Citation:
Yan Ma, Qian-Yun Tang, Ji Zhu, Li-Hong Wang, Cheng Yao. Fluorescent and thermal properties of siloxane-polyurethanes based on 1, 8-naphthalimide[J]. Chinese Chemical Letters,
;2014, 25(05): 680-686.
doi:
10.1016/j.cclet.2014.01.048

-
A series of fluorescent siloxane-polyurethanes (HPMS-PUs) containing an amino-functionalized, 1,8-naphthalimide, fluorescent monomer (AABD) as a chain extender were synthesized. The properties of the HPMS-PUs were investigated by UV-vis and fluorescence spectroscopies, thermogravimetric analysis and thermal migration behavior. The maximum absorption and emission wavelengths of HPMS-PUs showed a red shift of about 4 nmand a blue shift of about 9 nm, respectively, compared to those of AABD. The Stokes shifts of AABD and HPMS-PU2 were 3514 and 2931 cm-1, respectively. The quantum yield of HPMS-PU2 was 0.79, which was six times higher than that of AABD. Concentration self-quenching was observed in both AABD and HPMS-PUs. The fluorescence of HPMS-PUs was quite stable with respect to both temperature and fluorescence quencher effects. The thermal stability of HPMS-PUs increased with AABD content. The fluorophore units in the HPMS-PUs did not readily migrate.
-
Keywords:
- Polysiloxane,
- Polyurethane,
- 1,8-Naphthalimide,
- Fluorescence,
- Thermal properties
-
Sulfone-containing compounds are frequently found in pharmacologically active compounds, natural products, and materials [1]. Furthermore, they can also be employed as important intermediates in various organic transformations to access a series of useful molecules [2]. Consequently, the introduction of sulfone functionality into organic framework has attracted considerable synthetic pursuit of chemists because of their interesting biological activities and versatile synthetic applications [3]. During the past decades, a number of sulfonylating agents such as sulfonyl chlorides [4], sulfonyl hydrazides [5], sulfinates [6], sulfinic acids [7], thiophenols [8], sulfonyl cyanides [9], sulfonyl selenides [10] and SO2 surrogates [11] have been developed for the construction of organic sulfones. Nevertheless, most of these sulfonylation reactions usually require the use of catalysts or a stoichiometric amount of oxidants, or suffer from complex reaction conditions. It is still highly desirable to develop new strategies to construct sulfone-containing compounds from simple sulfonylating agents under mild conditions.
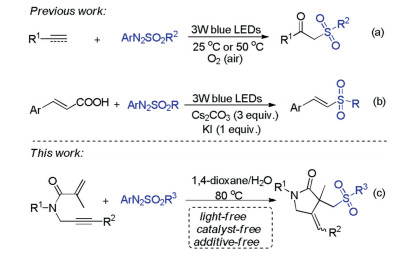
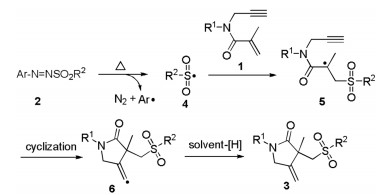
Arylazo sulfones are colored and bench stable compounds, which could generate aryl radical and sulfonyl radical along with the release of N2 through the homolytic cleavage of C-N and S-N bonds under visible-light irradiation or heating conditions [12]. Generally, arylazo sulfones are utilized as arylating reagents in photoinduced reactions in the absence of photocatalyst [13]. Recently, arylazo sulfones have alternatively emerged as sulfonylating agents for constructing sulfone-containing compounds [14]. In 2019, our group reported oxysulfonylation of alkenes or alkynes with arylazo sulfones and dioxygen leading to β-oxo sulfones under visible-light irradiation conditions (Scheme 1a) [14a, b]. In 2020, Yadav and co-workers also described an efficient visible-light mediated decarboxylative sulfonylation of cinnamic acids and arylazo sulfones for the synthesis of (E)-vinyl sulfones (Scheme 1b) [14c]. As our ongoing interest in sulfonylation reactions [15], herein, we wish to present a catalyst- and light-free selective sulfonylation/cyclization of 1,6-enynes with arylazo sulfones to synthesize sulfonated γ-butyrolactams under simple heating conditions (Scheme 1c).
Scheme 1
The cyclization of 1, n-enynes has become as a powerful protocol for the construction of various important heterocycles such as quinolines, pyrroles, pyridines, furans, indoles and pyrans in terms of its step-economy and high reaction efficiency [16-18]. γ-Butyrolactam is the core structure of a large number of natural and biologically active compounds, which covered a wide spectrum of biological activities [19]. Recently, some functionalized γ-butyrolactams have been elegantly synthesized through the radical 1,6-enyne cyclization strategies under light or strong oxidant mediated conditions [20]. The present reaction, which simply utilizes arylazo sulfones as sulfonylating agents, offers a convenient and regioselective approach to access various sulfonylated γ-butyrolactams under mild and catalyst-free conditions.
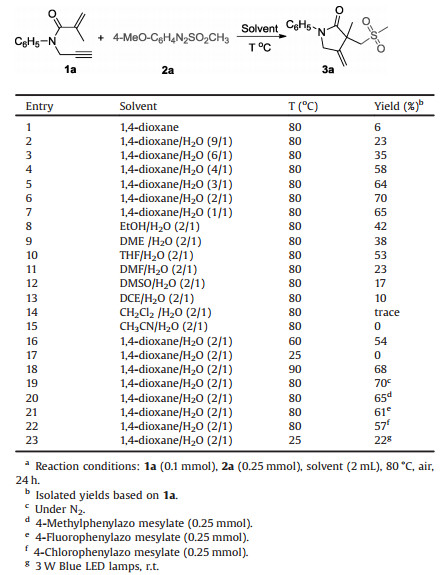
At the beginning of the experiment, the model reaction of N-phenyl-N-(prop-2-yn-1-yl)methacrylamide (1a) with 4-methoxyphenylazo mesylate (2a) was chosen to test the optimized reaction conditions. The product 3a was only isolated in 6% yield when the model reaction was conducted in 1, 4-dioxane at 80 ℃ (Table 1, entry 1). To our delight, the yield of 3a was largely increased when water was added in this reaction system (Table 1, entries 2–6). The highest yield of 3a (70%) was obtained when the ratio of 1, 4-dioxane and water was 2:1 (Table 1, entry 6). The yield would be slightly decreased if we continued to increase the amount of water (Table 1, entry 7). Next, various mixed solvents with organic solvent and water (2/1) were investigated (Table 1, entries 8–15). Generally, low to moderate yields were observed when the reaction was carried out in the mixture of EtOH, DME, THF, DMF, DMSO, or DCE with H2O (2/1). Only a trace amount of product 3a was detected when the reaction was performed in CH2Cl2/H2O (2/1). None of the product was observed in CH3CN/H2O (Table 1, entry 15). The decrease of reaction temperature would lead to lower reaction efficiency. No transformation was observed when the reaction was conducted at room temperature (Table 1, entry 17). The reaction was not improved when the reaction temperature was increased to 90 ℃ (Table 1, entry 18). Furthermore, product 3a was still obtained in good yield when the reaction was carried out under nitrogen atmosphere (Table 1, entry 19). Moreover, the use of other arylazo mesylates (4-methylphenylazo mesylate, 4-fluorophenylazo mesylate or 4-chlorophenylazo mesylate) to replace 4-methoxyphenylazo mesylate also gave the desired product 3a, but lower reaction efficiency was observed (Table 1, entries 20–22). Moreover, when the reaction was carried out under irradiation with 3 W Blue LED lamps, the corresponding product 3a was obtained in 22% yield (Table 1, entry 23).
Table 1
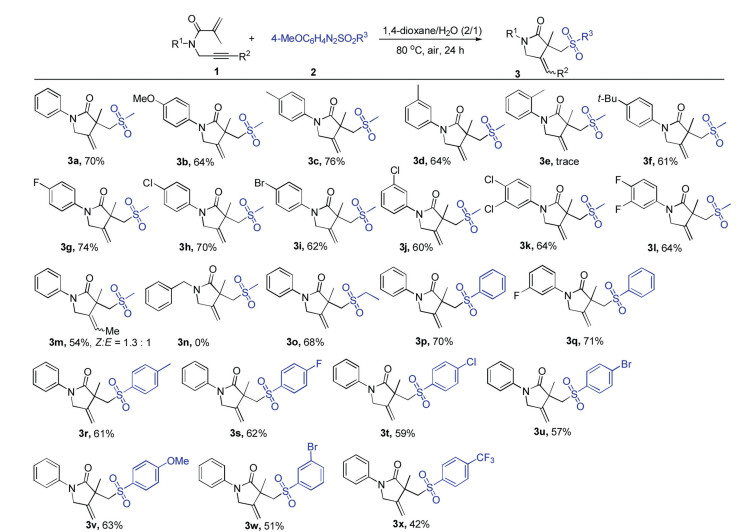
Under the optimized conditions, the scope and limitation of various 1,6-enyne derivatives and arylazo sulfones were further examined (Scheme 2). Generally, a series of 1,6-enynes with both electron-donating and electron-withdrawing groups on the N-aromatic ring could undergo the reaction efficiently to produce the desired products (3b-3l) in moderate to good yields. Notably, halogen substituents such as F, Cl and Br groups were all tolerated in this reaction system, which could be used for the further modification via coupling reactions. It was found that the reaction efficiency was greatly affected by the steric hindrance of substituent on the aromatic ring, and only a trace amount of product 3e was detected when ortho-methyl N-aromatic ring substrate was employed in this system. Unfortunately, when N-alkyl substituted 1,6-enyne such as N-benzyl-N-(prop-2-ynyl) methacrylamide was tested in the present reaction, none of the desired product 3n was detected. It should be noted that internal alkyne such as N-(but-2-yn-1-yl)-N-phenylmethacrylamide was suitable for this reaction, affording the product 3m in moderate yield. Then, the scope of different arylazo sulfones was investigated. Arylazo alkylsulfone (i.e., 4-methoxyphenylazo ethyl sulfone) could also be utilized in this system to afford the corresponding product 3o in 68% yield. Moreover, a variety of arylazo arylsulfones bearing an electron-donating (methyl and methoxy) or an electron-withdrawing group (fluoro, chloro, bromo and trifluoromethyl) on the aryl rings were suitable substrates, and the corresponding products (3r-3x) were obtained in moderate to good yields.
Scheme 2
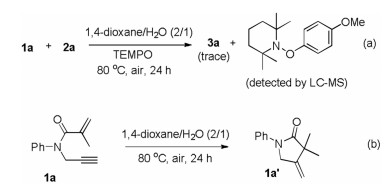
To investigate the possible reaction mechanism, two control experiments were carried out. When radical scavenger TEMPO (2, 2, 6, 6-tetramethyl-1-piperidinyloxy) was added in the model reaction system, the transformation was extremely inhibited and aryl-TEMPO adduct was observed by LC–MS. This result suggested that a radical process might be involved in the present reaction (Scheme 3a). Furthermore, the cyclization product 1a' was not generated from 1a under standard conditions in the absence of 4-methoxyphenylazo mesylate (2a) (Scheme 3b), suggesting that this cascade cyclization was triggered by in situ generated sulfonyl radical.
Scheme 3
Based on the above experimental results and previous reports [4, 7, 8, 13, 14], a possible reaction pathway was proposed as demonstrated in Scheme 4. Initially, the homolysis of N–S bond of arylazo sulfone 2 generated sulfonyl radical 4 and aryl radical along with the release of N2 under heating conditions. Subsequently, the selective addition of sulfonyl radical 4 to C=C bond gave alkyl radical 5, which further underwent the intramolecular cyclization with the C-C triple bond to give the vinyl radical 6. Finally, the abstraction of a hydrogen from the solvent would produce the desired product 3.
Scheme 4
In summary, a convenient and efficient method has been developed for the construction of sulfonylated γ-butyrolactams through the regioselective sulfonylation/cyclization of 1,6-enynes with arylazo sulfones. The present transformation could be achieved under catalyst- and additive-free conditions via the sequential formation of C-S and C-C bonds. This protocol simply utilizes arylazo sulfones as sulfonylating agents offering a highly attractive routine to synthesize sulfonylated γ-butyrolactams, which is expected to exhibit potential application in synthetic chemistry.
Declaration of competing interest
The authors report no declarations of interest.
Acknowledgments
This work was supported by the International Cooperation Project of Qinghai Province (No. 2018-HZ-806), the Youth Innovation and Technology Project of Higher School in Shandong Province (No. 2019KJC021), the Natural Science Foundation of Shandong Province (No. ZR2018MB009), the Qinghai Key Laboratory of Tibetan Medicine Research (No. 2017- ZJ-Y11) and CAS "Light of West China" Program 2018, and Entrepreneurship Training Program for College Students (No. 201910049).
Appendix A. Supplementary data
Supplementary material related tothis article can be found, in the online version, at doi:https://doi.org/10.1016/j.cclet.2020.11.059.
* Corresponding author at: School of Chemistry and Chemical Engineering, Qufu Normal University, Qufu 273165, China.
** Corresponding author.
E-mail addresses: xhzhao@nwipb.cas.cn (X. Zhao), weiweiqfnu@163.com (W. Wei).
References
-
[1]
[1] G. Moad, M. Chen, M. Häussler, et al., Functional polymers for optoelectronic applications by RAFT polymerization, Polym. Chem. 2 (2011) 492-519.
-
[2]
[2] T. Klingstedt, K.P.R. Nilsson, Conjugated polymers for enhanced bioimaging, Biochim. Biophys. Acta - Gen. Subjects 1810 (2011) 286-296.
-
[3]
[3] D. Liu, Y.H. Duan, Synthesis of novel thieno-[3,4-b]-pyrazine-cored molecules as red fluorescent materials, Chin. Chem. Lett. 24 (2013) 809-812.
-
[4]
[4] J.H. Kim, K. Park, H.Y. Nam, et al., Polymers for bioimaging, Prog. Polym. Sci. 32 (2007) 1031-1053.
-
[5]
[5] M.O. Liu, H.F. Lin, M.C. Yang, et al., Thermal and fluorescent properties of optical brighteners and their whitening effect for pelletization of cycloolefin copolymers, Mater. Lett. 60 (2006) 2132-2137.
-
[6]
[6] L. Torini, J.F. Argillier, N. Zydowicz, Interfacial polycondensation encapsulation in miniemulsion, Macromolecules 38 (2005) 3225-3236.
-
[7]
[7] K. Kojio, Y. Mitsui, M. Furukawa, Synthesis and properties of highly hydrophilic polyurethane based on diisocyanate with ether group, Polymer 50 (2009) 3693- 3697.
-
[8]
[8] C.L. Wang, Z.J. Zhang, Y.M. Kuo, D.Y. Chao, Fluorescence from fluorescent dyebased polyurethane ionomer (Ⅱ), Polym. Adv. Technol. 15 (2004) 93-99.
-
[9]
[9] O. Kim, M.S. Gong, Fluorescent polyurethane foams containing red fluorescent perylene chromophore, J. Ind. Eng. Chem. 10 (2004) 801-805.
-
[10]
[10] M.M. Rahman, A. Hasneen, H.D. Kim, W.K. Lee, Preparation and properties of polydimethylsiloxane (PDMS)/polytetramethyleneadipate glycol (PTAd)-based waterborne polyurethane adhesives: effect of PDMS molecular weight and content, J. Appl. Polym. Sci. 125 (2012) 88-96.
-
[11]
[11] J.P. Sheth, A. Aneja, G.L. Wilkes, et al., Influence of system variables on the morphological and dynamic mechanical behavior of polydimethylsiloxane based segmented polyurethane and polyurea copolymers: a comparative perspective, Polymer 45 (2004) 6919-6932.
-
[12]
[12] J.C. McDonald, G.M. Whitesides, Poly(dimethylsiloxane) as a material for fabricating microfluidic devices, Acc. Chem. Res. 35 (2002) 491-499.
-
[13]
[13] G. Kwak, T. Masuda, Poly(silyleneethynylenephenylene) and poly(silylenephenyleneethynylenephenylene) s: Synthesis and photophysical properties related to charge transfer, Macromolecules 35 (2002) 4138-4142.
-
[14]
[14] G. Kwak, A. Takagi, M. Fujiki, T. Masuda, Facile preparation of transparent, homogeneous, fluorescent gel film based on s-p-conjugated, hyperbranched polymer with siloxane linkages by means of hydrosilylation and aerial oxidation, Chem. Mater. 16 (2004) 781-785.
-
[15]
[15] O. Jaudouin, J.J. Robin, J.M. Lopez-Cuesta, D. Perrin, C. Imbert, Ionomer-based polyurethanes: a comparative study of properties and applications, Polym. Int. 61 (2012) 495-510.
-
[16]
[16] Y. Zhang, S.B. Feng, Q. Wu, et al., Microwave-assisted synthesis and evaluation of naphthalimides derivatives as free radical scavengers, Med. Chem. Res. 20 (2011) 752-759.
-
[17]
[17] J.E. Rogers, L.A. Kelly, Nucleic acid oxidation mediated by naphthalene and benzophenone imide and diimide derivatives: consequences for DNA redox chemistry, J. Am. Chem. Soc. 121 (1999) 3854-3861.
-
[18]
[18] H.B. Xiao, M.J. Chen, G.H. Shi, et al., A novel fluorescent molecule based on 1,8- naphthalimide: synthesis, spectral properties, and application in cell imaging, Res. Chem. Intermed. 36 (2010) 1021-1026.
-
[19]
[19] I. Grabchev, S. Sali, R. Betcheva, V. Gregoriou, New green fluorescent polymer sensors for metal cations and protons, Eur. Polym. J. 43 (2007) 4297-4305.
-
[20]
[20] I. Grabchev, V. Bojinov, Synthesis and characterisation of fluorescent polyacrylonitrile copolymers with 1,8-naphthalimide side chains, Polym. Degrad. Stabil. 70 (l) (2000) 47-153.
-
[21]
[21] Y. Wang, X.G. Zhang, B. Han, et al., The synthesis and photoluminescence characteristics of novel blue light-emitting naphthalimide derivatives, Dyes Pigments 86 (2010) 190-196.
-
[22]
[22] Y.Y. Zhang, C.H. Zhou, Synthesis and activities of naphthalimide azoles as a new type of antibacterial and antifungal agents, Bioorg. Med. Chem. Lett. 21 (2011) 4349-4352.
-
[23]
[23] Y.C. Chen, R.R. Chiou, H.L. Huang, et al., Fluorescence from fluorescent dye based polyurethane ionomer (Ⅲ), J. Appl. Polym. Sci. 97 (2005) 455-465.
-
[24]
[24] J.V. Crivello, B. Daoshen, Regioselective hydrosilations. I. The hydrosilation ofa,vdihydrogen functional oligopolydimethylsiloxanes with 3-vinyl-7-oxabicyclo[ 4.1.0]heptanes, J. Polym. Sci. A 31 (1993) 2563-2572.
-
[25]
[25] D.J. David, H.B. Staley, Analytical Chemistry of the Polyurethanes, vol. 16, Wiley-Interscience, New York, 1969.
-
[26]
[26] F.M. Li, S.J. Chen, Z.I. Li, J. Qiu, Vinyl monomers bearing chromophore moieties and their polymers. 1. Initiation and photochemical behavior of N-acryloyl-N-phenylpiperazines and their polymers, J. Polym. Sci. A 34 (1996) 1881-1888.
-
[27]
[27] X.H. Hu, X.Y. Zhang, J.B. Dai, Synthesis and characterization of a novel waterborne stilbene-based polyurethane fluorescent brightener, Chin. Chem. Lett. 22 (2011) 997-1000.
-
[28]
[28] N.Z. Galunov, B.M. Krasovitskii, O.N. Lyubenko, et al., Spectral properties and applications of the new 7H-benzo[de]pyrazolo[5,1-a]isoquinolin-7-ones, J. Lumin. 102 (2003) 119-124.
-
[29]
[29] J. Qiu, Z.C. Li, Q.Y. Gao, et al., Vinyl monomers bearing chromophore moieties and their polymers. 3. Synthesis and photochemical behavior of acrylic monomers having phenothiazine moieties and their polymers, J. Polym. Sci. A 34 (1996) 3015-3023.
-
[30]
[30] J. McCall, C. Alexander, M.M. Richter, Quenching of electrogenerated chemiluminescence by phenols, hydroquinones, catechols, and benzoquinones, Anal. Chem. 71 (1999) 2523-2527.
-
[31]
[31] C. Turro, S.H. Bossmann, Y. Jenkins, J.K. Barton, N.J. Turro, Proton transfer quenching of the MLCT excited state of Ru(phen)2dppz2+ in homogeneous solution and bound to DNA, J. Am. Chem. Soc. 117 (1995) 9026-9032.
-
[32]
[32] L. Biczok, H. Linschitz, Concerted electron and proton movement in quenching of triplet C60 and tetracene fluorescence by hydrogen-bonded phenol-base pairs, J. Phys. Chem. 99 (1995) 1843-1845.
-
[33]
[33] G. Camino, S.M. Lomakin, M. Lazzari, Polydimethylsiloxane thermal degradation. Part 1. Kinetic aspects, Polymer 42 (2001) 2395-2402.
-
[34]
[34] G. Deshpande, M.E. Rezac, The effect of phenyl content on the degradation of poly (dimethyl diphenyl) siloxane copolymers, Polym. Degrad. Stabil. 74 (2001) 363- 370.
-
[35]
[35] P. Krol, K. Pielichowska, L. Byczynski, Thermal degradation kinetics of polyurethane- siloxane anionomers, Thermochim. Acta 507 (2010) 91-98.
-
[36]
[36] W.J. Zhou, H. Yang, X.Z. Guo, J.J. Lu, Thermal degradation behaviors of some branched and linear polysiloxanes, Polym. Degrad. Stabil. 91 (2006) 1471-1475.
-
[37]
[37] AATCC Test Method 140-2001, Dye and pigment migration in a pad-dry process, in: AATCC Technical Manual, American Association of Textile Chemists and Colorists, 2006.
-
[1]
-
-
[1]
[1] G. Moad, M. Chen, M. Häussler, et al., Functional polymers for optoelectronic applications by RAFT polymerization, Polym. Chem. 2 (2011) 492-519.
-
[2]
[2] T. Klingstedt, K.P.R. Nilsson, Conjugated polymers for enhanced bioimaging, Biochim. Biophys. Acta - Gen. Subjects 1810 (2011) 286-296.
-
[3]
[3] D. Liu, Y.H. Duan, Synthesis of novel thieno-[3,4-b]-pyrazine-cored molecules as red fluorescent materials, Chin. Chem. Lett. 24 (2013) 809-812.
-
[4]
[4] J.H. Kim, K. Park, H.Y. Nam, et al., Polymers for bioimaging, Prog. Polym. Sci. 32 (2007) 1031-1053.
-
[5]
[5] M.O. Liu, H.F. Lin, M.C. Yang, et al., Thermal and fluorescent properties of optical brighteners and their whitening effect for pelletization of cycloolefin copolymers, Mater. Lett. 60 (2006) 2132-2137.
-
[6]
[6] L. Torini, J.F. Argillier, N. Zydowicz, Interfacial polycondensation encapsulation in miniemulsion, Macromolecules 38 (2005) 3225-3236.
-
[7]
[7] K. Kojio, Y. Mitsui, M. Furukawa, Synthesis and properties of highly hydrophilic polyurethane based on diisocyanate with ether group, Polymer 50 (2009) 3693- 3697.
-
[8]
[8] C.L. Wang, Z.J. Zhang, Y.M. Kuo, D.Y. Chao, Fluorescence from fluorescent dyebased polyurethane ionomer (Ⅱ), Polym. Adv. Technol. 15 (2004) 93-99.
-
[9]
[9] O. Kim, M.S. Gong, Fluorescent polyurethane foams containing red fluorescent perylene chromophore, J. Ind. Eng. Chem. 10 (2004) 801-805.
-
[10]
[10] M.M. Rahman, A. Hasneen, H.D. Kim, W.K. Lee, Preparation and properties of polydimethylsiloxane (PDMS)/polytetramethyleneadipate glycol (PTAd)-based waterborne polyurethane adhesives: effect of PDMS molecular weight and content, J. Appl. Polym. Sci. 125 (2012) 88-96.
-
[11]
[11] J.P. Sheth, A. Aneja, G.L. Wilkes, et al., Influence of system variables on the morphological and dynamic mechanical behavior of polydimethylsiloxane based segmented polyurethane and polyurea copolymers: a comparative perspective, Polymer 45 (2004) 6919-6932.
-
[12]
[12] J.C. McDonald, G.M. Whitesides, Poly(dimethylsiloxane) as a material for fabricating microfluidic devices, Acc. Chem. Res. 35 (2002) 491-499.
-
[13]
[13] G. Kwak, T. Masuda, Poly(silyleneethynylenephenylene) and poly(silylenephenyleneethynylenephenylene) s: Synthesis and photophysical properties related to charge transfer, Macromolecules 35 (2002) 4138-4142.
-
[14]
[14] G. Kwak, A. Takagi, M. Fujiki, T. Masuda, Facile preparation of transparent, homogeneous, fluorescent gel film based on s-p-conjugated, hyperbranched polymer with siloxane linkages by means of hydrosilylation and aerial oxidation, Chem. Mater. 16 (2004) 781-785.
-
[15]
[15] O. Jaudouin, J.J. Robin, J.M. Lopez-Cuesta, D. Perrin, C. Imbert, Ionomer-based polyurethanes: a comparative study of properties and applications, Polym. Int. 61 (2012) 495-510.
-
[16]
[16] Y. Zhang, S.B. Feng, Q. Wu, et al., Microwave-assisted synthesis and evaluation of naphthalimides derivatives as free radical scavengers, Med. Chem. Res. 20 (2011) 752-759.
-
[17]
[17] J.E. Rogers, L.A. Kelly, Nucleic acid oxidation mediated by naphthalene and benzophenone imide and diimide derivatives: consequences for DNA redox chemistry, J. Am. Chem. Soc. 121 (1999) 3854-3861.
-
[18]
[18] H.B. Xiao, M.J. Chen, G.H. Shi, et al., A novel fluorescent molecule based on 1,8- naphthalimide: synthesis, spectral properties, and application in cell imaging, Res. Chem. Intermed. 36 (2010) 1021-1026.
-
[19]
[19] I. Grabchev, S. Sali, R. Betcheva, V. Gregoriou, New green fluorescent polymer sensors for metal cations and protons, Eur. Polym. J. 43 (2007) 4297-4305.
-
[20]
[20] I. Grabchev, V. Bojinov, Synthesis and characterisation of fluorescent polyacrylonitrile copolymers with 1,8-naphthalimide side chains, Polym. Degrad. Stabil. 70 (l) (2000) 47-153.
-
[21]
[21] Y. Wang, X.G. Zhang, B. Han, et al., The synthesis and photoluminescence characteristics of novel blue light-emitting naphthalimide derivatives, Dyes Pigments 86 (2010) 190-196.
-
[22]
[22] Y.Y. Zhang, C.H. Zhou, Synthesis and activities of naphthalimide azoles as a new type of antibacterial and antifungal agents, Bioorg. Med. Chem. Lett. 21 (2011) 4349-4352.
-
[23]
[23] Y.C. Chen, R.R. Chiou, H.L. Huang, et al., Fluorescence from fluorescent dye based polyurethane ionomer (Ⅲ), J. Appl. Polym. Sci. 97 (2005) 455-465.
-
[24]
[24] J.V. Crivello, B. Daoshen, Regioselective hydrosilations. I. The hydrosilation ofa,vdihydrogen functional oligopolydimethylsiloxanes with 3-vinyl-7-oxabicyclo[ 4.1.0]heptanes, J. Polym. Sci. A 31 (1993) 2563-2572.
-
[25]
[25] D.J. David, H.B. Staley, Analytical Chemistry of the Polyurethanes, vol. 16, Wiley-Interscience, New York, 1969.
-
[26]
[26] F.M. Li, S.J. Chen, Z.I. Li, J. Qiu, Vinyl monomers bearing chromophore moieties and their polymers. 1. Initiation and photochemical behavior of N-acryloyl-N-phenylpiperazines and their polymers, J. Polym. Sci. A 34 (1996) 1881-1888.
-
[27]
[27] X.H. Hu, X.Y. Zhang, J.B. Dai, Synthesis and characterization of a novel waterborne stilbene-based polyurethane fluorescent brightener, Chin. Chem. Lett. 22 (2011) 997-1000.
-
[28]
[28] N.Z. Galunov, B.M. Krasovitskii, O.N. Lyubenko, et al., Spectral properties and applications of the new 7H-benzo[de]pyrazolo[5,1-a]isoquinolin-7-ones, J. Lumin. 102 (2003) 119-124.
-
[29]
[29] J. Qiu, Z.C. Li, Q.Y. Gao, et al., Vinyl monomers bearing chromophore moieties and their polymers. 3. Synthesis and photochemical behavior of acrylic monomers having phenothiazine moieties and their polymers, J. Polym. Sci. A 34 (1996) 3015-3023.
-
[30]
[30] J. McCall, C. Alexander, M.M. Richter, Quenching of electrogenerated chemiluminescence by phenols, hydroquinones, catechols, and benzoquinones, Anal. Chem. 71 (1999) 2523-2527.
-
[31]
[31] C. Turro, S.H. Bossmann, Y. Jenkins, J.K. Barton, N.J. Turro, Proton transfer quenching of the MLCT excited state of Ru(phen)2dppz2+ in homogeneous solution and bound to DNA, J. Am. Chem. Soc. 117 (1995) 9026-9032.
-
[32]
[32] L. Biczok, H. Linschitz, Concerted electron and proton movement in quenching of triplet C60 and tetracene fluorescence by hydrogen-bonded phenol-base pairs, J. Phys. Chem. 99 (1995) 1843-1845.
-
[33]
[33] G. Camino, S.M. Lomakin, M. Lazzari, Polydimethylsiloxane thermal degradation. Part 1. Kinetic aspects, Polymer 42 (2001) 2395-2402.
-
[34]
[34] G. Deshpande, M.E. Rezac, The effect of phenyl content on the degradation of poly (dimethyl diphenyl) siloxane copolymers, Polym. Degrad. Stabil. 74 (2001) 363- 370.
-
[35]
[35] P. Krol, K. Pielichowska, L. Byczynski, Thermal degradation kinetics of polyurethane- siloxane anionomers, Thermochim. Acta 507 (2010) 91-98.
-
[36]
[36] W.J. Zhou, H. Yang, X.Z. Guo, J.J. Lu, Thermal degradation behaviors of some branched and linear polysiloxanes, Polym. Degrad. Stabil. 91 (2006) 1471-1475.
-
[37]
[37] AATCC Test Method 140-2001, Dye and pigment migration in a pad-dry process, in: AATCC Technical Manual, American Association of Textile Chemists and Colorists, 2006.
-
[1]
-

-
-
[1]
Junqing Wu , Yiyang Zhang , Qingqing Hong , Hui Yang , Lifeng Zhang , Ming Zhang , Lei Yu . Organometallic modification of silica with europium endowing the fluorescence properties: The key technique for numerical quality monitoring. Chinese Chemical Letters, 2025, 36(4): 110165-. doi: 10.1016/j.cclet.2024.110165
-
[2]
Xinyu Liu , Jialin Yang , Zonglin He , Jiaoyan Ai , Lina Song , Baohua Liu . Linear polyurethanes with excellent comprehensive properties from poly(ethylene carbonate) diol. Chinese Chemical Letters, 2025, 36(1): 110236-. doi: 10.1016/j.cclet.2024.110236
-
[3]
Ying Xu , Chengying Shen , Hailong Yuan , Wei Wu . Mapping multiple phases in curcumin binary solid dispersions by fluorescence contrasting. Chinese Chemical Letters, 2024, 35(9): 109324-. doi: 10.1016/j.cclet.2023.109324
-
[4]
Deshuai Zhen , Chunlin Liu , Qiuhui Deng , Shaoqi Zhang , Ningman Yuan , Le Li , Yu Liu . A review of covalent organic frameworks for metal ion fluorescence sensing. Chinese Chemical Letters, 2024, 35(8): 109249-. doi: 10.1016/j.cclet.2023.109249
-
[5]
Manman Ou , Yunjian Zhu , Jiahao Liu , Zhaoxuan Liu , Jianjun Wang , Jun Sun , Chuanxiang Qin , Lixing Dai . Polyvinyl alcohol fiber with enhanced strength and modulus and intense cyan fluorescence based on covalently functionalized graphene quantum dots. Chinese Chemical Letters, 2025, 36(2): 110510-. doi: 10.1016/j.cclet.2024.110510
-
[6]
Kuan Deng , Fei Yang , Zhi-Qi Cheng , Bi-Wen Ren , Hua Liu , Jiao Chen , Meng-Yao She , Le Yu , Xiao-Gang Liu , Hai-Tao Feng , Jian-Li Li . Construction of wavelength-tunable DSE quinoline salt derivatives by regulating the hybridization form of the nitrogen atom and intramolecular torsion angle. Chinese Chemical Letters, 2024, 35(10): 109464-. doi: 10.1016/j.cclet.2023.109464
-
[7]
Mengfan Zhang , Lingyan Liu , Peng Wei , Wei Feng , Tao Yi . A proximity tagging strategy utilizing an activated aldehyde group as the active site. Chinese Chemical Letters, 2025, 36(4): 110127-. doi: 10.1016/j.cclet.2024.110127
-
[8]
Ying Wang , Hong Yang , Caixia Zhu , Qing Hong , Xuwen Cao , Kaiyuan Wang , Yuan Xu , Yanfei Shen , Songqin Liu , Yuanjian Zhang . Cascading oxidoreductases-like nanozymes for high selective and sensitive fluorescent detection of ascorbic acid. Chinese Chemical Letters, 2025, 36(4): 110153-. doi: 10.1016/j.cclet.2024.110153
-
[9]
Hao Zhang , Hao Liu , Ke Huang , Qingxiu Xia , Hongjie Xiong , Xiaohui Liu , Hui Jiang , Xuemei Wang . Ionic exchange based intracellular self-assembly of pitaya-structured nanoparticles for tumor imaging. Chinese Chemical Letters, 2025, 36(6): 110281-. doi: 10.1016/j.cclet.2024.110281
-
[10]
Shaonan Liu , Shuixing Dai , Minghua Huang . The impact of ester groups on 1,8-naphthalimide electron transport material in organic solar cells. Chinese Journal of Structural Chemistry, 2024, 43(6): 100277-100277. doi: 10.1016/j.cjsc.2024.100277
-
[11]
YanYuan Jia , Rong Rong , Jie Liu , Jing Guo , GuoYu Jiang , Shuo Guo . Unity is Strength, and Independence Shines: A Science Popularization Experiment on AIE and ACQ Effects. University Chemistry, 2024, 39(9): 349-358. doi: 10.12461/PKU.DXHX202402035
-
[12]
Qin Li , Kexin Yang , Qinglin Yang , Xiangjin Zhu , Xiaole Han , Tao Huang . Illuminating Chlorophyll: Innovative Chemistry Popularization Experiment. University Chemistry, 2024, 39(9): 359-368. doi: 10.3866/PKU.DXHX202309059
-
[13]
Zehua Zhang , Haitao Yu , Yanyu Qi . 多重共振TADF分子的设计策略. Acta Physico-Chimica Sinica, 2025, 41(1): 2309042-. doi: 10.3866/PKU.WHXB202309042
-
[14]
Feng Lu , Tao Wang , Qi Wang . Preparation and Characterization of Water-Soluble Silver Nanoclusters: A New Design and Teaching Practice in Materials Chemistry Experiment. University Chemistry, 2025, 40(4): 375-381. doi: 10.12461/PKU.DXHX202406005
-
[15]
Shuwen SUN , Gaofeng WANG . Two cadmium coordination polymers constructed by varying Ⅴ-shaped co-ligands: Syntheses, structures, and fluorescence properties. Chinese Journal of Inorganic Chemistry, 2024, 40(3): 613-620. doi: 10.11862/CJIC.20230368
-
[16]
Lulu DONG , Jie LIU , Hua YANG , Yupei FU , Hongli LIU , Xiaoli CHEN , Huali CUI , Lin LIU , Jijiang WANG . Synthesis, crystal structure, and fluorescence properties of Cd-based complex with pcu topology. Chinese Journal of Inorganic Chemistry, 2025, 41(4): 809-820. doi: 10.11862/CJIC.20240171
-
[17]
Kaimin WANG , Xiong GU , Na DENG , Hongmei YU , Yanqin YE , Yulu MA . Synthesis, structure, fluorescence properties, and Hirshfeld surface analysis of three Zn(Ⅱ)/Cu(Ⅱ) complexes based on 5-(dimethylamino) isophthalic acid. Chinese Journal of Inorganic Chemistry, 2024, 40(7): 1397-1408. doi: 10.11862/CJIC.20240009
-
[18]
Fangzhou Wang , Wentong Gao , Chenghui Li . A weak but inert hindered urethane bond for high-performance dynamic polyurethane polymers. Chinese Chemical Letters, 2024, 35(5): 109305-. doi: 10.1016/j.cclet.2023.109305
-
[19]
Gaofeng WANG , Shuwen SUN , Yanfei ZHAO , Lixin MENG , Bohui WEI . Structural diversity and luminescence properties of three zinc coordination polymers based on bis(4-(1H-imidazol-1-yl)phenyl)methanone. Chinese Journal of Inorganic Chemistry, 2024, 40(5): 849-856. doi: 10.11862/CJIC.20230479
-
[20]
Wu-Yang Liu , Xin-Xiang Lei , Wen-Ji Wang , Jun-Mian Tian , Yu-Qi Gao , Jin-Ming Gao . Hyperforatone A, the 1,8-seco rearranged polycyclic polyprenylated acylphloroglucinol with a unique bicyclo[5.4.0]undecane core from Hypericum perforatum. Chinese Chemical Letters, 2025, 36(4): 110478-. doi: 10.1016/j.cclet.2024.110478
-
[1]
Metrics
- PDF Downloads(0)
- Abstract views(892)
- HTML views(17)

 Login In
Login In




 DownLoad:
DownLoad:
 DownLoad:
DownLoad: