Figure 3
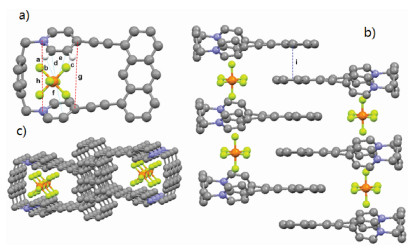 Figure 3. Crystal structure of 12+·2PF6− (a). Crystal structure of self-assembly 12+·2PF6− side view (b), top view (c). Dashed lines denote the noncovalent interactions between the two components. CH···F hydrogen bond distances (Å): a=2.461, b=2.404, c=2.557. PF6− ions and hydrogen atoms not involved in the noncovalent interactions are omitted for clarity.
Figure 3. Crystal structure of 12+·2PF6− (a). Crystal structure of self-assembly 12+·2PF6− side view (b), top view (c). Dashed lines denote the noncovalent interactions between the two components. CH···F hydrogen bond distances (Å): a=2.461, b=2.404, c=2.557. PF6− ions and hydrogen atoms not involved in the noncovalent interactions are omitted for clarity.Inspired by the formation of complex 1·2 in water, we further investigated the capability of 12+·2Br− to accommodate a variety of polycyclic aromatic hydrocarbons in water. As shown in Fig. 4a, 1H NMR spectroscopy revealed that 12+·2Br− can encapsulate naphthalene to formation of 1:1 complex. However, the formation of 1:1 complexes between 12+·2Br− and anthracene, phenanthrene, triphenylene, pyrene and perylene were not observed (Figs. 4b–f). These observations could be explained by the fact that host 12+·2Br− had a small cavity and could only recognize small polycyclic aromatic hydrocarbons such as naphthalene. However, because of the extremely poor solubility of naphthalene in water, the corresponding binding constants of host 12+·2Br− and naphthalene could not be determined. The highly selective binding behavior of host 12+·2Br− toward naphthalene could be further used for the separation of naphthalene from a variety of polycyclic aromatic hydrocarbons.
Figure 4
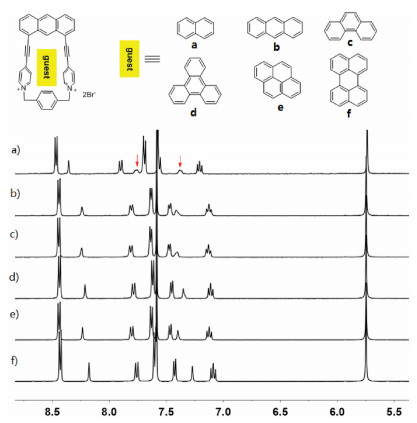 Figure 4. Partial 1H NMR spectra (400 MHz, D2O, 298 K) of 12+·2Br− after addition of a variety of water-insoluble guests including (a) naphthalene, (b) anthracene, (c) phenanthrene, (d) triphenylene, (e) pyrene and (f) perylene. All the spectra were recorded after sonicating the corresponding suspensions for no less than 24 h to allow the host–guest complexation to reach the equilibrium. The resonance signals of the guests are labelled with red arrows.
Figure 4. Partial 1H NMR spectra (400 MHz, D2O, 298 K) of 12+·2Br− after addition of a variety of water-insoluble guests including (a) naphthalene, (b) anthracene, (c) phenanthrene, (d) triphenylene, (e) pyrene and (f) perylene. All the spectra were recorded after sonicating the corresponding suspensions for no less than 24 h to allow the host–guest complexation to reach the equilibrium. The resonance signals of the guests are labelled with red arrows.In conclusion, by taking advantage of anthracene-based "clip" structure, we developed an efficient approach to construct a water-soluble macrocycle and studied its binding ability toward neutral guests containing naphthalene or phenyl units in water. The formation of 1:1 complexes between host 12+·2Br− and 2 or 3 were confirmed by the 1H NMR titrations experiment. Additionally, we demonstrated that the major driving force for the formation of 2⊂12+·2Br− or 3⊂12+·2Br− in water might be hydrophobic interactions. We further investigated its ability to host a variety of polycyclic aromatic hydrocarbons in aqueous solution. It was found that host 12+·2Br− could selectively encapsulate of naphthalene to formation of 1:1 complexes over a variety of polycyclic aromatic hydrocarbons. This highly selective accommodation hydrophobic guest in water could be explained by the fact that host 12+·2Br− had a relatively small hydrophobic cavity. The application of this novel water-soluble host for the separation of naphthalene from a variety of polycyclic aromatic hydrocarbons and removal of naphthalene from sewage, are underway in our laboratory.
Declaration of competing interest
The authors report no declarations of interest.
Acknowledgments
The authors are grateful for the financial support from the National Natural Science Foundation of China (Nos. 21602055 and 51772091); Natural Science Foundation of Hunan Province (No. 2017JJ3094) and Research Foundation of Education Bureau of Hunan Province (No. 18C1072).
Appendix A. Supplementary data
Supplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.cclet.2020.11.019.
* Corresponding author.
E-mail address: zengfei@iccas.ac.cn (F. Zeng).
References
-
[1]
[1] M. Friedman, Chemistry, nutrition, and microbiology of D-amine acids, J. Agric. Food Chem. 47 (1999) 3457-3479.
-
[2]
[2] C. Henneberger, T. Papouin, S.H.R. Oliet, et al., Long-term potentiation depends on release of D-serine from astrocytes, Nature 463 (2010) 232-236.
-
[3]
[3] I. Azua, I. Goiriena, Z. Bana, et al., Release and consumption of D-amino acids during growth of marine prokaryotes, Microb. Ecol. 67 (2014) 1-12.
-
[4]
[4] V. Vranova, H. Zahradnickova, D. Janous, et al., The significance of D-amino acids in soil, fate and utilization by microbes and plants: review and identification of knowledge gaps, Plant Soil 354 (2012) 21-39.
-
[5]
[5] S.A. Fuchs, R. Berger, L.W.J. Klomp, et al., D-amino acids in the central nervous system in health and disease, Mol. Genet. Metab. 85 (2005) 168-180.
-
[6]
[6] H. Wolosker, E. Dumin, L. Balan, V.N. Foltyn, D-amino acids in the brain: D-serine in neurotransmission and neurodegeneration, FEBS J. 275 (2008) 3514-3526.
-
[7]
[7] G.H. Fisher, L. Petrucelli, C. Gardner, et al., Free D-amino acids in human cerebrospinalfluid of Alzheimer-disease, multiple-sclerosis, and healthy control subjects, Mol. Chem. Neuropathol. 23 (1994) 115-124.
-
[8]
[8] S. Kato, Y. Kito, H. Hemmi, T. Yoshimura, Simultaneous determination of D-amino acids by the coupling method of D-amino acid oxidase with high-performance liquid chromatography, J. Chromatogr. B 879 (2011) 3190-3195.
-
[9]
[9] C. Mueller, J.R. Fonseca, T.M. Rock, S. Krauss-Etschmann, P. Schmitt-Kopplin, Enantioseparation and selective detection of D-amino acids by ultra-high-performance liquid chromatography/mass spectrometry in analysis of complex biological samples, J. Chromatogr. A 1324 (2014) 109-114.
-
[10]
[10] Y. Gogami, K. Okada, T. Oikawa, High-performance liquid chromatography analysis of naturally occurring D-amino acids in sake, J. Chromatogr. B 879 (2011) 3259-3267.
-
[11]
[11] H. Brückner, A. Schieber, Determination of free D-amino acids in mammalia by chiral gas chromatography-mass spectrometry, J. High Resolut. Chromatogr. 23 (2000) 576-582.
-
[12]
[12] R. Patzold, H. Bruckner, Gas chromatographic determination and mechanism of formation of D-amino acids occurring in fermented and roasted cocoa beans, cocoa powder, chocolate and cocoa shell, Amino Acids 31 (2006) 63-72.
-
[13]
[13] R. Patzold, A. Schieber, H. Bruckner, Gas-chromatographic quantification of free Damino acids in higher vertebrates, Biomed. Chromatogr. 19 (2005) 466-473.
-
[14]
[14] C.H. Nieh, Y. Kitazumi, O. Shirai, K. Kano, Sensitive D-amino acid biosensor based on oxidase/peroxidase system mediated by pentacyanoferrate-bound polymer, Biosens. Bioelectron. 47 (2013) 350-355.
-
[15]
[15] S. Lata, B. Batra, C.S. Pundir, Construction of D-amino acid biosensor based on Damino acid oxidase immobilized onto poly (indole-5-carboxylic acid)/zinc sulfide nanoparticles hybrid film, Process Biochem. 47 (2012) 2131-2138.
-
[16]
[16] S. Lata, B. Batra, P. Kumar, et al., Construction of an amperometric D-amino acid biosensor based on D-amino acid oxidase/carboxylated mutliwalled carbon nanotube/ copper nanoparticles/polyalinine modified gold electrode, Anal. Biochem. 437 (2013) 1-9.
-
[17]
[17] E. Rosini, G. Molla, C. Rossetti, et al., A biosensor for all D-amino acids using evolved D-amino acid oxidase, J. Biotechnol. 135 (2008) 377-384.
-
[18]
[18] Y.C. Cao, R.C. Jin, S. Thaxton, et al., A two-color-change, nanoparticle-based method for DNA detection, Talanta 67 (2005) 449-455.
-
[19]
[19] H. Chi, B.H. Liu, G.J. Guan, et al., A simple, reliable and sensitive colorimetric visualization of melamine in milk by unmodified gold nanoparticles, Analyst 135 (2010) 1070-1075.
-
[20]
[20] F. Li, Y. Feng, C. Zhao, et al., Simple colorimetric sensing of trace bleomycin using unmodified gold nanoparticles, Biosens. Bioelectron. 26 (2011) 4628-4631.
-
[21]
[21] C.D. Medley, J.E. Smith, Z. Tang, et al., Gold nanoparticle-based colorimetric assay for the direct detection of cancerous cells, Anal. Chem. 80 (2008) 1067-1072.
-
[22]
[22] H. Li, Y.W. Yang, Gold nanoparticles functionalized with supramolecular macrocycles, Chin. Chem. Lett. 24 (2013) 545-552.
-
[23]
[23] B.H. Wu, H.Y. Yang, H.Q. Huang, et al., Solvent effect on the synthesis of monodisperse amine-capped Au nanoparticles, Chin. Chem. Lett. 24 (2013) 457-462.
-
[24]
[24] S. Link, M.A. El-Sayed, Spectral properties and relaxation dynamics of surface plasmon electronic oscillations in gold and silver nanodots and nanorods, J. Phys. Chem. B 103 (1999) 8410-8426.
-
[25]
[25] Y.P. Li, L. Jiang, T. Zhang, et al., Colorimetric detection of glucose using a boronic acid derivative receptor attached to unmodified AuNPs, Chin. Chem. Lett. 25 (2014) 77-79.
-
[26]
[26] R. Elghanian, J.J. Storhoff, R.C. Mucic, et al., Selective colorimetric detection of polynucleotides based on the distance-dependent optical properties of gold nanoparticles, Science 277 (1997) 1078-1081.
-
[27]
[27] C.Y. Lin, C.J. Yu, Y.H. Lin, W.L. Tseng, Colorimetric sensing of silver(I) and mercury(II) ions based on an assembly of Tween 20-stabilized gold nanoparticles, Anal. Chem. 82 (2010) 6830-6837.
-
[28]
[28] N. Ding, H. Zhao, W.B. Peng, et al., A simple colorimetric sensor based on antiaggregation of gold nanoparticles for Hg2+ detection, Colloids Surf. A-Physicochem. Eng. Aspect 395 (2012) 161-167.
-
[29]
[29] J.S. Lee, M.S. Han, C.A. Mirkin, Colorimetric detection of mercuric ion (Hg2+) in aqueous media using DNA-functionalized gold nanoparticles, Angew. Chem. Int. Ed. 46 (2007) 4093-4096.
-
[30]
[30] Y. Xue, H. Zhao, Z.J. Wu, et al., Colorimetric detection of Cd2+ using gold nanoparticles cofunctionalized with 6-mercaptonicotinic acid and L-cysteine, Analyst 136 (2011) 3725-3730.
-
[31]
[31] Y. Zhou, P.L. Wang, X.O. Su, et al., Colorimetric detection of ractopamine and salbutamol using gold nanoparticles functionalized with melamine as a probe, Talanta 112 (2013) 20-25.
-
[32]
[32] X.F. Zhang, H. Zhao, Y. Xue, et al., Colorimetric sensing of clenbuterol using gold nanoparticles in the presence of melamine, Biosens. Bioelectron. 34 (2012) 112-117.
-
[33]
[33] X.F. Zhang, Y. Zhang, H. Zhao, et al., Highly sensitive and selective colorimetric sensing of antibiotics in milk, Anal. Chim. Acta 778 (2013) 63-69.
-
[34]
[34] X.F. Zhang, Z.J. Wu, Y. Xue, et al., Colorimetric detection of melamine based on the interruption of the synthesis of gold nanoparticles, Anal. Methods 5 (2013) 1930-1934.
-
[35]
[35] Z.J. Wu, H. Zhao, Y. Xue, et al., Colorimetric detection of melamine during the formation of gold nanoparticles, Biosens. Bioelectron. 26 (2011) 2574-2578.
-
[36]
[36] Q.A. Cao, H. Zhao, Y.J. He, et al., Hydrogen-bonding-induced colorimetric detection of melamine by nonaggregation-based Au-NPs as a probe, Biosens. Bioelectron. 25 (2010) 2680-2685.
-
[37]
[37] Y. Zhou, H. Zhao, Y.J. He, N. Ding, Q. Cao, Colorimetric detection of Cu2+ using 4-mercaptobenzoic acid modified silver nanoparticles, Colloid Surf. A-Physicochem. Eng. Aspect 391 (2011) 179-183.
-
[38]
[38] Y.C. Shiang, C.C. Huang, H.T. Chang, Gold nanodot-based luminescent sensor for the detection of hydrogen peroxide and glucose, Chem. Commun. (2009) 3437-3439.
-
[39]
[39] A.R. Quesada, R.W. Byrnes, S.O. Krezoski, et al., Direct reaction of H2O2 with sulfhydryl groups in HL-60 cells: zinc-metallothionein and other sites, Arch. Biochem. Biophys. 334 (1996) 241-250.
-
[40]
[40] B. Cardey,M. Enescu, Selenocysteine versus cysteine reactivity: a theoretical study of their oxidation by hydrogen peroxide, J. Phys. Chem. A 111 (2007) 673-678.
-
[41]
[41] J. Wang, D.M. Wang, Y.F. Li, Study of cysteine modified gold nanoparticles as a colorimetric detection platform for oxidants, Chin. Sci. Bull. 56 (2011) 1196-1203.
-
[42]
[42] L.F. Yuan, Y.J. He, Effect of surface charge of PDDA-protected gold nanoparticles on the specificity and efficiency of DNA polymerase chain reaction, Analyst 138 (2013) 539-545.
-
[43]
[43] X.H. Ji, X.N. Song, J. Li, et al., Size control of gold nanocrystals in citrate reduction: the third role of citrate, J. Am. Chem. Soc. 129 (2007) 13939-13948.
-
[44]
[44] A. Daniello, A. Vetere, G.H. Fisher, et al., Presence of D-alanine in proteins of normal and Alzheimer human brain, Brain Res. 592 (1992) 44-48.
-
[45]
[45] K. Wiesehan, K. Buder, R.P. Linke, et al., Selection of D-amino-acid peptides that bind to Alzheimer's disease amyloid peptide Aβ1-42 by mirror image phage display, Chembiochem 4 (2003) 748-753.
-
[46]
[46] Y.S. Shim, W.J. Yoon, J. Ha, et al., Method validation of 16 types of structural amino acids using an automated amino acid analyzer, Food Sci. Biotechnol. 22 (2013) 1567-1571.
-
[47]
[47] S.V. Khoronenkova, V.I. Tishkov, D-amino acid oxidase: physiological role and applications, Biochemistry (Mosc.) 73 (2008) 1511-1518.
-
[1]
-
-
[1]
[1] M. Friedman, Chemistry, nutrition, and microbiology of D-amine acids, J. Agric. Food Chem. 47 (1999) 3457-3479.
-
[2]
[2] C. Henneberger, T. Papouin, S.H.R. Oliet, et al., Long-term potentiation depends on release of D-serine from astrocytes, Nature 463 (2010) 232-236.
-
[3]
[3] I. Azua, I. Goiriena, Z. Bana, et al., Release and consumption of D-amino acids during growth of marine prokaryotes, Microb. Ecol. 67 (2014) 1-12.
-
[4]
[4] V. Vranova, H. Zahradnickova, D. Janous, et al., The significance of D-amino acids in soil, fate and utilization by microbes and plants: review and identification of knowledge gaps, Plant Soil 354 (2012) 21-39.
-
[5]
[5] S.A. Fuchs, R. Berger, L.W.J. Klomp, et al., D-amino acids in the central nervous system in health and disease, Mol. Genet. Metab. 85 (2005) 168-180.
-
[6]
[6] H. Wolosker, E. Dumin, L. Balan, V.N. Foltyn, D-amino acids in the brain: D-serine in neurotransmission and neurodegeneration, FEBS J. 275 (2008) 3514-3526.
-
[7]
[7] G.H. Fisher, L. Petrucelli, C. Gardner, et al., Free D-amino acids in human cerebrospinalfluid of Alzheimer-disease, multiple-sclerosis, and healthy control subjects, Mol. Chem. Neuropathol. 23 (1994) 115-124.
-
[8]
[8] S. Kato, Y. Kito, H. Hemmi, T. Yoshimura, Simultaneous determination of D-amino acids by the coupling method of D-amino acid oxidase with high-performance liquid chromatography, J. Chromatogr. B 879 (2011) 3190-3195.
-
[9]
[9] C. Mueller, J.R. Fonseca, T.M. Rock, S. Krauss-Etschmann, P. Schmitt-Kopplin, Enantioseparation and selective detection of D-amino acids by ultra-high-performance liquid chromatography/mass spectrometry in analysis of complex biological samples, J. Chromatogr. A 1324 (2014) 109-114.
-
[10]
[10] Y. Gogami, K. Okada, T. Oikawa, High-performance liquid chromatography analysis of naturally occurring D-amino acids in sake, J. Chromatogr. B 879 (2011) 3259-3267.
-
[11]
[11] H. Brückner, A. Schieber, Determination of free D-amino acids in mammalia by chiral gas chromatography-mass spectrometry, J. High Resolut. Chromatogr. 23 (2000) 576-582.
-
[12]
[12] R. Patzold, H. Bruckner, Gas chromatographic determination and mechanism of formation of D-amino acids occurring in fermented and roasted cocoa beans, cocoa powder, chocolate and cocoa shell, Amino Acids 31 (2006) 63-72.
-
[13]
[13] R. Patzold, A. Schieber, H. Bruckner, Gas-chromatographic quantification of free Damino acids in higher vertebrates, Biomed. Chromatogr. 19 (2005) 466-473.
-
[14]
[14] C.H. Nieh, Y. Kitazumi, O. Shirai, K. Kano, Sensitive D-amino acid biosensor based on oxidase/peroxidase system mediated by pentacyanoferrate-bound polymer, Biosens. Bioelectron. 47 (2013) 350-355.
-
[15]
[15] S. Lata, B. Batra, C.S. Pundir, Construction of D-amino acid biosensor based on Damino acid oxidase immobilized onto poly (indole-5-carboxylic acid)/zinc sulfide nanoparticles hybrid film, Process Biochem. 47 (2012) 2131-2138.
-
[16]
[16] S. Lata, B. Batra, P. Kumar, et al., Construction of an amperometric D-amino acid biosensor based on D-amino acid oxidase/carboxylated mutliwalled carbon nanotube/ copper nanoparticles/polyalinine modified gold electrode, Anal. Biochem. 437 (2013) 1-9.
-
[17]
[17] E. Rosini, G. Molla, C. Rossetti, et al., A biosensor for all D-amino acids using evolved D-amino acid oxidase, J. Biotechnol. 135 (2008) 377-384.
-
[18]
[18] Y.C. Cao, R.C. Jin, S. Thaxton, et al., A two-color-change, nanoparticle-based method for DNA detection, Talanta 67 (2005) 449-455.
-
[19]
[19] H. Chi, B.H. Liu, G.J. Guan, et al., A simple, reliable and sensitive colorimetric visualization of melamine in milk by unmodified gold nanoparticles, Analyst 135 (2010) 1070-1075.
-
[20]
[20] F. Li, Y. Feng, C. Zhao, et al., Simple colorimetric sensing of trace bleomycin using unmodified gold nanoparticles, Biosens. Bioelectron. 26 (2011) 4628-4631.
-
[21]
[21] C.D. Medley, J.E. Smith, Z. Tang, et al., Gold nanoparticle-based colorimetric assay for the direct detection of cancerous cells, Anal. Chem. 80 (2008) 1067-1072.
-
[22]
[22] H. Li, Y.W. Yang, Gold nanoparticles functionalized with supramolecular macrocycles, Chin. Chem. Lett. 24 (2013) 545-552.
-
[23]
[23] B.H. Wu, H.Y. Yang, H.Q. Huang, et al., Solvent effect on the synthesis of monodisperse amine-capped Au nanoparticles, Chin. Chem. Lett. 24 (2013) 457-462.
-
[24]
[24] S. Link, M.A. El-Sayed, Spectral properties and relaxation dynamics of surface plasmon electronic oscillations in gold and silver nanodots and nanorods, J. Phys. Chem. B 103 (1999) 8410-8426.
-
[25]
[25] Y.P. Li, L. Jiang, T. Zhang, et al., Colorimetric detection of glucose using a boronic acid derivative receptor attached to unmodified AuNPs, Chin. Chem. Lett. 25 (2014) 77-79.
-
[26]
[26] R. Elghanian, J.J. Storhoff, R.C. Mucic, et al., Selective colorimetric detection of polynucleotides based on the distance-dependent optical properties of gold nanoparticles, Science 277 (1997) 1078-1081.
-
[27]
[27] C.Y. Lin, C.J. Yu, Y.H. Lin, W.L. Tseng, Colorimetric sensing of silver(I) and mercury(II) ions based on an assembly of Tween 20-stabilized gold nanoparticles, Anal. Chem. 82 (2010) 6830-6837.
-
[28]
[28] N. Ding, H. Zhao, W.B. Peng, et al., A simple colorimetric sensor based on antiaggregation of gold nanoparticles for Hg2+ detection, Colloids Surf. A-Physicochem. Eng. Aspect 395 (2012) 161-167.
-
[29]
[29] J.S. Lee, M.S. Han, C.A. Mirkin, Colorimetric detection of mercuric ion (Hg2+) in aqueous media using DNA-functionalized gold nanoparticles, Angew. Chem. Int. Ed. 46 (2007) 4093-4096.
-
[30]
[30] Y. Xue, H. Zhao, Z.J. Wu, et al., Colorimetric detection of Cd2+ using gold nanoparticles cofunctionalized with 6-mercaptonicotinic acid and L-cysteine, Analyst 136 (2011) 3725-3730.
-
[31]
[31] Y. Zhou, P.L. Wang, X.O. Su, et al., Colorimetric detection of ractopamine and salbutamol using gold nanoparticles functionalized with melamine as a probe, Talanta 112 (2013) 20-25.
-
[32]
[32] X.F. Zhang, H. Zhao, Y. Xue, et al., Colorimetric sensing of clenbuterol using gold nanoparticles in the presence of melamine, Biosens. Bioelectron. 34 (2012) 112-117.
-
[33]
[33] X.F. Zhang, Y. Zhang, H. Zhao, et al., Highly sensitive and selective colorimetric sensing of antibiotics in milk, Anal. Chim. Acta 778 (2013) 63-69.
-
[34]
[34] X.F. Zhang, Z.J. Wu, Y. Xue, et al., Colorimetric detection of melamine based on the interruption of the synthesis of gold nanoparticles, Anal. Methods 5 (2013) 1930-1934.
-
[35]
[35] Z.J. Wu, H. Zhao, Y. Xue, et al., Colorimetric detection of melamine during the formation of gold nanoparticles, Biosens. Bioelectron. 26 (2011) 2574-2578.
-
[36]
[36] Q.A. Cao, H. Zhao, Y.J. He, et al., Hydrogen-bonding-induced colorimetric detection of melamine by nonaggregation-based Au-NPs as a probe, Biosens. Bioelectron. 25 (2010) 2680-2685.
-
[37]
[37] Y. Zhou, H. Zhao, Y.J. He, N. Ding, Q. Cao, Colorimetric detection of Cu2+ using 4-mercaptobenzoic acid modified silver nanoparticles, Colloid Surf. A-Physicochem. Eng. Aspect 391 (2011) 179-183.
-
[38]
[38] Y.C. Shiang, C.C. Huang, H.T. Chang, Gold nanodot-based luminescent sensor for the detection of hydrogen peroxide and glucose, Chem. Commun. (2009) 3437-3439.
-
[39]
[39] A.R. Quesada, R.W. Byrnes, S.O. Krezoski, et al., Direct reaction of H2O2 with sulfhydryl groups in HL-60 cells: zinc-metallothionein and other sites, Arch. Biochem. Biophys. 334 (1996) 241-250.
-
[40]
[40] B. Cardey,M. Enescu, Selenocysteine versus cysteine reactivity: a theoretical study of their oxidation by hydrogen peroxide, J. Phys. Chem. A 111 (2007) 673-678.
-
[41]
[41] J. Wang, D.M. Wang, Y.F. Li, Study of cysteine modified gold nanoparticles as a colorimetric detection platform for oxidants, Chin. Sci. Bull. 56 (2011) 1196-1203.
-
[42]
[42] L.F. Yuan, Y.J. He, Effect of surface charge of PDDA-protected gold nanoparticles on the specificity and efficiency of DNA polymerase chain reaction, Analyst 138 (2013) 539-545.
-
[43]
[43] X.H. Ji, X.N. Song, J. Li, et al., Size control of gold nanocrystals in citrate reduction: the third role of citrate, J. Am. Chem. Soc. 129 (2007) 13939-13948.
-
[44]
[44] A. Daniello, A. Vetere, G.H. Fisher, et al., Presence of D-alanine in proteins of normal and Alzheimer human brain, Brain Res. 592 (1992) 44-48.
-
[45]
[45] K. Wiesehan, K. Buder, R.P. Linke, et al., Selection of D-amino-acid peptides that bind to Alzheimer's disease amyloid peptide Aβ1-42 by mirror image phage display, Chembiochem 4 (2003) 748-753.
-
[46]
[46] Y.S. Shim, W.J. Yoon, J. Ha, et al., Method validation of 16 types of structural amino acids using an automated amino acid analyzer, Food Sci. Biotechnol. 22 (2013) 1567-1571.
-
[47]
[47] S.V. Khoronenkova, V.I. Tishkov, D-amino acid oxidase: physiological role and applications, Biochemistry (Mosc.) 73 (2008) 1511-1518.
-
[1]
-

-
-
[1]
Xiangqian Cao , Chenkai Yang , Xiaodong Zhu , Mengxin Zhao , Yilin Yan , Zhengnan Huang , Jinming Cai , Jingming Zhuang , Shengzhou Li , Wei Li , Bing Shen . Synergistic enhancement of chemotherapy for bladder cancer by photothermal dual-sensitive nanosystem with gold nanoparticles and PNIPAM. Chinese Chemical Letters, 2024, 35(8): 109199-. doi: 10.1016/j.cclet.2023.109199
-
[2]
Yiyang Shen , Zhen Zhang , Ruyi Liang , Tongbo Wu . Unraveling the interplay of DNAzyme and interfacial factors for enhanced biosensing. Chinese Chemical Letters, 2024, 35(12): 109638-. doi: 10.1016/j.cclet.2024.109638
-
[3]
Guorong Li , Yijing Wu , Chao Zhong , Yixin Yang , Zian Lin . Predesigned covalent organic framework with sulfur coordination: Anchoring Au nanoparticles for sensitive colorimetric detection of Hg(Ⅱ). Chinese Chemical Letters, 2024, 35(5): 108904-. doi: 10.1016/j.cclet.2023.108904
-
[4]
Gengchen Guo , Tianyu Zhao , Ruichang Sun , Mingzhe Song , Hongyu Liu , Sen Wang , Jingwen Li , Jingbin Zeng . Au-Fe3O4 dumbbell-like nanoparticles based lateral flow immunoassay for colorimetric and photothermal dual-mode detection of SARS-CoV-2 spike protein. Chinese Chemical Letters, 2024, 35(6): 109198-. doi: 10.1016/j.cclet.2023.109198
-
[5]
Chuan-Zhi Ni , Ruo-Ming Li , Fang-Qi Zhang , Qu-Ao-Wei Li , Yuan-Yuan Zhu , Jie Zeng , Shuang-Xi Gu . A chiral fluorescent probe for molecular recognition of basic amino acids in solutions and cells. Chinese Chemical Letters, 2024, 35(10): 109862-. doi: 10.1016/j.cclet.2024.109862
-
[6]
Zhen Dai , Linzhi Tan , Yeyu Su , Kerui Zhao , Yushun Tian , Yu Liu , Tao Liu . Site-specific incorporation of reduction-controlled guest amino acids into proteins for cucurbituril recognition. Chinese Chemical Letters, 2024, 35(5): 109121-. doi: 10.1016/j.cclet.2023.109121
-
[7]
Xiang Huang , Dongzhen Xu , Yang Liu , Xia Huang , Yangfan Wu , Dongmei Fang , Bing Xia , Wei Jiao , Jian Liao , Min Wang . Asymmetric synthesis of difluorinated α-quaternary amino acids (DFAAs) via Cu-catalyzed difluorobenzylation of aldimine esters. Chinese Chemical Letters, 2024, 35(12): 109665-. doi: 10.1016/j.cclet.2024.109665
-
[8]
Qian Ren , Xue Dai , Ran Cen , Yang Luo , Mingyang Li , Ziyun Zhang , Qinghong Bai , Zhu Tao , Xin Xiao . A cucurbit[8]uril-based supramolecular phosphorescent assembly: Cell imaging and sensing of amino acids in aqueous solution. Chinese Chemical Letters, 2024, 35(12): 110022-. doi: 10.1016/j.cclet.2024.110022
-
[9]
Min-Hang Zhou , Jun Jiang , Wei-Min He . EDA-complexes-enabled photochemical synthesis of α-amino acids with imines and tetrabutylammonium oxalate. Chinese Chemical Letters, 2025, 36(1): 110446-. doi: 10.1016/j.cclet.2024.110446
-
[10]
Shaonan Tian , Yu Zhang , Qing Zeng , Junyu Zhong , Hui Liu , Lin Xu , Jun Yang . Core-shell gold-copper nanoparticles: Evolution of copper shells on gold cores at different gold/copper precursor ratios. Chinese Journal of Structural Chemistry, 2023, 42(11): 100160-100160. doi: 10.1016/j.cjsc.2023.100160
-
[11]
Zhi Li , Wenpei Li , Shaoping Jiang , Chuan Hu , Yuanyu Huang , Maxim Shevtsov , Huile Gao , Shaobo Ruan . Legumain-triggered aggregable gold nanoparticles for enhanced intratumoral retention. Chinese Chemical Letters, 2024, 35(7): 109150-. doi: 10.1016/j.cclet.2023.109150
-
[12]
Jia Chen , Yun Liu , Zerong Long , Yan Li , Hongdeng Qiu . Colorimetric detection of α-glucosidase activity using Ni-CeO2 nanorods and its application to potential natural inhibitor screening. Chinese Chemical Letters, 2024, 35(9): 109463-. doi: 10.1016/j.cclet.2023.109463
-
[13]
Shaofeng Gong , Zi-Wei Deng , Chao Wu , Wei-Min He . Stabilized carbon radical-mediated three-component functionalization of amino acid/peptide derivatives. Chinese Chemical Letters, 2025, 36(5): 110936-. doi: 10.1016/j.cclet.2025.110936
-
[14]
Min Huang , Ru Cheng , Shuai Wen , Liangtong Li , Jie Gao , Xiaohui Zhao , Chunmei Li , Hongyan Zou , Jian Wang . Ultrasensitive detection of microRNA-21 in human serum based on the confinement effect enhanced chemical etching of gold nanorods. Chinese Chemical Letters, 2024, 35(9): 109379-. doi: 10.1016/j.cclet.2023.109379
-
[15]
Kun Zhang , Ni Dan , Dan-Dan Ren , Ruo-Yu Zhang , Xiaoyan Lu , Ya-Pan Wu , Li-Lei Zhang , Hong-Ru Fu , Dong-Sheng Li . A small D-A molecule with highly heat-resisting room temperature phosphorescence for white emission and anti-counterfeiting. Chinese Journal of Structural Chemistry, 2024, 43(3): 100244-100244. doi: 10.1016/j.cjsc.2024.100244
-
[16]
Yiyue Ding , Qiuxiang Zhang , Lei Zhang , Qilu Yao , Gang Feng , Zhang-Hui Lu . Exceptional activity of amino-modified rGO-immobilized PdAu nanoclusters for visible light-promoted dehydrogenation of formic acid. Chinese Chemical Letters, 2024, 35(7): 109593-. doi: 10.1016/j.cclet.2024.109593
-
[17]
Hengying Xiang , Nanping Deng , Lu Gao , Wen Yu , Bowen Cheng , Weimin Kang . 3D core-shell nanofibers framework and functional ceramic nanoparticles synergistically reinforced composite polymer electrolytes for high-performance all-solid-state lithium metal battery. Chinese Chemical Letters, 2024, 35(8): 109182-. doi: 10.1016/j.cclet.2023.109182
-
[18]
Yunxia Liu , Guandong Wu , Lin Li , Yiming Niu , Bingsen Zhang , Botao Qiao , Junhu Wang . Construction of sintering-resistant gold catalysts via ascorbic-acid inducing strong metal-support interactions. Chinese Chemical Letters, 2025, 36(4): 110608-. doi: 10.1016/j.cclet.2024.110608
-
[19]
Yuxin Wang , Zhengxuan Song , Yutao Liu , Yang Chen , Jinping Li , Libo Li , Jia Yao . Methyl functionalization of trimesic acid in copper-based metal-organic framework for ammonia colorimetric sensing at high relative humidity. Chinese Chemical Letters, 2024, 35(6): 108779-. doi: 10.1016/j.cclet.2023.108779
-
[20]
Xiaoning Li , Quanyu Shi , Meng Li , Ningxin Song , Yumeng Xiao , Huining Xiao , Tony D. James , Lei Feng . Functionalization of cellulose carbon dots with different elements (N, B and S) for mercury ion detection and anti-counterfeit applications. Chinese Chemical Letters, 2024, 35(7): 109021-. doi: 10.1016/j.cclet.2023.109021
-
[1]
Metrics
- PDF Downloads(0)
- Abstract views(777)
- HTML views(1)

 Login In
Login In




 DownLoad:
DownLoad:
 DownLoad:
DownLoad: