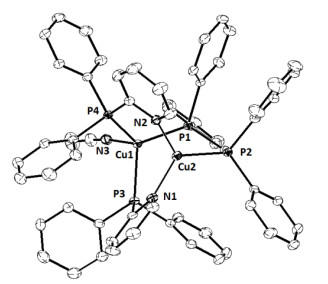
| Bond | Dist. | Bond | Dist. | |
| Cu(1)–P(1) | 2.3172(15) | Cu(1)–P(3) | 2.3122(14) | |
| Cu(1)–P(4) | 2.3057(15) | Cu(1)–N(3) | 2.027(5) | |
| Cu(2)–P(2) | 2.2044(15) | Cu(2)–N(1) | 2.031(5) | |
| Cu(2)–N(2) | 2.034(5) | Cu(1)···Cu(2) | 2.8739(9) | |
| Angle | (º) | Angle | (º) | |
| P(1)–Cu(1)–P(3) P(3)–Cu(1)–P(4) P(3)–Cu(1)–N(3) P(2)–Cu(2)–N(1) N(1)–Cu(2)–N(2) |
114.03(5) 111.99(5) 98.93(14) 127.58(13) 103.10(19) |
P(1)–Cu(1)–P(4) P(1)–Cu(1)–N(3) P(4)–Cu(1)–N(3) P(2)–Cu(2)–N(2) |
123.26(5) 102.21(14) 101.58(14) 124.09(14) |

Synthesis, Structure and Photophysical Properties of a Dinuclear Copper(I) Complex Based on Phosphino-pyridine and Diphosphine Mixed-ligands
English
Synthesis, Structure and Photophysical Properties of a Dinuclear Copper(I) Complex Based on Phosphino-pyridine and Diphosphine Mixed-ligands
-
-
-
[1]
Uoyama, H.; Goushi, K.; Shizu, K.; Nomura, H.; Adachi, C. Highly efficient organic light-emitting diodes from delayed fluorescence. Nature 2012, 492, 234–238. doi: 10.1038/nature11687
-
[2]
Bizzarri, C.; Spuling, E.; Knoll, D. M.; Volz, D.; Bräse, S. Sustainable metal complexes for organic light-emitting diodes (OLEDs). Coord. Chem. Rev. 2018, 373, 49–82. doi: 10.1016/j.ccr.2017.09.011
-
[3]
Yang, M.; Chen, X. L.; Lu, C. Z. Efficiently luminescent copper(I) iodide complexes with crystallization-induced emission enhancement (CIEE). Dalton Trans. 2019, 48, 10790–10794. doi: 10.1039/C9DT01910C
-
[4]
Fleetham, T.; Li, G.; Li, J. Phosphorescent Pt(Ⅱ) and Pd(Ⅱ) complexes for efficient, high-color-quality, and stable OLEDs. Adv. Mater. 2017, 29, 1601861. doi: 10.1002/adma.201601861
-
[5]
Wu, Y.; Tan, X.; Lv, A.; Yu, F.; Ma, H.; Shen, K.; Sun, Z.; Chen, F.; Chen, Z. K.; Hang, X. C. Triplet excited-state engineering of phosphorescent Pt(Ⅱ) complexes. J. Phys. Chem. Lett. 2019, 10, 5105–5110. doi: 10.1021/acs.jpclett.9b01685
-
[6]
Femoni, C.; Muzzioli, S.; Palazzi, A.; Stagni, S.; Zacchini, S.; Monti, F.; Accorsi, G.; Bolognesi, M.; Armaroli, N.; Massi, M.; Valenti, G.; Marcaccio, M. New tetrazole-based Cu(I) homo- and hetero-leptic complexes with various P^P ligands: synthesis, characterization, redox and photophysical properties. Dalton Trans. 2013, 42, 997–1010. doi: 10.1039/C2DT32056H
-
[7]
Yersin, H.; Rausch, A. F.; Czerwieniec, R.; Hofbeck, T.; Fischer, T. The triplet state of organo-transition metal compounds. Triplet harvesting and singlet harvesting for efficient OLEDs. Coord. Chem. Rev. 2011, 255, 2622–2652. doi: 10.1016/j.ccr.2011.01.042
-
[8]
Deaton, J. C.; Switalski, S. C.; Kondakow, D. Y.; Young, R. H.; Pawlik, T. D.; Giesen, D. J.; Harkins, S. B.; Miller, A. J. M.; Mickenberg, S. F.; Peters, J. C. E-Type delayed fluorescence of a phosphine-supported Cu2(μ-NAr2)2 diamond core: harvesting singlet and triplet excitons in OLEDs. J. Am. Chem. Soc. 2010, 132, 9499–9508. doi: 10.1021/ja1004575
-
[9]
Hofbeck, T.; Monkowius, U.; Yersin, H. Highly efficient luminescence of Cu(I) compounds: thermally activated delayed fluorescence combined with short-lived phosphorescence. J. Am. Chem. Soc. 2015, 137, 399–404. doi: 10.1021/ja5109672
-
[10]
Czerwieniec, R.; Leitl, M. J.; Homeier, H. H. H.; Yersin, H. Cu(I) complexes-thermally activated delayed fluorescence. photophysical approach and material design. Coord. Chem. Rev. 2016, 325, 2–28. doi: 10.1016/j.ccr.2016.06.016
-
[11]
Mara, M. W.; Fransted, K. A.; Chen, L. X. Interplays of excited state structures and dynamics in copper(I) diimine complexes: implications and perspectives. Coord. Chem. Rev. 2015, 282, 2–18.
-
[12]
Jia, J. H.; Chen, X. L.; Liao, J. Z.; Liang, D.; Yang, M. X.; Yu, R.; Lu, C. Z. Highly luminescent copper(I) halide complexes chelated with a tetradentate ligand (PNNP): synthesis, structure, photophysical properties and theoretical studies. Dalton Trans. 2019, 48, 1418–1426. doi: 10.1039/C8DT03452D
-
[13]
Cuttell, D. G.; Kuang, S. M.; Fanwick, P. E.; McMillin, D. R.; Walton, R. A. Simple Cu(I) complexes with unprecedented excited-state lifetimes. J. Am. Chem. Soc. 2002, 124, 6–7. doi: 10.1021/ja012247h
-
[14]
Harkins, S. B.; Peters, J. C. A highly emissive Cu2N2 diamond core complex supported by a [PNP]-ligand. J. Am. Chem. Soc. 2005, 127, 2030–2031. doi: 10.1021/ja043092r
-
[15]
Schinabeck, A.; Rau, N.; Klein, M.; Sundermeyer, J.; Yersin, H. Deep blue emitting Cu(I) tripod complexes. Design of high quantum yield materials showing TADF-assisted phosphorescence. Dalton Trans. 2018, 47, 17067–17076. doi: 10.1039/C8DT04093A
-
[16]
Kubas, G. J. Inorg. Synth. 1990, 26, 68.
-
[17]
Sheldrick, G. M. SHELXS-97, Program for Crystal Structure Solution. University of Göttigen, Germany 1997.
-
[18]
Sheldrick, G. M. SHELXL-97, Program for Crystal Structure Refinement. University of Göttigen, Germany 1997.
-
[19]
Kang, L.; Chen, J.; Teng, T.; Chen, X. L.; Yu, R.; Lu, C. Z. Experimental and theoretical studies of highly emissive dinuclear Cu(I) halide complexes with delayed fluorescence. Dalton Trans. 2015, 44, 11649–11659. doi: 10.1039/C5DT01292A
-
[20]
Huang, C. H.; Wen, M.; Wang, C. Y.; Lu, Y. F.; Huang, X. H.; Li, H. H.; Wu, S. T.; Zhuang, N. F.; Hu, X. L. A series of pure-blue-light emitting Cu(I) complexes with thermally activated delayed fluorescence: structural, photophysical, and computational studies. Dalton Trans. 2017, 46, 1413–1419. doi: 10.1039/C6DT03965K
-
[21]
Linfoot, C. L.; Leitl, M. J.; Richardson, P.; Rausch, A. F.; Chepelin, O.; White, F. J.; Yersin, H.; Robertson, N. Thermally activated delayed fluorescence (TADF) and enhancing photoluminescence quantum yields of [CuI(diimine)(diphosphine)]+ complexes-photophysical, structural, and computational studies. Inorg. Chem. 2014, 53, 10854–10861. doi: 10.1021/ic500889s
-
[22]
Nitsch, J.; Lacemon, F.; Lorbach, A.; Eichhorn, A.; Cisnetti, F.; Steffen, A. Cuprophilic interactions in highly luminescent dicopper(I)-NHC-picolyl complexes-fast phosphorescence or TADF? Chem. Commun. 2016, 52, 2932–2935. doi: 10.1039/C5CC09659F
-
[23]
Czerwieniec, R.; Leitl, M. J.; Homeier, H. H. H.; Yersin, H. Cu(I) complexes-thermally activated delayed fluorescence. Photophysical approach and material design. Coord. Chem. Rev. 2016, 325, 2–28. doi: 10.1016/j.ccr.2016.06.016
-
[1]
-
Table 1. Selected Bond Lengths (Å) and Angles (º) of Complex 1
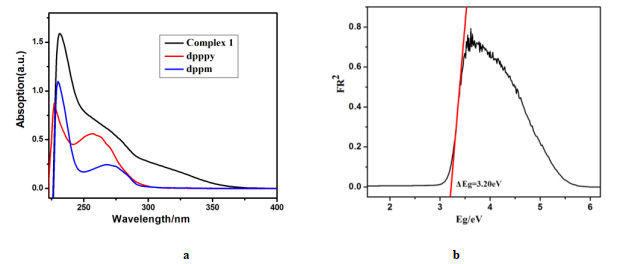
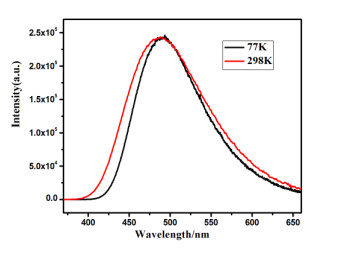
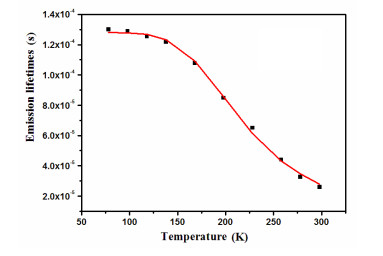
Table 2. Emission Properties of 1 in the Solid State at 298 and 77 K, and the Fitting Parameters Based on Eq. (1)
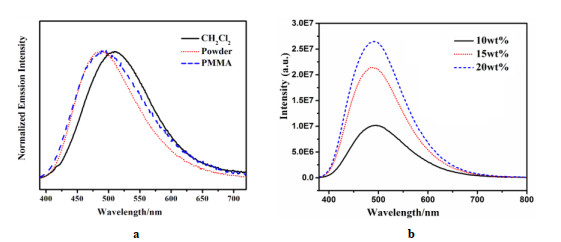
Emission properties Fitting parameters λmax (298 K) (nm) 488 ΔE(S1 – T1) (cm–1/eV) 812/0.10 τ (298 K) (μs) 25.9 τT1 (μs) 130 ΦPL (298K) (%) 57.3 τS1 (ns) 232 λmax (77 K) (nm) 493 τ (77 K) (μs) 130.1 Table 3. Emission Properties of 1 in Different Environments at Ambient Temperature
λmax (nm) ΦPL (%) Powders 488 57.3 PMMA (10wt%)a 492 27.2 PMMA (15wt%)a 494 33.4 PMMA (20wt%)a 494 36.1 CH2Cl2b 510 1.2 a) Dopant concentrations in PMMA matrices are 10%, 15% and 20% weight, respectively
b) The concentration in CH2CL2 solution is 1.0 × 10–5 mol/L -

计量
- PDF下载量: 3
- 文章访问数: 590
- HTML全文浏览量: 11

 下载:
下载:
 下载:
下载:

