
|

Green-synthesized, low-cost tetracyanodiazafluorene (TCAF) as electron injection material for organic light-emitting diodes
English
Green-synthesized, low-cost tetracyanodiazafluorene (TCAF) as electron injection material for organic light-emitting diodes
-
-
-
[1]
M. Schwarze, C. Gaul, R. Scholz, et al., Nat. Mater. 18 (2019) 242-248. doi: 10.1038/s41563-018-0277-0
-
[2]
B. Hu, C. An, M. Wagner, et al., J. Am. Chem. Soc. 141 (2019) 5130-5134. doi: 10.1021/jacs.9b01082
-
[3]
C. Wang, J. Zhang, G. Long, et al., Angew. Chem. Int. Ed 54 (2015) 6292-6296. doi: 10.1002/anie.201500972
-
[4]
W. Chen, Q. Zhang, J. Mater. Chem. C 5 (2017) 1275-1302. doi: 10.1039/C6TC05066B
-
[5]
X.P. Xu, G.J. Zhang, Y. Li, et al., Chin. Chem. Lett. 30 (2019) 809-825. doi: 10.1016/j.cclet.2019.02.030
-
[6]
Y. Lin, Y. Li, X. Zhan, Chem. Soc. Rev. 41 (2012) 4245-4272. doi: 10.1039/c2cs15313k
-
[7]
C.L. Wang, H.L. Dong, et al., Chem. Rev. 112 (2012) 2208-2267. doi: 10.1021/cr100380z
-
[8]
J.H. Dou, Y.Q. Zheng, Z.F. Yao, et al., Adv. Mater. 27 (2015) 8051-8055. doi: 10.1002/adma.201503803
-
[9]
C. Bronnbauer, A. Osvet, C.J. Brabec, et al., ACS Photonics 3 (2016) 1233-1239. doi: 10.1021/acsphotonics.6b00234
-
[10]
E.F. Gomez, A.J. Steckl, ACS Photonics 2 (2015) 439-445. doi: 10.1021/ph500481c
-
[11]
J. Liang, L. Li, X. Niu, et al., Nat. Photonics 7 (2013) 817-824. doi: 10.1038/nphoton.2013.242
-
[12]
J. Liu, D. Chen, X. Luan, et al., ACS Appl. Mater. Interfaces 9 (2017) 12647-12653. doi: 10.1021/acsami.7b00463
-
[13]
H. Niikura, F. Legare, R. Hasbani, et al., Nature 421 (2003) 826-829. doi: 10.1038/nature01430
-
[14]
Y.R. Sun, N.C. Giebink, H. Kanno, et al., Nature 440 (2006) 908-912. doi: 10.1038/nature04645
-
[15]
M.S. White, M. Kaltenbrunner, E.D. Glowacki, et al., Nat. Photonics 7 (2013) 811-816. doi: 10.1038/nphoton.2013.188
-
[16]
M. Segal, M. Singh, K. Rivoire, et al., Nat. Mater. 6 (2007) 374-378. doi: 10.1038/nmat1885
-
[17]
T. Earmme, S.A. Jenekhe, Adv. Funct. Mater. 22 (2012) 5126-5136. doi: 10.1002/adfm.201201366
-
[18]
Z.C. Wen, X.J. Ma, X.Y. Yang, et al., Chin. Chem. Lett. 30 (2019) 995-999. doi: 10.1016/j.cclet.2019.01.028
-
[19]
N. Wang, W.T. Yang, S.X. Li, et al., Chin. Chem. Lett. 30 (2019) 1277-1281. doi: 10.1016/j.cclet.2019.01.010
-
[20]
Z.Q. Gao, Z.H. Li, P.F. Xia, et al., Adv. Funct. Mater. 17 (2007) 3194-3199. doi: 10.1002/adfm.200700238
-
[21]
K.H. Kim, J.L. Liao, S.W. Lee, et al., Adv. Mater. 28 (2016) 2526-2532. doi: 10.1002/adma.201504451
-
[22]
K.S. Yook, S.E. Jang, S.O. Jeon, et al., Adv. Mater. 22 (2010) 4479-4483. doi: 10.1002/adma.201002034
-
[23]
M. Pfeiffer, S.R. Forrest, K. Leo, et al., Adv. Mater. 14 (2002) 1633-1636. doi: 10.1002/1521-4095(20021118)14:22<1633::AID-ADMA1633>3.0.CO;2-%23
-
[24]
J. Kido, T. Matsumoto, Appl. Phys. Lett. 73 (1998) 2866-2868. doi: 10.1063/1.122612
-
[25]
G.F. He, M. Pfeiffer, K. Leo, et al., Appl. Phys. Lett. 85 (2004) 3911-3913. doi: 10.1063/1.1812378
-
[26]
X. Guan, K. Zhang, F. Huang, et al., Adv. Funct. Mater. 22 (2012) 2846-2854. doi: 10.1002/adfm.201200199
-
[27]
J. Lee, Y. Park, D.Y. Kim, et al., Appl. Phys. Lett. 82 (2003) 173-175. doi: 10.1063/1.1537048
-
[28]
J.S. Huang, G. Li, E. Wu, et al., Adv. Mater. 18 (2006) 114-117. doi: 10.1002/adma.200501105
-
[29]
S. Lee, H. Shin, J.J. Kim, Adv. Mater. 26 (2014) 5864-5868. doi: 10.1002/adma.201400330
-
[30]
T.W. Lee, O.O. Park, L.M. Do, et al., J. Appl. Phys. 90 (2001) 2128-2134. doi: 10.1063/1.1391215
-
[31]
C. Duan, K. Zhang, X. Guan, et al., Chem. Sci. 4 (2013) 1298-1307. doi: 10.1039/c3sc22258f
-
[32]
J. Fang, B.H. Wallikewitz, F. Gao, et al., J. Am. Chem. Soc. 133 (2011) 683-685. doi: 10.1021/ja108541z
-
[33]
C.V. Hoven, A. Garcia, G.C. Bazan, et al., Adv. Mater. 20 (2008) 3793-3810. doi: 10.1002/adma.200800533
-
[34]
Z. Hu, K. Zhang, F. Huang, et al., Chem. Commun. 51 (2015) 5572-5585. doi: 10.1039/C4CC09433F
-
[35]
F. Huang, Y. Zhang, M.S. Liu, et al., Adv. Funct. Mater. 19 (2009) 2457-2466. doi: 10.1002/adfm.200801898
-
[36]
H. Ma, H.L. Yip, F. Huang, et al., Adv. Funct. Mater. 20 (2010) 1371-1388. doi: 10.1002/adfm.200902236
-
[37]
J. Park, R. Yang, C.V. Hoven, et al., Adv. Mater. 20 (2008) 2491-2496. doi: 10.1002/adma.200702995
-
[38]
S. Xiao, Q. Zhang, W. You, Adv. Mater. 29 (2017) 1601391.
-
[39]
G. Zhou, Y. Geng, Y. Cheng, et al., Appl. Phys. Lett. 89 (2006) 233501.
-
[40]
R. Yang, Y. Xu, X.D. Dang, et al., J. Am. Chem. Soc. 130 (2008) 3282-3283. doi: 10.1021/ja711068d
-
[41]
C.H. Wu, K.W. Tsai, W.J. Huang, et al., Adv. Mater. Inter. 3 (2016) 1500621.
-
[42]
H. Li, Y. Xu, C.V. Hoven, et al., J. Am. Chem. Soc. 131 (2009) 8903-8912. doi: 10.1021/ja9018836
-
[43]
D.G. Georgiadou, L.C. Palilis, M. Vasilopoulou, et al., J. Mater. Chem. 21 (2011) 9296-9301. doi: 10.1039/c0jm04567e
-
[44]
D.G. Georgiadou, M. Vasilopoulou, L.C. Palilis, et al., ACS Appl. Mater. Interfaces 5 (2013) 12346-12354. doi: 10.1021/am402991b
-
[45]
T. Chiba, Y.J. Pu, T. Ide, et al., ACS Appl. Mater. Interfaces 9 (2017) 18113-18119. doi: 10.1021/acsami.7b02658
-
[46]
S. Stolz, Y. Zhang, U. Lemmer, et al., ACS Appl. Mater. Interfaces 9 (2017) 2776-2785. doi: 10.1021/acsami.6b15062
-
[47]
M. Takada, T. Nagase, T. Kobayashi, et al., Org. Electron. 50 (2017) 290-295. doi: 10.1016/j.orgel.2017.07.049
-
[48]
K.W. Tsai, C.H. Wu, J.Y. Jan, et al., J. Mater. Chem. C 4 (2016) 8559-8564. doi: 10.1039/C6TC03051C
-
[49]
R. Grover, R. Srivastava, M.N. Kamalasanan, et al., J. Lumin. 146 (2014) 53-56. doi: 10.1016/j.jlumin.2013.09.004
-
[50]
C.A. Di, G. Yu, Y. Liu, et al., Appl. Phys. Lett. 90 (2007) 133508.
-
[51]
Y.K. Kim, J. Won Kim, Y. Park, Appl. Phys. Lett. 94 (2009) 063305.
-
[52]
K.S. Lee, L. Lim, S.H. Han, et al., Org. Electron. 15 (2014) 343-347. doi: 10.1016/j.orgel.2013.11.023
-
[53]
J. Niederhausen, P. Amsalem, J. Frisch, et al., Phys. Rev. B 84 (2011) 165302.
-
[54]
S.M. Park, Y.H. Kim, Y. Yi, et al., Appl. Phys. Lett. 97 (2010) 063308.
-
[55]
D.S. Acker, W.R. Hertler, J. Am. Chem. Soc. 84 (1962) 3370-3374. doi: 10.1021/ja00876a028
-
[56]
D. Dong, D. Fang, H.R. Li, et al., Chem.-Asian J. 12 (2017) 920-926. doi: 10.1002/asia.201700112
-
[57]
Z.Q. Lin, P.J. Sun, Y.Y. Tay, et al., ACS Nano 6 (2012) 5309-5319. doi: 10.1021/nn3011398
-
[58]
M. Mamada, C. Perez-Bolivar, P. Anzenbacher Jr., Org. Lett. 13 (2011) 4882-4885. doi: 10.1021/ol201973w
-
[59]
J.F. Zhao, L. Chen, P.J. Sun, et al., Tetrahedron 67 (2011) 1977-1982. doi: 10.1016/j.tet.2010.12.065
-
[60]
W.J. Li, B. Liu, Y. Qian, et al., Polym. Chem. 4 (2013) 1796-1802. doi: 10.1039/c2py20971c
-
[61]
Y. Yu, L.Y. Bian, J.G. Chen, et al., Adv. Sci. 5 (2018) 1800747.
-
[62]
G. Li, J.F. Zhao, S.F. Yang, et al., Chem.-Asian J. 13 (2018) 250-254. doi: 10.1002/asia.201701674
-
[63]
Q. Miao, Adv. Mater. 26 (2014) 5541-5549. doi: 10.1002/adma.201305497
-
[64]
U.H.F. Bunz, Acc. Chem. Res. 48 (2015) 1676-1686. doi: 10.1021/acs.accounts.5b00118
-
[65]
Y. Liu, X. Wu, Z. Xiao, et al., Appl. Surf. Sci. 413 (2017) 302-307. doi: 10.1016/j.apsusc.2017.04.038
-
[66]
C. Fan, L. Zhu, B. Jiang, et al., J. Phys. Chem. C 117 (2013) 19134-19141. doi: 10.1021/jp406220c
-
[67]
C. Adachi, M.A. Baldo, M.E. Thompson, et al., J. Appl. Phys. 90 (2001) 5048-5051. doi: 10.1063/1.1409582
-
[68]
Y. Dai, H. Zhang, Z. Zhang, et al., J. Mater. Chem. C 3 (2015) 6809-6814. doi: 10.1039/C4TC02875A
-
[69]
H. Glowatzki, B. Bröker, R.P. Blum, et al., Nano Lett. 8 (2008) 3825-3829. doi: 10.1021/nl8021797
-
[70]
Z. Bin, H. Guo, Z. Liu, et al., ACS Appl. Mater. Interfaces 10 (2018) 4882-4886. doi: 10.1021/acsami.7b17385
-
[71]
H.Y. Oh, J.W. Kim, Appl. Phy. Lett. 97 (2010) 063308.
-
[72]
W. Chen, Y. Zhou, L. Wang, et al., Adv. Mater. 30 (2018) 1800515.
-
[73]
W. Kaim, M. Moscherosch, Coord. Chem. Rev. 129 (1994) 157-193. doi: 10.1016/0010-8545(94)85020-8
-
[74]
R. Xu, Y. Guo, S.J. Shao, Chin. Chem. Lett. 17 (2006) 377-379.
-
[1]
-
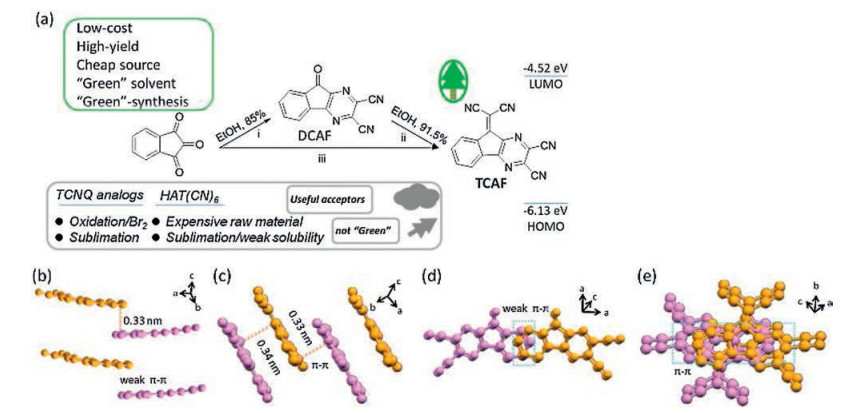
Scheme 1 (a) The synthetic routes of DCAF and TCAF: (ⅰ) 1H-indene-1, 2, 3-trione, 2, 3-diaminomaleonitrile (DAMN), EtOH, refluxed, 10 h, 85.0% for DCAF; (ⅱ) DCAF, malononitrile, EtOH, refluxed, 10 h, 91.5% for TCAF; (ⅲ) 1, 2, 3-indantrione, DAMN, and EtOH, refluxed, 10 h; then malononitrile, refluxed, another 10 h. Packing modes of DCAF (b, d) and TCAF (c, e) in single crystals.
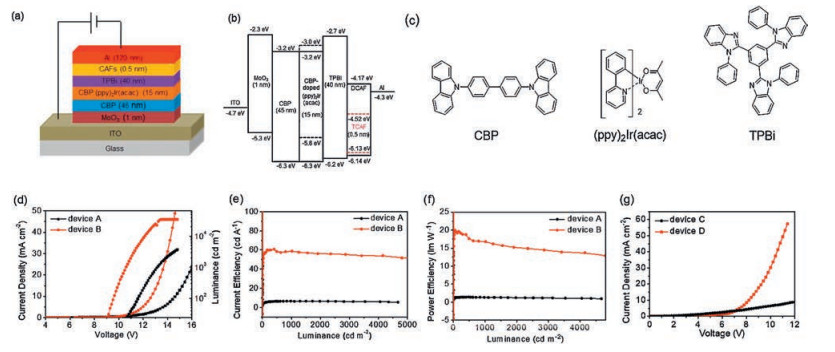
Figure 2 (a) Schematic configuration of devices A and B; (b) Energy level diagrams of devices A and B; (c) molecule structures of organic materials in devices A and B; (d) J–V–L characteristics of devices A and B; (e) LE–L characteristics of devices A and B; (f) PE–L characteristics of devices A and B; (g) L–V characteristics of DCAF/TCAF-based electrononly devices C and D.
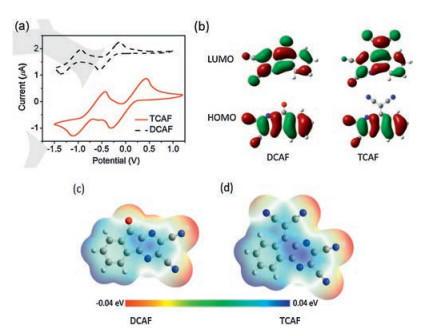
Table 1. Electrochemical and thermal characteristics of DCAF and TCAF.
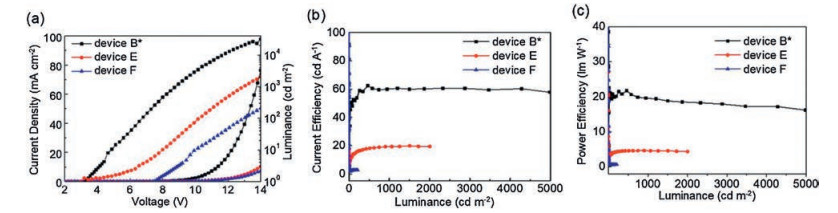
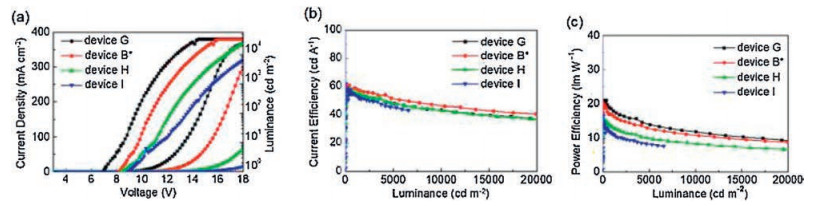
Table 2. Three groups of device characteristics: Ⅰ. Devices A and B with different CAFs as EIM; Ⅱ. Devices B, E, and F with different thicknesses of TCAF; Ⅲ. Devices G, B, H and I with different thicknesses of TPBi (30, 40, 50, and 60 nm).

-

计量
- PDF下载量: 8
- 文章访问数: 1148
- HTML全文浏览量: 31

 下载:
下载:
 下载:
下载:

